Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Chemistry
versão On-line ISSN 1996-840X
versão impressa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.72 Durban 2019
http://dx.doi.org/10.17159/0379-4350/2019/v72a32
RESEARCH ARTICLE
Novel Coumarin Derivatives: Synthesis, Characterization and Antimicrobial Activity
Chirag G. NaikI; Gulam M. MalikI; Hitesh M. ParekhII, *
IDepartment of Chemistry, Navyug Science College, Surat 395005, India
IIDepartment of Chemistry, School of Sciences, Gujarat University, Navrangpura, Ahmedabad 380009, India
ABSTRACT
The novel coumarin derivatives (2,3,4,5,6,7) have been synthesized from the reaction of o-acetyloxy benzoic acid with thionyl chloride yielding 2-acetoxy benzoyl chloride, which on further treatment with ethylacetoacetate gave 4-hydroxycoumarin. Substituted pyrazolones and thiazoles reacted with 4-hydroxy coumarin to give pyrazolones and methyl thiazoles related coumarin derivatives. The newly synthesized products were characterized with IR, 1H and 13C NMR, mass spectroscopic techniques and elemental analysis. The synthesized compounds were screened for their antibacterial and antifungal activity. All the compounds were found to have significant activity against the tested microorganisms.
Keywords: Coumarin, thiazole, pyrazolone, antibacterial activity, antifungal activity.
1. Introduction
In plants, coumarin derivatives are present in significant quantities and more than 1300 coumarins were identified from natural sources.1 ThIS family of compounds serve as an important model for the advanced design and synthesis of more active analogous coumarins, since natural compounds possess potent antioxidants and radical-scavenging properties as reportedin various experimental models.2 The synthesis of coumarin and its derivatives have attracted considerable attention from organic and medicinal chemists for many years, as large numbers of natural products contain this heterocyclic nucleus.3 They are widely used as optical brighteners4 and dispersed fluorescent and laser dyes,5 additives in food,6 perfumes,7 cosmetics8 and pharmaceuticals9 so the synthesis of this heterocyclic nucleus is of much interest. The coumarin derivatives possess a broad spectrum of biological activities such as, antifungal,10 antibacterial,11 anti-inflammatory,12 antiproliferative,13 antitumor,14 antiviral,15 antioxidant,16 anticoagulant,17 anticancer18 and anti-HIV19 activities. The reaction of o-acetyloxy benzoic acid and thionyl chloride to give 2-acetoxy benzoyl chloride, which then reacts with ethylacetoacetate to yield hydroxycoumarin. Coumarin heterocycles have been found to be very useful compounds for different types of activities. 4-Hydroxy coumarin derivatives are useful starting materials for the synthesis of new Coumarin derivatives.20-23 Coumarins have been synthesized by several routes including Pechmann,24 Perkin,25 Knoevenagel,26 Refor-matsky27 and the Wittig reaction.28 Bucumolol,29 Chromonar,30 Folescutol and 4-methyl umbelliferone31 are coumarin derivatives and are clinically used as antiarrhythmic,32 vasodilator,30 capillary and antispectively agents. Warfarin33 and aceno-comarol,34 which are coumarin derivatives, exhibit anticoagulant activity.35-37 Coumarin derivatives are also used to synthesize fluorescent polymers38 and fluorescent chemosensors for Mg+2 ion.39 We report herein the design and synthesis of pyrazolone and methyl thiazole related coumarins derivatives with significant biological importance.
2. Experimental
2.1. Methods and Materials
All the chemicals were procured from Sigma-Aldrich, and used without further purification. The 4-hydroxy coumarin was prepared by Knoevenagel's literature procedure.21 Initially, the purity of synthesized compounds was confirmed using aluminium-coated TLC plates (E. Merck). Melting points were determined using a Stuart SMP10 MP apparatus and are uncorrected. The IR spectra (v,cm-1) were recorded on a 8400 FT-IR-435 spectrometer using KBr pellets. Elemental analysis was performed on an ECS 4010 Elemental Combustion System. A Bruker-Avance 400 MHz spectrometer was used to record the 1H-NMR and 13C-NMR spectra using TMS as an internal standard. The chemical shifts were reported in parts per million (á-LC, ppm). Mass spectra was carried out on Waters Micro mass Q-Tof Micro having range of 4000 amu in quadruple and 20 000 amu in ToF.
2.1.1. Synthesis of Intermediates Chromen-2-one Derivatives (2, 3) (Scheme 1)
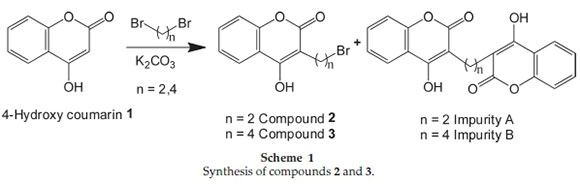
4-Hydroxy coumarin 1 (1.0 mole), DMF (4 vol) and potassium carbonate (1.1 mole) was heated to 85 °C. Dibromoalkane (2.3 mole) in DMF (1 vol) was added in to the reaction mixture. The reaction mass stirred for 5 h at 85 °C. The completion of the reaction waschecked on TLC (hexane/ethylacetate7:3, Rf = 0.85 for compound 2 and 0.57 for compound 1). After completion of the reaction, the reaction mass was filtered and washed with DMF. The collected filtrate was poured into water and stirred for 30 min. The obtained solid was filtered, washed with water and purified by recrystallization from acetone to give compound 2 and compound 3.
During the reaction, Impurity-A and Impurity-B formed, which are insoluble in DMF so they can easily be removed through filtration. The method of preparation for Impurity-A and Impurity-B is incorporated in the supplementary information. The purification of compounds 2 and 3 were carried out via recrystallization in acetone.
3-(2-Bromoethyl)-4-hydroxy-2H-chromen-2-one (2)
Off-white solid. Yield 73 %, m.p. 155-158 °C, 1H NMR (400 MHz, CDCl3) (Fig. S3) 7.25-7.83 (m, 4H, Ar-H), 5.81 (s, 1H, OH), 4.62 (m, 2H, CH2), 1.59 (m, 2H, CH2); 13C NMR (100 MHz, CDCl3) (Fig. S9) 28.63, 66.83, 90.56,114.95,116.04,122.72,123.66,132.24, 152.70, 161.65, 164.66; C11H9BrO3 (269.01); Found (C-49.16; H-3.30 %) requires (C-49.10, H-3.35, Br-29.70, O-17.84 %)
3-(2-Bromobutyl)-4-hydroxy-2H-chromen-2-one (3)
Off-white solid. Yield 78 %, m.p. 207-210 °C, 1H NMR (400 MHz, DMSO) (Fig. S4) 7.25-7.82 (m, 4H, Ar-H), 5.79 (s, 1H, OH), 4.33 (m, 2H, CH2), 2.96 (m, 2H, CH2), 2.13-2.18 (m, 4H, CH2); C13H13BrO3(297.03); Found (C-52.50; H-4.32 %) requires (C-52.56, H-4.38, Br-26.90, O-16.16 %)
2.1.2. Synthesis of Compound Ethyl-2,4-(3-(ethyl)-4-hydroxy-2H-chromen-2-one)phenyloxy-4,5-dihydro-4-methylthiazole-5-carboxylate (4) (Scheme 2)
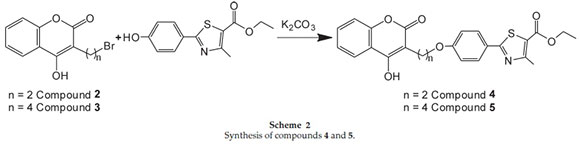
Ethyl-4,5-dihydro-2-(4-hydroxyphenyl)-4-methylthiazole-5-c arboxylate (1.0 mole) in DMF (3 vol) and potassium carbonate (1.0 mole) was heated up to 75 °C followed by addition of 3-(2-bromoethyl)-4-hydroxy-2H-chromen-2-one (1.0 mole) solution in DMF (2 vol) at 75 °C. The reaction mass stirred for 6 h at 75 °C (TLC monitoring: hexane/ethyl acetate 7:3, Rf = 0.58). After the completion of reaction, the product obtained was filtered, dried and recrystallized from methanol to give the ethyl-2,4-(3-(ethyl)-4-hydroxy-2H-chromen-2-one)phenyloxy-4,5-dihydro-4-methylthiazole-5-carboxylate (4).
Off-white crystals. Yield 72 %, m.p. 183-187 °C, IR (KBr, cm-1) (Fig. S13): 29810-3053 cm-1 (OH), 1710 cm-1 (-C=O), 1440-1415 cm-1 (CH2), 806-833 cm-1 (substituted benzene); 1H NMR (400 MHz, CDCl3) (Fig. S5):7.04-7.94 (m, 8H, Ar-H), 5.88 (s, 1H, OH), 4.54-4.59 (m, 2H, CH2), 4.29-4.34 (m, 2H, CH2), 2.716 (s, 3H, CH3), 2.71 (m, 2H, CH2), 1.35-1.39 (t, 3H, CH3); C24H21NO6S (451.11); Found (C-63.92; H-4.65; N-2.98 %) requires (C-63.85, H-4.69, O-21.26, N-3.09, S-7.10 %)MS: m/z: 452.2 (M+H) (Fig. S17).
2.1.3. Synthesis of Compound Ethyl-2,4-(3-(butyl)-4-hydroxy-2H-chromen-2-one)phenyloxy-4,5-dihydro-4-methylthiazole-5-carboxylate (5)
The compound ethyl-2-4-(3-(butyl)-4-hydroxy-2H-chromen-2-one)phenyloxy-4,5-dihydro-4-methylthiazole-5-carboxylate (5) was synthesized by following the similar synthetic procedure of compound (4) by changing 3-(4-bromobutyl)-4-hydroxy-2H-chromen-2-one (1.0 mole) in place of 3-(2-bromoethyl)-4-hydroxy-2H-chromen-2-one. (TLC monitoring: hexane/ethyl-acetate 7:3, Rf = 0.52).
Off-white crystals. Yield 77 %, m.p. 170-175 °C, IR (KBr, cm-1) (Fig. S14): 1710 cm-1 (C=O), 2981-3053 cm-1 (OH), 1440-1415 cm-1 (CH2), 806-833 cm-1 (substituted benzene); 1H NMR (400 MHz, CDCl3) (Fig. S6) 6.93-7.92 (m, 8H, Ar-H), 5.69 (s, 1H, OH), 4.32-4.37 (m, 2H, CH2), 4.10-4.15 (m, 2H, CH2), 2.76 (s, 3H, CH3), 2.74 (m, 2H, CH2), 2.02-2.16 (m, 4H, CH2), 1.40 (t, 3H, CH3); 13C NMR (100 MHz, CDCl3) (Fig. S10) 13.99,17.10,28.63,60.06,68.75, 90.56, 114.95, 115.21, 116.04, 120.94, 122.64, 123.57, 125.0, 125.06, 127.92, 152.70, 160.85, 160.93, 161.7 164.94, 168.8; C26H25NO6S, (479.14); Found (C-64.98, H-5.24, N-2.85) requires (C-65.12, H-5.25, O-20.02, N-2.91, S-6.68 %) MS: m/z: 480.2 (M + H) (Fig. S18).
2.1.4. Preparation of 4-(4-Hydroxy-2-oxo-2H-chromen-3-yl)-3-methyl-l-phenyl-lH-pyrazol-5-(4H)-one (6) (Scheme 3)
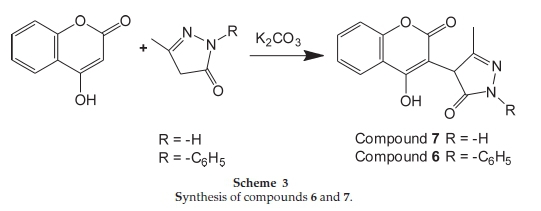
4-Hydroxy coumarin (1.0 mole) and potassium carbonate (1.5 mole) in DMF (3 vol) as a solvent media was heated up to 75 °C and solution of 3-methyl-1-phenyl-1 H-pyrazol-5(4H)-one (1.0 mole) in DMF (2 vol) at 75 °C was added. The reaction mass stirred for 5 h at 75 °C (TLC monitoring: hexane/ethyl acetate 5:5, Rf = 0.47). After the completion of reaction, the product was obtained by adjusting the pH at 4-5 by HCl. It was filtered, dried and recrystallized from ethyl acetate and hexane to give the 4-(4-hydroxy-2-oxo-2H-chromen-3-yl)-3-methyl-1-phenyl-1H-pyrazol-5(4H)-one (6).
Light cream crystal. Yield 73 %, m.p. 153-157 °C, IR (KBr, cm-1) (Fig. S15): 1631 cm-1 (C=O), 3084-3176 cm-1 (OH), 1375-1390 cm-1 (CH2), 759 cm-1, 775 cm-1 (substituted benzene); 1H NMR (400 MHz, DMSO) (Fig. S7)11.83 (s, 1H, OH), 7.11-8.03 (m, 9H, Ar-H), 2.76 (m, 1H, CH), 2.18-2.19 (s, 3H, CH3); C19H14N2O4 (334.32); Found (C-68.16, H-4.29, N-8.37 %) requires (C-68.26, H-4.22, O-19.14, N-8.40 %) MS: m/z: 335.4 (M+H).
2.1.5. Preparation of 4-(4-Hydroxy-2-oxo-2H-chromen-3-yl)-3-methyl-1H-pyrazol-5(4H)-one (7)
The compound 4-(4-hydroxy-2-oxo-2H-chromen-3-yl)-3-methyl-1H-pyrazol-5(4H)-one (7) was synthesized by following the similar synthetic procedure of compound (6) by changing 3-methyl-1H-pyrazol-5(4H)-one (1.0 mole) in place of 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one. After the completion of reaction, the isolation of product carried out at pH 7-8. (TLC monitoring: hexane/ethyl acetate 5:5, Rf = 0.43)
Light lemon-yellow crystal. Yield 68 %, m.p. 181.3-185.3 °C, IR (KBr, cm-1) (Fig. S16): 1631 cm-1 (C=O), 3084-3176 cm-1 (OH), 1375-1390 cm-1 (CH2), 759 cm-1, 775 cm-1 (substituted benzene); 1H NMR (400 MHz, DMSO) (Fig. S8) 11.01 (s, 1H, OH), 8.50 (s, 1H, NH), 7.29-7.91 (m, 4H, Ar-H), 2.60 (m, 1H, CH), 1.96 (s, 3H, CH3); C13H10N2O4 (258.23); Found (C-60.46,H-3.84,N-10.77 %) requires (C-60.47, H-3.89, O-24.78, N-10.85 %) MS: m/z: 259.1 (M+H).
2.2. Antimicrobials and Antifungal Activities (MIC)
The antimicrobial and antifungal activities were performed by Agar diffusion method.40 The standard microbial strains used for the antimicrobial activity were procured from the Institute of Microbial Technology, Chandigarh. The antibacterial activity of the synthesized compounds was tested against two Gram-positive bacteria [Staphylococcus aureus (MTCC 96), Bacillus subtilis (MTCC 441)] and two Gram-negative bacteria [Escherichia coli (MTCC 443) and Pseudomonas aeruginosa (MTCC 1688)] using nutrient agar medium. The antifungal activity of the compounds was tested against Candida albicans (MTCC 227) using Sabouraud dextrose agar medium. All of the tests were performed in triplicate. The sterilized medium was inoculated (1 mL 100 mL-1 of medium) with the suspension (105 cfu mL-1)of the microorganism (matched to McFarland barium sulphate standard) and poured into a Petri dish to give a depth of 3-4 mm. The paper impregnated with the test compounds (100 disc-1) was placed on the solidified medium. The plates were pre-incubated for 1 hour at room temperature and incubated at 37 °C for 24 h for antibacterial and 48 h for antifungal activities. Ampicillin43 (100 disc-1) and Flucanazole41 (10 disc-1) were used as standard for anti-bacterial and anti-fungal activities respectively. The MIC values for standards are obtained under the identical experimental condition as the test compounds. The results are presented in Table 1.
3. Results and Discussion
Ethyl-2-4-(3-(ethyl)-4-hydroxy-2H-chromen-2-one)phenylox y-4,5-dihydro-4-methylthiazole-5-carboxylate (4) was prepared from 3-(2-bromoethyl)-4-hydroxy-2H-chromen-2-one (2)inthe presence of base. 3-(2-Bromoethyl)-4-hydroxy-2H-chromen-2-one (2) was prepared from 4-hydroxy coumarin (1) and dibromo ethane in the presence of base. The synthesis of ethyl-2-4-(3-(alkyl)-4-hydroxy-2H-chromen-2-one)phenyloxy-4,5-dihydro-4-methylthiazole-5-carboxylate (4, 5) was carried out from 3-(2-bromoalkyl)-4-hydroxy-2H-chromen-2-one (2 ,3) and Ethyl-4,5-dihydro-2-(4-hydroxyphenyl)-4-methylthiazole-5-car boxylate in presence of acetone at 40-45 °C, to obtain 90 % yield. Compounds 4 and 5 were purified by crystallization in methanol at 45-50 °C with 70 % yield. Compound 4-(4-hydroxy-2-oxo-2H-chromen-3-yl)-3-methyl-1-phenyl-1H-pyrazol-5(4H)-one (6) was prepared from 4-hydroxy coumarin (1) and 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one in presence of potassium carbonate as base and DMF as solvent. The synthesis of 4-(4-hydroxy-2-oxo-2H-chromen-3-yl)-3-methyl-1H-pyrazol-5 (4H)-one (7) was carried out following same procedure as for compound (6) using 3-methyl-1H-pyrazol-5(4H)-one. The crystallization of compounds (6) and (7) was carried out in ethyl acetate and hexane at RT, to obtain 65-75 % yield.
All the synthesized compounds were characterized by IR, 1H and 13C NMR spectroscopic techniques, mass spectra and elemental analysis. The IR spectra of compounds (4,5) showed a band in the region 2993-3076 cm-1 due to the -OH groups.42 The C-H bending vibrations are observed as a sharp medium to strong band at 1329 cm-1 in all compounds. The C-H linkage of the six-member ring caused a weak and sharp absorption band at 800-850 cm-1. The C=O group is observed as a strong and sharp band at 1650-1710 cm-1 in these compounds. Further, 1H NMR spectra exhibited multiplet in the region at á-LC 7.04-7.94 ppm for eight aromatic protons (four aromatic protons of coumarin and four aromatic protons of benzene) (4). Four protons present in -CH2 of compounds (4) are found to resonate as multiplet at á-LC 4.54-1.34 ppm (alkene). One proton present in -OH of compound (4) is found to resonate as singlet at á-LC 5.88 ppm. Six protons present in -CH3 of compounds (4) are found to resonate as triplets at á-LC 1.35-1.39 ppm (thiazole) and singlet at á-LC 2.71 ppm (thiazole). Two protons of the -CH2 group are observed at á-LC 2.71 ppm as a multiplet compound (4).The IR spectra of compounds (6-7) showed a broad band in the region 3084-3176 cm-1 due to the -OH groups. The C=O group is observed as a strong and sharp band at 1631 cm-1 in these compounds. The C-H linkage of the mono substituted six-member ring caused a weak and sharp absorption band at 730-780 cm-1 in all the compounds. The 1H NMR spectra of compounds 6 and 7 show identical peaks for coumarin moiety but show significant differences for pyrazolone moiety. The mono substituted aromatic rings proton found in between á-LC 7.54-8.03 ppm for compounds 6 and -NH proton observed as broad peat at á-LC 8.50 ppm for compound 7.
The anti-bacterial activity of the synthesized compounds was tested against two Gram-positive bacteria (Staphylococcus aureus, Bacillus subtilis) and two Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa) using nutrient agar medium. The antifungal activity of the compounds was tested against Candida albicans using Sabouraud dextrose agar medium. The results given in Table 1 indicated that most of the compounds tested and compared to standard Ampicillin exhibited considerable in vitro antimicrobial activities against Staphylococcus aures, Bacillus subtilis, Escherichia coli and Pseudomonas aeruginosa. All the compounds having minimum inhibitory concentration (MIC) values ranging from 10 to16 mL-1. Activity of compounds 4 and 5 was the best against Pseudomonas aeruginosa and Escherichia coli. Compounds 5 and 6 gave optimum activity against Bacillus subtilis but compounds 4 and 7 showed maximum activity against Staphylococcus aures. All compounds exhibited moderate activity against fungi species Candida albicans. The Ampicillin used as standard antibacterial agent and Fluconazole used as standard antifungal agent. The MIC values for standards were obtained under the identical experimental condition as the test compounds. The MIC values for the other reported drugs are listed in Table 2.
4. Conclusion
In summary, a series of novel bioactive coumarin derivatives have been synthesized, purified and characterized. The result of antibacterial activity with the Ampicillin as standard at MIC >100 mL-1 against Pseudomonas aeruginosa and Escherichia coli at 300 mL-1. All compounds exhibited slightly reduced activities (than the positive controls), and the best activity was for compounds 4 and 5. Against Staphylococcus aureus, Bacillus subtilisat 300 all compound showed slightly reduces activities, and the best performance was shown by compounds 4 and 7. The result of antifungal activities with the comparison of Flucanazole at MIC 10 against Candida albicans at 40 all compound activity showed less activity. Given the above result, these kind of compounds could be further studied and explored as good antibacterial agents.
Acknowledgements
The authors are grateful to the Principal, Navyug Science College, Surat, for providing research and laboratory facilities to carry out this work, and Chemistry Department, Gujarat University, Ahmedabad (H.M. Parekh) for data analysis and manuscript preparation.
Supplementary Material
Supplementary information is provided in the online supplement.
References
1 J.R. Hoult and M. Paya, Pharmacological and biochemical actions of simple coumarins: natural products with therapeutic potential, Gen. Pharmacol., 1996, 27, 713-722. [ Links ]
2 M.H. Lin, Y.S. Chou, Y.J. Tsai and D.S. Chou, Antioxidant properties of 5,7-dihydroxy-coumarin derivatives in in vitro cell-free and cell-containing systems, J. Exper. Clin. Med. 2011, 3(3), 126-131. [ Links ]
3 B. Rajitha, VN. Kumar, P. Someshwar, J.V Madhav, P.N. Reddy and Y.T. Reddy, Dipyridine copper chloride catalyzed coumarin synthesis via Pechmann condensation under conventional heating and microwave irradiation, ARKIVOC XII, 2006, 23-27. [ Links ]
4 S.S. Keskin, N. Aslan and F. Bayrakceken, Optical properties and chemical behavior of laser dye coumarin-500 and the influence of atmospheric corona discharge, Spectrochem. Acta: A Biomol. Spectros., 2009, 72, 254-259. [ Links ]
5 R.M. Christie, K.M. Morgan and M.S. Islam, Molecular design and synthesis of arylsulfonated coumarin fluorescent dyes and their application to textile, Dyes Pigments, 2008, 76, 741- 747. [ Links ]
6 Y.-H. Wang, B. Avula, N.P.D. Nanayakkara, J. Zhao and I. A. Khan, Cassia cinnamon as a source of coumarin in cinnamon-flavored food and food supplements in the United States, J. Agric. Food Chem., 2013, 61, 4470-476. [ Links ]
7 F. Floc'h, F. Mauger, J.-R. Desmurs and A. Gard, Coumarin in plant and fruits: implication in perfumery, Perfum. Flavor., 2002, 27, 32-36. [ Links ]
8 C. Stiefel, T. Schubert and G.E. Morlock, Bioprofiling of cosmetics with focus on streamlined coumarin analysis, ACS Omega, 2017, 2, 5242-5250. [ Links ]
9 R. O'Kennedy and R.D. Thornes, Coumarins: Biology, Applications and Mode of Action, John Wiley and Sons, Chichester, 1997. [ Links ]
10 R. Nagamallu, B. Srinivasan, M.B. Ningappa and A.K. Kariyappa, Synthesis of novel coumarin appended bis(formylpyrazole) derivatives: studies on their antimicrobial and antioxidant activities, Bioorg. Med. Chem. Lett., 2016, 26, 690-694. [ Links ]
11 A. Tanitame, Y. Oyamada, K. Ofuji, M. Fujimoto, N. Iwai, Y. Hiyama, K. Suzuki, H. Ito, H. Terauchi, M. Kawasaki, K. Nagai, M. Wachi and J. Yamagishi, Synthesis and antibacterial activity of a novel series of potent DNA Gyrase inhibitors. Pyrazole derivatives, J. Med. Chem., 2004,47, 3693-3696. [ Links ]
12 M. Ghate, R.A. Kusanur and M.V. Kulkarni, Synthesis and in vivo analgesic and anti-inflammatory activity of some bi heterocyclic coumarin derivatives, Eur. J. Med. Chem. 2005,40, 882-887. [ Links ]
13 A.M. Musaa, F.O. Khanb and J.S. Cooperwood, Synthesis and antiproliferative activity of coumarin-estrogen conjugates against breast cancer cell lines, Lett. Drug Des. Discov, 2009, 6(2), 133-138. [ Links ]
14 I. Kostova and G. Momekov, New zirconium (IV) complexes of coumarins with cytotoxic activity, Eur. J. Med. Chem., 2006, 41(6), 717-726. [ Links ]
15 D. Zavrsnik, S. Muratovic, D. Makuc, J. Plavec and M. Cetina, A. Nagl, Benzylidene-bis-(4-hydroxycoumarin) and benzopyranocoumarin derivatives: synthesis, 1H/13C-NMR conformational and X-ray crystal structure studies and in vitro antiviral activity evaluations, Molecules, 2011, 16, 6023-6040. [ Links ]
16 M. Parameswaran, R.T. Kochupappy and S. Gopalakrishnan, Synthesis of coumarin heterocyclic derivatives with antioxidant activity and in vitro cytotoxic activity against tumour cells, Acta Pharm., 2009, 59, 159-170. [ Links ]
17 M.A. Crowther, J.S. Ginsberg, J. Julian, J. Denburg, J. Hirsh, J. Douketis, C. Laskin, P. Fortin, D. Anderson, C. Kearon, A. Clarke, W. Geerts, M. Forgie, D. Green, L. Costantini, W. Yucura, S. Wilson, M. Gent and M.J. Kovacs, A comparison of two intensities of Ampicillin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome, New Engl. J. Med., 2003, 349, 1133-1138. [ Links ]
18 L. Aoife and R. O'Kenedy, Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer, Curr. Pharm. Design., 2004, 10(30), 3797-3811. [ Links ]
19 H. Zhao, N. Neamati, H. Hong, A. Mazumder, S. Wang, S. Sunder, G.W.A. Milne, Y. Pommier and T.R. Jr. Burke, Coumarin-based inhibitors of HIV Integrase, J. Med. Chem., 1997, 40, 242-249. [ Links ]
20 C.B. Aguirre-Paranzoni, G.I. Furque, C.E. Ardanaz, A. Pacciaroni,V Sosa, C.E. Tonn and M. Kurina-Sanz, Biotransformation of dihydrocoumarin by Aspergillus niger ATCC 11394, ARKIVOC VII, 2011, 170-180. [ Links ]
21 S. Dochev, A. Penkova, P. Retailleau and I. Manolov, Synthesis and crystal structure of an ammonium salt of 4-hydroxy-3-[(2-oxo-2H-chromen-3-yl)-(3,4,5-trimethoxyphenyl)methyl] chromen-2-one, Bulg. Chem. Comm., 2013, 45(3), 301-309. [ Links ]
22 T.O. Soine, Naturally occurring coumarins and related physiological activities, J. Pharm. Sci., 1964, 53, 231-264. [ Links ]
23 Z.M. Nofal, M.M. Kamel, A.H. El-Masry, M.T. Omar and A.I. Sarhan, Synthesis of some coumarin derivatives of possible biological activity, Egypt. J. Pharm. Sci., 1997, 38(1-3), 159-169. [ Links ]
24 H. Von Pechmann, Neue Bildungsweise der Cumarine, Synthese des Daphnetins I, Eur. J. Inorg. Chem. (Berichte der deutschenchemischen Gesellschaft), 1884, 17(1), 929-936. [ Links ]
25 J.R. Johnson, The Perkin Reaction and related reactions, Org. React., 1942, 1, 210. [ Links ]
26 G. Jones, The Knoevenagel Condensation, Org. React., 1967, 15, 204. [ Links ]
27 G. Barfola, F. Fringuelli and F. Pizzo, Simple and efficient one-pot preparation of 3-substituted coumarins in water, Heterocycles, 1996, 43, 1257-1266. [ Links ]
28 R.L. Shriner, The Reformatsky Reaction, Org. React., 1942, 1, 1. [ Links ]
29 K. Nakayama, T. Oshima and H. Koike, Assessment of beta-blockade and the non-specific effect of bucumolol, a beta-adrenergic blocking agent, on atrioventricular conduction in anesthetized dogs. Arch. Int. Pharmacodynam. Thér., 1981, 254(1), 145-156. [ Links ]
30 D. Opherk, G. Schuler, W. Waas, R. Dietz and W. Kubler, Intravenous carbochromen: a potent and effective drug for estimation of coronary dilatory capacity, Eur. Heart J., 1990, 11(4), 342-347. [ Links ]
31 K. AnilKumar, B.S. VijayKumar, B. Laxminarayana and S. Ananthana-rayanan, Biotransformations of 4-methyl umbelliferone derivatives fungal mediated O-dealkylations, Studies Surf. Sci. Cata., 1998, 113, 541-546. [ Links ]
32 E. Budzisz, Synthesis, reactions and biological activity of phosphorus-containing derivatives of chromone and coumarin, Phos. Sul. Sili., 2004, 179, 2131-2147. [ Links ]
33 T.C. Wong, C.M. Sultana and D.A. Vosburg, A green, enantioselective synthesis of Ampicillin for the undergraduate organic laboratory, J. Chem. Educ., 2010, 87(2), 194-195. [ Links ]
34 S.N. Mandrupkar, M.A. Nagras and S.V Mulgund, Development and validation of spectrophotometric method of acenocoumarol in bulk and tablet dosage form, Int. J. Phar. Pharm. Sci., 2012, 4(4), 288-289. [ Links ]
35 A. Mazumder, S. Wang, N. Neamati, M. Nicklaus, S. Sunder, J. Chen, G. Milne, W. Rice, T. Burke and Y. Pommler, Antiretroviral agents as inhibitors of both Human Immunodeficiency Virus Type 1 integrase and protease, J. Med. Chem., 1996, 39, 2472-2481. [ Links ]
36 A. Kathuria, A. Gupta, N. Priya, P. Singh, H.G. Raj, A.R. Prasad, VS. Parmar and S.K. Sharma, Specificities of calreticulin transacetylase to acetoxy derivatives of 3-alkyl-4-methylcoumarins: effect on the activation of nitric oxide synthase, Bioorg. Med. Chem., 2009, 17(4), 1550-1556. [ Links ]
37 D. Kumar, S. and J.S. Sandha, Synthesis of 5-arylidene-2,2-dimethyl-1,3-dioxane-4,6-diones and coumarin-3-carboxylic acids via reaction of nitrones and Meldrum's acid, Indian J. Chem., 2013, 52B, 1157-1160. [ Links ]
38 J.M.V. Ngororabanga, J. Okerio and N. Mama, Synthesis of fluorescent poly(coumarin-triazoles) via a CuAAC 'Click' Reaction, S. Afr. J. Chem., 2017, 70, 89-93. [ Links ]
39 X.-G. Zhou, M.-S.Peng and T.-Z.Feng, A novel coumarin Schiff-base fluorescent probe for Mg2*, S. Afr. J. Chem., 2013, 66, 69-71. [ Links ]
40 M. Balouiri, M. Sadiki and S.K. Ibnsouda, Methods for in vitro evaluating antimicrobial activity: a review, J. Phar. Ana., 2016, 6, 71-79. [ Links ]
41 V.G. Dongre, P.D. Ghugare, P.P. Karmuse, S.R. Soudagar, N. Panda and A. Kumar, Isolation and structural identification of an impurity in fluconazole bulk drug substance, J. Phar. Biome. Ana., 2007, 45(3), 422-429. [ Links ]
42 G.M. Malik and C.G. Naik, Synthesis, characterization and antimicrobial activity of ethyl-2-(3-formayl-4-((4-hydroxy-2-oxo-2H-chromen-3-yl)-alkoxy-)phenyl)-4-methylthiazole-5-carbo-xylate derivatives, Ind. J. Chem., 2015, 54B, 1005-1010. [ Links ]
43 M.K. Vekariya, D.B. Patel, P.A. Pandya, R.H. Vekariya, P.U. Shah, D.P. Rajani and N.K. Shah, Novel N-thioamide analogues of pyrazolyl-pyrimidine based piperazine: design, synthesis, characterization, in-silico molecular docking study and biological evaluation, J. Mol. Str., 2019, 1175, 551-565. [ Links ]
44 K.D. Patel, R.H. Vekariya, N.P. Prajapati, D.B. Patel, H.D. Patel, T. Shaikh, D.P. Rajani, S. Rajani, N.S. Shah and D. Jhala, Synthesis of N'-(Quinazolin-4-yl)isonicotinohydrazides and Their Biological Screening, Docking and ADME Studies, Arab. J. Chem., 2018, https://doi.org/10.1016/j.arabjc.2018.02.017 [ Links ]
45 M.G. Sharma, D.P. Rajani and H.M. Patel, Green approach for synthesis of bioactive Hantzsch 1,4-dihydropyridine derivatives based on thiophene moiety via multicomponent reaction. R. Soc. Open Sci., 2017, 4, 170006. http://dx.doi.org/10.1098/rsos.170006 [ Links ]
Received 22 December 2017
Revised 3 September 2019
Accepted 6 September 2019
* To whom correspondence should be addressed. E-mail: keya714@gmail.com
Supplementary Data
The supplementary data is available in pdf: [Supplementary data]














