Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.73 Durban 2020
http://dx.doi.org/10.17159/0379-4350/2020/v73a18
RESEARCH ARTICLE
Anti-corrosion Behaviour of Expired Tobramycin Drug on Carbon Steel in Acidic Medium
F.E. AbengI, *; V.C. AnadebeII; V.D. IdimI; M.M. EdimI
IMaterial and Electrochemistry Research Group, Department of Chemistry, Cross River University of Technology, P.M.B. 1123, Calabar, Nigeria
IIDepartment of Chemical Engineering, Federal University Ndufu Alike, Ebonyi State, P.M.B. 1010, Abakaliki, Nigeria
ABSTRACT
The anti-corrosion behaviour of the expired Tobramycin (ETo) drug for carbon steel in 2 M HCl solution was investigated by thermometric methods (Thermo), potentiodynamic polarization (PDP), electrochemical impedance spectroscopy (EIS) and scanning electron microscopy (SEM). The results obtained from Thermo, PDP and EIS show that the level of anti-corrosion behaviour is in direct association to the ETo application. The model of adsorption isotherm fitted Langmuir, confirming a chemical adsorption system. The SEM image of carbon steel immersed in optimum concentration of ETo has confirmed the film formation on the carbon steel surface. The PDP technique, indicated by the Tafel plots, demonstrated that the ETo inhibitor acted as a mixed-type inhibitor. The results obtained from activation energy, Ea, the quantity of heat adsorbed, Qads, and standard free energy of adsorption, AG°ads, show a chemical adsorption mechanism of the ETo inhibitor.
Keywords: Tobramycin, thermometric, potentiodynamic polarization, electrochemical impedance spectroscopy, carbon steel, corrosion.
1. Introduction
Carbon steel plays vital roles in manufacturing companies, due to its low cost of production. However, various corrosive media can easily oxidize carbon steel, due to its high chemical reactivity. Corrosion of metals, which lead to massive loss of resources, is one of the major challenges in industries. Prevention of corrosion of metal is important to increase the life span of equipment that are made from iron, in order to protect the environment and save cost of maintenance.1 Most operations in the manufacturing industries are associated with an acid solution, for instance, industrial acid cleaning, acid descaling, and oil well acidifying processes. Because of the strong corrosivity of acid solutions, anti-corrosive agents, called inhibitors are the best and cheapest method for protecting metals in aqueous media. The application of an inhibitor to the corrodent decreases the rate of dissolution of the metal. An organic compound containing electronegative heteroatoms, such as N, P, O and S1, are mostly considered as organic corrosion inhibitors. Pharmaceutical drug molecules fall under this class of organic compounds. Current research in the field of corrosion inhibition is geared towards the use of these drugs as anti-corrosive agent.2 Our group have made a series of contribution in this area of research, using Levofloxacin, Moxifloxacin and Nifedipine as corrosion inhibitors.3-5 Karthikeyan et al.6investigated Vancomycin as a corrosion inhibitor for mild steel ina1MH2SO4 solution, using weight loss, potentiodynamic polarization (PDP) and electrochemical impedance spectroscopy (EIS). Abdullatef7 studied the inhibitory action of Azithromycin as an inhibitor for corrosion of mild steel, copper and zinc in a 0.5 M H2SO4 solution using PDP and EIS. Karthikeyan et al.8also reported Dicloxacillin as an anti-corrosion additive for mild steel in a sulphuric acid solution using gravimetric and electrochemical methods. The drug, Farcolin, was reported as an organic inhibitor for acid corrosion of metals by Attia.9 Farcolin was tested as a corrosion inhibitor using gravimetric and electrochemical methods. Ade et al.10studied the inhibitory performance of an anti-fungal drug as an organic inhibitor for mild steel in HCl, HNO3 and H2SO4 using gravimetric method. However, most of the pharmaceutical drugs are much more expensive than the organic inhibitors which are currently used in industries. Thus, using fresh drugs as a corrosion inhibitor is not economically viable. Therefore, it was thought worthwhile to investigate the corrosion inhibition properties of expired drugs. It is well reported that a drug retains at least 90 % of its original potency even after its expiration date, but the use for medicinal purposes is restricted. Thus, the use of expired drugs as a corrosion inhibitor can solve two major problems: environmental pollution with pharmaceuti-cally active compounds and the reduction of the disposal costs of expired drugs.2,11-14 Dohare et al.2reported the application of expired Tramadol as an anti-corrosion agent for mild steel in hydrochloric acid. However, studies on expired drugs as anti-corrosion agents are scanty. Given the observation above, it was thought worthwhile to study the corrosion inhibition behaviour of expired Tobramycin (ETo) for carbon steel in acidic medium using thermometric and electrochemical methods. Tobramycin plays a very important role in the management of lung disease for both the adult and paediatric populations, because of its activity against P. aeruginosa. Tobramycin is also used in treating urinary tract infections due to its excellent inhibiting properties for urinary tract infection. The IUPAC name for Tobramycin is given as (2S,3R,4S,5S,6R)-4-amino-2-[(1S,2S,3R,4S,6R)-4,6-diamino-3-[(2R,2S,5S,6R)-3-amino-6-(aminomethyl)-5-hydro-xyoxan-2-yl]oxy-2-hydroxylcyclohexyl]oxy-6-hydroxymethyl)-oxane-3,5-diol. It has a molecular mass of 467.575 g mol-1 with the molecular formula C18H37N5O9. The chemical structure of Tobramycin is given in Fig. 1.

2. Experimental
2.1. Acid Inhibitor Solution Preparation
The stock solution of the inhibitor, ETo, was prepared by dissolving the appropriate amount of the expired drug in a previously prepared 2 M HCl standard solution. A serial dilution method was used to obtain the concentration for the corrosion experiments.
2.2. Preparation of Carbon Steel Specimen
The carbon steel specimens were measured (5 cm x 4 cm x 0.1 cm) and mechanically cut. Each coupon was abraded using 220, 320 and 400 and 600 emery papers to obtain a mirror-polished surface for thermometric experiment.
The working electrode for the electrochemical experiment was prepared by soldering copper wire to the coupon. Two components epoxy resin was used to mount the carbon steel coupons, with the copper wire, in a PVC holder leaving an exposed surface area of 1 cm2 of the coupon. Before running the electrochemical experiments, the coupons were polished, rinsed in deionized water, followed by acetone, and dried in air.
The chemical composition of carbon steel used in this research is listed in Table 1.
2.3. Thermometric Measurement
The reaction vessel used was a well-lagged calorimeter, containing carbon steel in inhibited and uninhibited (blank) solution. The reaction vessel was placed in a water bath. The temperature was allowed to rise and was recorded per minute for 30 min. Equation 1 was used to calculate the reaction number (RN).

where Tm and Ti represent the maximum and initial temperatures in °C, t is the time taken in minutes to reach the maximum temperature. The inhibition efficiency was calculated using Equation 2.

where RNblank and RNinh are reaction numbers for carbon steel corrosion in uninhibited (blank) and inhibited solutions of HCl.
2.4. Electrochemical Methods
The electrochemical measurement experiments were performed in 2.0 M HCl solution in the absence and presence of different concentrations of ETo (inhibitor) at 303 K. A conventional three-electrode cell assembly, consisting of the working electrode representing the carbon steel, counter electrode represented by (1 cm x 1 cm) platinum foil and the reference electrode (the saturated calomel electrode) at a constant temperature was connected to a Gamry 600 electrochemical system for the electrochemical experiments. The electrochemical measurement was performed in a glass flask filled with 250 mL test solution. The test solution was 2 M HCl both in the absence and presence of five different concentrations, namely 50 ppm, 100 ppm, 200 ppm, 300 ppm and 500 ppm of ETo. This setup was kept at room temperature for 1800 s (30 min) and then electrochemical measurements were carried out. EIS measurement was carried out over the frequency range of 100 kHz to 0.5 Hz with a signal amplitude of 5 mV. PDP measurements were performed at the potential range of 0.25-0.5 mV and scan rate of 0.5 mV s-1.3,15 The inhibition efficiencies of the studied compound were calculated using Equations 3 and 4 in PDP and EIS experimental measurement, respectively.
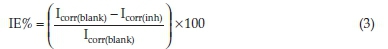
where Icorr(blank) and Icorr(inh) are corrosion current densities measured in the blank solution and solution containing inhibitor, respectively.

where Rct(blank) and Rct(inh) are charge transfer resistance of uninhibited and inhibited solution, respectively.
2.5. Scanning Electron Microscopy (SEM)
The carbon steel coupons were immersed in 2 M HCl solution in the absence and presence of the inhibitor for 24 h at room temperature. The morphological analyses of the corroded coupons were carried out at Chemical Engineering Department Ahmadu Bello University, Zaria, using the SEM (Model MVE-016477830, Phenom-world Eindhoven, Netherlands).
3. Results and Discussion
3.1. Thermometric Method
The variation of temperature as a function of time is regarded as a thermometric study. Fig. 2 shows the variation of temperature with respect to time in uninhibited (blank) and inhibited ETo media. At 30 min the maximum temperature Tm of 68 °C was recorded in the blank solution. This gave a reaction number (RN) of 1.3 °C min-1 listed in Table 2.


The results from the thermometric method shown in Table 2 revealed that ETo decrease the corrosion of carbon steel. The values of inhibition efficiency (IE) of ETo were observed to increase with an increase in concentration of the inhibitor. This shows that the inhibitive properties of ETo have been adsorbed on the metal surface, leading to a decrease in the dissolution rate of the carbon steel.3,16
3.2. Potentiodynamic Polarization (PDP)
Figures 3 and 4 represent carbon steel Tafel plots of various concentrations of ETo in 2 M HCl solution at 303 K and 323 K, respectively. The cathodic and anodic corrosion current densities of the branches decreased through the addition of ETo, suggesting that both the cathodic and anodic reaction rates were resisted as a result of the adsorption of the inhibitor at the active sites on the carbon steel surface. The inhibitive effect was due to the adsorption of ETo molecules at the surface of the steel.17 An inhibitor or molecule is regarded as anodic or cathodic when the shifting mechanism in the Ecorr value on the addition of the studied inhibitor is more than 85 mV compare to the blank solution. From our investigation, the deviation of Ecorr is less than 85 mV, this describes that ETo acted as a mixed-type inhibitor for corrosion of carbon steel.


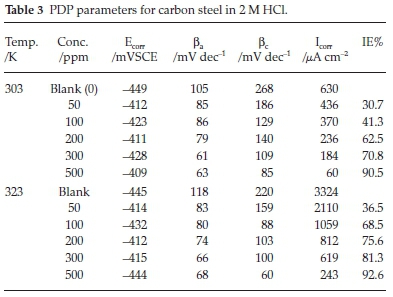
The values of Ecorr, cathodic (ßc) and anodic (ßa) Tafel slopes, Icorr and the percentage of IE are presented in Table 3. The inhibitor concentration increased from zero (that is the blank) to 500 ppm with corresponding values of Icorr decreasing gradually from 630 cm-2 to 60 cm-2 at 303 K and from 3324 cm-2 to 243 jiA cm-2 for 323 K. This signifies that ETo was adsorbed on the carbon steel surface.18 Thus the percentage IE exhibited a maximum value of 90.5 % and 92.6 % at 303 K and 323 K, respectively. The Tafel constants, namely, ßa and ßc, decreased with the increasing of in ETo concentration. ßa was more deviated compared to ßc, showing that ETo controls mainly the anodic metal dissolution at both temperatures.
3.3. Electrochemical Impedance Spectroscopy (EIS)
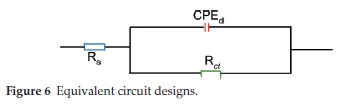
Nyquist plots for carbon steel dissolution in hydrochloric acid at 303 K in an uninhibited (blank) and ETo inhibited solution are illustrated in Fig. 5. Addition of ETo in the acidic media considerably increases the diameter of the Nyquist plots, thus demonstrating a decrease in the dissolution process of the carbon steel. The depressed capacitive-loop of the uninhibited (blank) and ETo inhibited solution corresponds to the double-layer capacitance or the constant phase element as shown in Fig. 6. The double-layer capacitance decreased as the concentration of the inhibitor increased. This is attributed to the surface coverage or blockage of the surface of the steel by ETo, thereby shielding the surface of the metal from corrosive agents.19-20 The EIS kinetics are shown in Table 4. The values of the constant phase element representing double-layer capacitance (Cdl) were obtained from Equation 5.

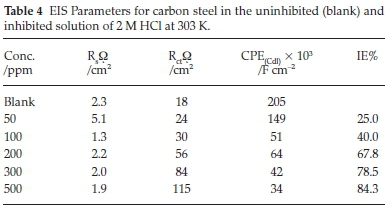

where fmax is the frequency at which the imaginary component of impedance -zmax is maximal and Rct is charge transfer resistance. Table 4 shows an increase in inhibition efficiency with an increased in ETo concentration.
3.3.1. Adsorption and Thermodynamic Study
To describe the adsorption behaviour of ETo, several adsorption isotherms have been tested and the Langmuir kinetic ther-modynamic model fits the experimental data well. Langmuir isotherm is given by Equation 6.21,22

where C is the concentration of inhibitor, Kads is the adsorption equilibrium constant and 9 is the degree of surface coverage determined from the values of inhibition efficiency (IE%) according to Equation 7.

The plots of C/0versus C gave a straight line with an intercept as shown in Fig. 7. The correlation coefficient (R2) was almost equal to unity, which confirmed that the adsorption of the inhibitor on the steel surface obeyed the Langmuir adsorption iso-therm.23,24 Equation 8 shows the relationship between adsorption equilibrium constant (Kads) and standard free energy of adsorption denoted as ΔG°.


Csolution represents the concentration of water in the solution. This is obtained by considering the equation of mass-mole relationship given as Equation 9,

where n is the number of mole substance dissolve, M is the molar mass of water and m is the mass of 1000 mL of water, which is equivalent to 1000 g. When these parameters are substituted in Equation 10 it gives 55.5 mole of the substance dissolve.

In substituting Equation 10 into Equation 11 it gives us the value of the concentration of water used in the inhibitor preparation.
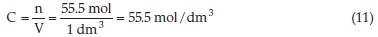
where C is the concentration of water used in the inhibitor preparation and V is the volume of water used in the inhibitor stock solution preparation. When the exponential of Equation 8 is removed, it results in Equation 12. Relating the free energy of adsorption, ΔGads, and the concentration of water used in the inhibitor preparation.

The values of ΔGads, obtained in all the methods and listed in Table 5, reflected a chemical adsorption mechanism. The negative sign and high values of AGads is an indication of ionic bonding between the inhibitor and carbon steel, which enhances the efficient adsorption.24

Equation 13 is known as Arrhenius condensed equation, which is used in determining the activation energy, Ea,of ETo in the corrosion reaction.

where icorr(1) and icorr(2) are corrosion current density at the temperature of 303 K (T1) and 323 K (T2), respectively. The Eavalues of the corrosion inhibition of carbon steel in inhibited and uninhibited solution are shown in Table 6. The results show that the Ea value of the uninhibited solution was higher than the Eavalue of the inhibited solution. This suggests a chemical adsorption, which confirmed the mechanism of ΔGads.24 The quantity of heat adsorbed, Qads, was also calculated using Equation 14.


where 81 and 82 represent the degree of surface coverage at T1 and T2, respectively, while R is the gas constant. The values of Qads were recorded in Table 6. The values were positive and consistent with an inhibitor indicating chemical adsorption and endothermic nature on the surface of the carbon steel.3,24
3.4. Mechanism of Corrosion Inhibition
Generally, it is believed that the ETo molecule was adsorbed on the metal surface by displacing the water molecules, thereby forming a protective film. The adsorption occurs through the interaction between the n-electrons of the rings of the ETo compound and the empty d-orbitals of the iron (Fe), that is present in the carbon steel. Secondly, the natural N and O (nitrogen and oxygen) atoms present in the ETo compound can be protonated in acid solution, thereby adsorbing on the cationic steel surface (that has already been saturated by negative Cl-(X) ions from the acids) leading to the electrostatic interaction illustrated in the Equation 15.
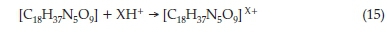
3.5. Scanning Electron Microscopy (SEM)
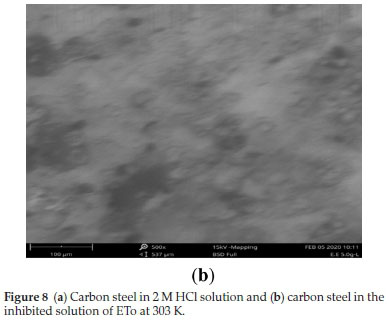
The surface texture of the carbon steel coupons, in the uninhibited and the inhibited solution at 303 K, was also analyzed using a scanning electron microscope. The morphological results of the carbon steel are shown in Fig. 8a,b. The examination of the Fig. 8a exhibited a harsh crack and plain pits on the steel surface in the absence of the inhibitor. In the presence of ETo (Fig. 8b) there were fewer pits and crack.21,25-28
4. Conclusion
Expired Tobramycin (ETo) is an excellent anti-corrosive agent for the studied carbon steel in acidic medium with inhibition efficiencies of 80 %, 90.5 %, 84.3 % at optimum concentration of 500 ppm. The nature of the adsorption mechanism of ETo molecules on the carbon steel surface followed a chemical adsorption mechanism and obeyed the Langmuir adsorption isotherm. Thus, the interaction between the inhibitor and the steel surface could be described as a monolayer adsorption. The EIS experimental measurement showed that the charge transfer resistance of the electrode increased, and double-layer capacitance decreased, which in turn resulted in an increase of inhibition efficiency with an increase in the concentration of ETo. PDPs techniques indicated that the ETo behaved as a mixed-type inhibitor.
ORCID iDs
EE. Abeng: orcid.org/0000-0002-2791-4034
V.C. Anadebe: orcid.org/0000-0002-7559-446X
References
1 A.K. Oyebamiji, B.M. Lasisi, E.O. Oyebamiji, A.K. Adegoke, B. Semire and B.B. Adeleke, Theoretical evaluation of pyrazole [3,4-d]pyrimi-dinethiones analogues as corrosion inhibitor for carbon steel in hydrochloric acid, Int. J. Modern Chem., 2018, 10(3), 138-153. [ Links ]
2 P. Dohare, D.S. Chauhan, A.A. Sorour and M.A. Quraishi, DFT and experimental studies on the inhibition potential of expired Tramadol drug on mild steel corrosion in hydrochloric acid, Mater. Disc., 2017, https://doi.org/10.1016/j.md.2017.11.001 [ Links ]
3 F.E. Abeng, M.E. Ikpi, V.E. Okpashi, O.A. Ushie and M.E. Obeten, Electrochemical and quantum chemical parameters of (-S)-9-fluoro-2,2-dihydro-3-3methyl-10 (4-methyl-1-pipera-zinyl)-7oxo-7H-Pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid as anti-corrosive agent for API 5L X-52 steel, J. Electrochem. Sci. Eng., 2020, 10(1), 21-28. [ Links ]
4 M.E. Ikpi and F.E. Abeng, Theoretical study on the corrosion inhibitor potential of moxifloxacin for API 5L X-52 steel in acidic environment, IOP Conf. Series: Earth Environ. Sci., 2018,173, DOI:10.1088/1755 -1315/173/1/012018 [ Links ]
5 F.E. Abeng, M.E. Ikpi, O.A. Ushie and M.M. Edim, Investigation of inhibition effect of nifedipine on API 5l X-52 steel corrosion in hydrochloric acid, J. Chem. Soc. Nig., 2019, 44(6), 1132-1141. [ Links ]
6 S. Karthikeyan, P.A. Jeeva and K. Raja, Experimental studies of an antibacterial agent on the corrosion of mild steel in1MH2SO4, J. Chem. Pharm. Res., 2015, 7(1), 906-912. [ Links ]
7 O.A. Abdullatef, Chemical and electrochemical studies on the corrosion mild steel, copper and Zinc in 0.5 M H2SO4 solution in presence of azithromycin as effective corrosion inhibitor, J. Adv. Chem., 2015,11, 3642-3655. [ Links ]
8 S. Karthikeyan, P.A. Jeeva and K. Raja, Docloxacillin: an effective retarder for mild steel dissolution in acid medium, Int. J. Chem. Tech. Res., 2015, 8(3), 1391-1395. [ Links ]
9 Attia, E.M, Expired farcolin drugs as corrosion inhibitor for carbon steel in 1 M HCl solution, J. Basic Appl. Chem., 2015, 5(1), 1-15 [ Links ]
10 S.B. Ade, N.V Shitole and S.M. Lonkar, Antifungal drugs used as metal corrosion inhibitor in various acid medium, Int. J. Chem. Tech. Res., 2014, 6, 3642-3650. [ Links ]
11 M. Kotchen, J. Kallaos, K. Wheeler, C. Wong and M. Zahller, Pharmaceutical in wastewater: behaviour, preferences and willingness to pay for a disposal program, J. Environ. Manage., 2009, 90, 1476-1482. [ Links ]
12 S.K. Khetan and T.J. Collins, Human pharmaceuticals in the aquatic environment: a challenge to green chemistry, Chem. Rev., 2007, 107, 2319-2364. [ Links ]
13 I.S. Ruthoy and C.G. Daughton, Types and quantities of leftover drugs entering the environment via disposal to sewage-revealed by coroner records, Sci. Total Environ., 2007, 388, 137-148. [ Links ]
14 P. Singh, E.E. Ebenso, L.O. Olasunkanmi, I. Obot and M. Quraishi, Electrochemical, theoretical and surface morphological studies of corrosion inhibition effect of green naphthyridine derivatives on mild steel in hydrochloric acid, J. Phys. Chem. C, 2016, 120, 3408-3419. [ Links ]
15 C. Verma, L.O. Olasunkanmi, E.E. Ebenso, M. Quraishi and I. Obot, Adsorption behaviour of glucosamine-base, pyrimidine-fused heterocyclic as green corrosion inhibitors for mild steel in acid: experimental and theoretical studies, J. Phys. Chem. C, 2016, 120, 11598-11611. [ Links ]
16 M.E. Ikpi and F.E. Abeng Electrochemical and quantum chemical investigation on adsorption of nifedipine at API 5L X-52 Steel / HCl acid interface as corrosion inhibitor, Arch. Metall. Mater., 2020, 65(1), 125-131. [ Links ]
17 I.A. Akpan and N.O. Offiong, Electrochemical investigation of the inhibitory action of ciprofloxacin drugs on the acid corrosion of mild steel, Chem. Process Eng. Res., 2014, 26, 20-23. [ Links ]
18 I.A. Akpan, and N.O. Offiong, Electrochemical study of the corrosion of mild steel in hydrochloric acid by amlodipine drugs, Int. J. Chem. Mater. Res., 2014, 2(3), 23-29. [ Links ]
19 R.S. Abdel-Hameed, E.A. Ismail, A.H. Abu-Nawwas and H.I. Al-Shafey, Expire voltaren drugs as corrosion inhibitor for aluminum in hydrochloric acid, Int. J. Electrochem. Sci., 2015,10, 2098-2109. [ Links ]
20 H.H. Zhang, C.K. Qin, Y. Chen and Z. Zhang, Inhibition behaviour of mild steel by three new benzaldehyde thiosemicarbazone derivatives in 0.5 M H2SO4: experimental and computational study, Royal Soc. Open Sci., 2019, 6, 190192-190211. [ Links ]
21 A.S. Fouda, M.N. El-Haddad and Y.M. Abdallah. Septazole: antibacterial drug as green corrosion inhibitor for copper in hydrochloric solution, Int. J. Innov. Res. Sci., Eng. Tech., 2013, 2(12), 7073-7085. [ Links ]
22 I. Naqui, A.R. Saleemi and S. Naveed, Cefixime: a drug as efficient corrosion inhibitor for mild steel in acidic media. Electrochemical thermodynamic studies, Int. J. Electrochem. Sci., 2011, 6, 146-161. [ Links ]
23 N.O. Eddy, S.R. Stoyanov and E.E. Ebenso, Fluoroquinolones as corrosion inhibitors for mild steel in acidic medium; experimental and theoretical studies, Int. J. Electrochem. Sci., 2010, 5, 1127-1150. [ Links ]
24 A.K. Singh and E.E. Ebenso, Effect of Ceftezole on the corrosion of mild steel inHCl solution, Int. J. Electrochem. Sci., 2012,7,2349-2360. [ Links ]
25 N. El-Aouni, A. Benabida, M. Cherkaoui and A. Elharfi, A new epoxy resin based organic molecule as an effective inhibitor of mild steel corrosion in sulfuric acid medium, J. Chem. Tech. Metall., 2018, 53(5), 878-890. [ Links ]
26 V.C. Anadebe, O.D. Onukwuli, M. Omotioma and N.A. Okafor, Optimization and electrochemical study on the control of mild steel corrosion in hydrochloric acid solution with bitter kola leaf extract as inhibitor, S. Afr. J. Chem., 2018, 71, 51-61. [ Links ]
27 T. He, W. Emori, R. Zhang, P.C. Okafor, M. Yang and C. Cheng, Detailed characterization of Phellodendron chinense schneid and its application in the corrosion inhibition of carbon steel in acidic media, Bio. Chem., 2019, 130, 107332-107346. [ Links ]
28 N.K. Gupta, C. Gopal,V. Srivastavaand M.A. Quraishi, Application of expired drugs in corrosion inhibition of mild steel, Int. J. Pharm. Chem. Anal., 2017,4(1): 8-12. [ Links ]
Received 26 March 2020
Revised 12 May 2020
Accepted 14 May 2020
* To whom correspondence should be addressed. E-mail: fidelisabeng@yahoo.com