Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Chemistry
versão On-line ISSN 1996-840X
versão impressa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.72 Durban 2019
http://dx.doi.org/10.17159/0379-4350/2019/v72a25
RESEARCH ARTICLE
2,4-Dioxo-1,3-diazaspiro[4,5]decane-3-sulfonic Acid as a Novel Solid Phase Halogen-free Acid Catalyst: Preparation, Characterization and Evaluation
Behzad Khalil*; Aref Atashrazm; Mona Rasoulian
Department of Chemistry, Faculty of Sciences, University of Guilan, P.O. Box 41335-1914, Rasht, Iran
ABSTRACT
N-sulfonated cyclohexylhydantoin (NSCH), a new N-sulfonic acid compound, was prepared and characterized using FT-IR, NMR, 13C NMR, UV-Vis and TGA analysis. The potential catalyst efficiency was examined, using synthesis of chromene, xanthene and hydroquinoline derivatives under solvent-free conditions. All of the examined model reactions gave excellent yields, and required short reaction times. Simple catalyst preparation and work-up under mild reaction condition, with low cost and the ability to recover the catalyst for reuse without significant loss of activity are some of the notable advantages of the introduced catalyst.
Keywords: Hydantoin, N-sulfonated acidic catalyst, chromene, xanthene, hydroquinoline.
1. Introduction
Common Bronsted acid hydrogen containing catalysts such as sulfuric acid, p-toluene sulfonic acid, hydrochloric acid, hydrofluoric acid and phosphoric acid are used as catalysts to promote many organic synthetic reactions and industrial processes. However, the above-mentioned Bronsted acid catalysts generally result in homogeneous reaction mixtures, causing problems with separation from reaction media, resulting in some disadvantages such as negative environmental impact, wasting energy and increased chemical wastes production. For these reasons, solid acid catalysts have received considerable attention to replace such conventional homogeneous Bronsted acid catalysts, and have shown higher efficiency, operational simplicity, easy reusability, recoverability, non-corrosiveness and higher environmental acceptance.1-5 Therefore, the development of solid acid catalysts could be very helpful for altering technologies using homogeneous acid catalysts. One of the simplest ways to access solid acidic materials is making use of nitrogen-containing organic compounds and introducing SO3H and HSO4 groups as Bronsetd acid functions into their chemical structure, which make them water soluble and enhance their polarity, which aids their use under solvent-free conditions. Many of such solid acid catalysts have been reported."0
Imidazolidine-2,4-diones (or hydantoins) are well known substructures found in a wide range of biologically active drugs and natural products.11 From the synthetic point of view, hydantoins could be useful precursors for preparation of acidic catalysts because their acidic N-H could be replaced by the sulfonic acid functional group. Accordingly, sulfonic acid-functionalized hydantoin is now examined as a potential solid acid catalyst in the multicomponent synthesis of xanthenes, chromenes and hydroquinolines.12-14
Xanthene, chromene and hydroquinoline derivatives are important organic compounds with a wide range ofapplicability and biological activity.15-20 Although some synthetic methodologies have been reported for each class of these compounds in the literature,21-32 each with their own advantages, many of them suffer from some disadvantages. The use of homogeneous acid catalysts may result in some difficulties with product separation and purification, low yield, long reaction time, use of toxic organic solvents and harsh reaction procedures. Therefore, we have developed a new and efficient catalyst to overcome these problems.
2. Experimental
2.1. General Procedure for the Synthesis of Cyclohexylhydantoin (CHH)
Cyclohexanone (5 mmol, 0.52 mL) and ammonium carbonate (10 mmol, 1 g) were added to 30 mL 1:1 ethanol/water. The mixture was heated at 60-70 °C until all of the ammonium carbonate dissolved. Then, a solution of potassium cyanide (10 mmol, 0.7 g) in 10 mL of distilled water was added dropwise over 10 min. After 48 h at 60-70 °C, the mixture was kept at room temperature for 45 min, and then below 0 °C for 2 h. The precipitate was filtered and recrystallized from ethanol. Finally, the desired hydantoin was obtained as needles (70%), mp 220222 °C.33
2.2. General Procedure for the Synthesis of Cyclohexylhydantoin- N-sulfonic Acid (NSCH)
A mixture of cyclohexylhydantoin (2 mmol, 0.2 g) and dichlo-romethane (10 mL) was stirred at 0 °C for 2 h, generating a uniform white paste. Then chlorosulfonic acid (2 mmol, 0.25 mL) in dichloromethane (2 mL) was added over a period of 10 min at 0 °C. The resulting clear solution was kept at 0 °C for 2 h and then allowed to reach room temperature and stirred for a further 2 h. After evaporation of the dichloromethane, the residue was washed with diethyl ether (3x5 mL). The final precipitate was obtained (95%), mp 198 °C.
2.3. General Procedure for the Synthesis of Chromenes
To a mixture of dimedone (1 mmol), the benzaldehyde (1 mmol) and malonitrile (1.2 mmol) was added the NSCH catalyst (15 mg) and the mixture was stirred at 80 °C for the appropri-ate time. The reaction progress was monitored by TLC. After completion of the reaction, chloroform (10 mL) was used to extract the catalyst, and the residual product was recrystallized from ethanol.
2.4. General Procedure for the Synthesis of Xanthenes
A mixture of,dimedone (2 mmol), the benzaldehyde(1 mmol). And the NSCH catalyst (15 mg) was stirred at 80 °C for a given time. At the end of the reaction (monitored by TLC), the reaction mixture was cooled to room temperature. The catalyst was separated from the reaction mixture by addition of chloroform (10 mL), then the crude residue was recrystallized from ethanol.
2.5. General Procedure for the Synthesis of Hydroquinolines
A stirred mixture of dimedone (1 mmol), benzaldehyde (1 mmol), ethyl acetoacetate (1 mmol), ammonium acetate (2 mmol) and the NSHH catalyst (15 mg) was heated at 80 °C and kept for a given time, and the reaction was monitored by TLC. The reaction mixture was cooled to room temperature and the catalyst separated from the reaction mixtures by addition of chloroform (10 mL). The crude product was recrystallized from ethanol.
2.6. Optimizing Reaction Conditions for Tetrahydrochromene Synthesis
A mixture of dimedone (1mmol), benzaldehyde (1 mmol) and malononitrile(1mmol) was reacted in the presence of different amounts of NSCH in the temperature range 25-120 °C in the absence and presence of different solvents. The results are shown in Fig. 4a.
2.7. Determination of the Acid Content of the Catalyst
The 0.04 M catalyst solution (20 mL) was titrated with the 0.05 M sodium hydroxide solution. The titration curve is given in Fig. 3. As seen in Fig. 3, the titration reached the end point when 18 mL of the titrant was added to the catalyst solution. This amount of the titrant is nearly same as that (16 mL) which is needed to complete neutralization of the 20 mL of the 0.04 M solution of the catalyst according to the calculations below (Equation 1). These results showed that the catalyst contains one acidic proton, verifying its sulfonated NH group.
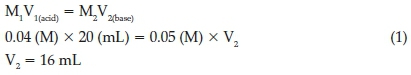
3. Results and Discussion
3.1. Catalyst Characterization
The structure of the cyclohexylhydantoin-N-sulfonic acid catalyst, which was synthesized as shown in Fig. 1, was characterized by FT-IR, 1H NMR, 13C NMR, TGA, DTG, and UV-Vis analysis.
The corresponding FT-IR spectra of cyclohexylhydantoin and cyclohexylhydantoin-N-sulfonicacid are shown in Fig. SI1 (Fig. 1 in supporting information). In the case of cyclohexylhydantoin-N-sulfonic acid, the OH stretching band of the SO3H functional group appeared as broad bands between 3200 and 3700 cm-1, centred at 3414 cm-1. Characteristic absorption bands related to the SO2 asymmetric and symmetric, S-N symmetric and S-OH bendingvibrations of appeared at 1262,1176,1069 and 879 cm-1.10
Thermogravimetric analysis of CHH and NSCH was carried out to investigate their structural stability during thermal changes. Fig. 2 displays a typical DTGA spectrum for CHH and NSCH. CHH shows a two-step thermal decomposition at 100 °C and 290 °C which corresponds to the loss of the moisture and complete decomposition of the CHH structure, respectively. In the case of NSCH, a three-step thermal decomposition appeared which includes firstly, weight loss in the range 30-120 °C, due to the removal of the local moisture. Secondly, the thermal decomposition of the sulfonic group appeared at 240 °C as a weight loss. The third decomposition, at 290 °C, corresponds to the complete decomposition of the NSCH. According to the DTGA results, the NSCH catalyst is thermally stable up to 240 °C.
1H NMR spectra of CHH and NSCH from 7ppm to 12ppm are shown in (Fig. SI2). The 1H NMR spectrum of CHH displayed two singlet peaks at 10.54 and 8.38 ppm due to the hydrogens attached to the nitrogen atoms. After functionalization of CHH with chlorosulfonic acid, the NH peak at 10.54 was replaced by a new peak at 11.45 ppm, which corresponds to the newly attached SO3H proton. In addition, the 13C NMR spectra of NSCH showed six peaks verifying its chemical structure.
3.2. Catalyst Acidity Power
One way to evaluate the acidic power of an acid in organic media is the use of the Hammett method (see equation below), using the UV-Vis technique. 7
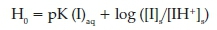
Inthis equation pK(I)aqrepresents the pKa value ofanindicator in aqueous solution. The concentration of the indicator in protonated and deprotonated forms are represented as [IH+]s and [I]s, respectively. The values of [I]s/[IH+]s were easily obtained using the UV-Visible spectrum according to the Lambert-Beer's Law. In this study, the indicator and organic solvent are 4-nitroaniline (pK(I)aq = 0.99) and CCl4.
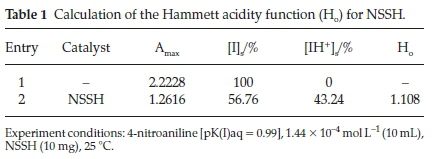
In the absence of the catalyst the mixture of the 4-nitroaniline and organic solvent shows the maximum absorbance at 330 nm with an intensity of (Amax = 2.2228), while as the catalyst was added to this mixture the intensity of the maximum absorbance at 330 nm decreased to (Amax = 2.2616). The decrease in the absorbance intensity of the indicator is due to the fact that some of the indicator molecules are protonated by the acidic protons which are supplied by the catalyst molecules, and the concentration of the non-protonated forms of the indicator molecules which are responsible for the absorption at 330 nm decreased. Table 1 lists the data obtained for the evaluation of the acidity power of the catalyst using Hammett acidity function.
3.3. Evaluation of the Catalytic Activity
After the characterization of NSCH, its catalytic activity was examined using it as solid acid catalyst to promote a number of synthetic organic reactions such as synthesis of the tetrahydro-chromenes (Fig. 4a), xanthenes (Fig. 4b) and hydroquinolines (Fig. 4c).
The results showed that the optimum amount of the NMCH was 6 mol%, and by using this amount of the catalyst the reaction proceeded best under solvent-free conditions at 80 °C. In the absence of the catalyst, the reaction was incomplete, even after 24 h.
The yield of products from different benzaldehydes, under different reaction conditions, is shown in Table 2. The presence of electron-donating substituents in the benzaldehyde, e.g. entry 5 in Table 2 required longer reaction times.
The proposed mechanism for tetrahydrochromene synthesis in the presence of NSCH is shown in Fig. SI3a.
Similarly, entries 13-17 report the optimized yields for the synthesis of 3,3,6,6-tetramethyl-1, 8-dioxooctahydroxanthenes from benzaldehyde derivatives (1 mmol) and dimedone (2 mmol) in presence of NSCH at 80 °C under solvent-free condition (Fig. 4b).
The synthesis of the hydroquinoline derivatives (Fig. 4c) was achieved efficiently from the benzaldehyde derivatives (1 mmol), dimedone (1 mmol) and ethyl acetoacetate (1 mmol) in the presence of NSCH at 80 °C under solvent-free condition. The results are listed in Table 2 (entries 18-22). Two alternative reaction pathways to achieve this outcome are shown in Fig. SI3c.
3.4. Reusability of the Catalyst
Recovery of the catalyst and its reuse were investigated under the optimized reaction procedure for the above three procedures. In each case, the catalyst was filtered off at the end of the reaction, washed with ethanol, dried in an oven and reused for the same reaction. This process was repeated three times and no significant changes in the reaction time or yield were observed, verifying the practical recyclability of this catalyst (Fig. 5).
3.5. Comparison of the Reported Efficiency with Previous Work
To evaluate the efficiency of our newly introduced procedures, comparison of the our results for the synthesis of chromenes, xanthenes and hydroquinolines using NSCH as catalyst with the results reported previously in the literature were carried out (Table 3).The newly developed method herein reduced reaction times, excess use of the reagents, especially catalyst amount, and considerably improved on lengthy conditions for catalyst preparation.
4. Conclusions
In summary, we have introduced a novel halogen-free solid acid catalyst, N-sulfonated cyclohexylhydantoin (NSCH), which has high acidic power and catalytic activity. The efficiency of the NSCH catalyst was evaluated in the synthesis of tetra-hydrochromene, 1, 8-dioxooctahydroxanthene and hydro-quinoline derivatives. The results clearly showed that the NSCH catalyst has high catalytic activity to enhance many of the organic synthetic reactions that require acidic conditions. The simple preparation of the catalyst, high availability of the starting materials, recyclability and reusability of the catalyst are advantages of this procedure. The solvent-free conditions, easy work-up and short reaction times are further advantages of the presented procedure.
Acknowledgements
Partial support of this work from the Research Council of the University of Guilan is gratefully acknowledged. The authors are thankful to the Iran National Science Foundation: INSF, Grant number 95815510, for supporting this work.
Conflict of interest
The authors declare no conflict of interest.
Supplementary Material
Supplementary information is provided in the online supplement.
§ORCID iD
B. Khalili: © orcid.org/0000-0002-0001-2824
References
1 N. Ahmed and Z.N. Siddiqui, Perchloric acid modified-cellulose: a versatile, novel and biodegradable heterogeneous solid acid catalyst for single-pot synthesis of novel bis-pyran annulated heterocyclic scaffolds under solvent-free conditions, J. Mol. Catal. A: Chem, 2014, 387, 45-56. [ Links ]
2 A. Praminik and S. Bhar, Alumina-sulfuric acid catalyzed eco-friendly synthesis of xanthenediones, Catal. Commun., 2012, 20, 17-24. [ Links ]
3 Z.N. Siddiqui, K. Khan and N. Ahmed, Nano fibrous silica sulphuric acid as an efficient catalyst for the synthesis of /S-enaminone, Catal. Lett., 2014,144, 623-632. [ Links ]
4 A. Rajack, K. Yuvaraju, Ch. Praveen and Y.L.N. Murthy, A facile synthesis of 3,4-dihydropyrimidinones/thiones and novel N-dihydro pyrimidinone-decahydroacridine-1, 8-diones catalyzed by cellulose sulfuric acid, J. Mol. Catal., 2013, 370, 197-204. [ Links ]
5 M.D. Gonz'alez Y. Cesteros, J. Llorca and P. Salagre, Boosted selectivity toward high glycerol tertiary butyl ethers by microwave-assisted sulfonic acid-functionalization of SBA-15 and beta zeolite, J. Catal., 2012, 290, 202-209. [ Links ]
6 O. Goli-Jolodar, F. Shirini and M. Seddighi, Introduction of O-sulfonated poly (vinylpyrrolidonium) hydrogen sulfate as an efficient, and reusable solid acid catalyst for some solvent-free multi-component reactions, RSC Adv., 2016, 6, 44794^4806. [ Links ]
7 O. Goli-Jolodar, F. Shirini and M. Seddighi, Introduction of a novel nanosized N-sulfonated Bronsted acidic catalyst for the promotion of the synthesis of polyhydroquinoline derivatives via Hantzsch condensation under solvent-free conditions, RSC Adv., 2016, 6,26026-26037. [ Links ]
8 K. Mohammadi, F. Shirini and A. Yahyazadeh, 1,3-Disulfonic acid imidazolium hydrogen sulfate: a reusable and efficient ionic liquid for the one-pot multi-component synthesis of pyrimido[4,5-b]quino-line derivatives, RSC Adv., 2015, 5, 23586-23590. [ Links ]
9 F. Shirini, M.P. Najafi, S. Moayedi and M. Seddighi, Introduction of O-sulfonated poly(4-vinylpyrrolidonium) chloride as a polymeric and reusable catalyst for the synthesis of xanthene derivatives, RSC Adv., 2014,4, 38581-38588. [ Links ]
10 F. Shirini, M. Abedini, M. Seddighi, O. Goli-Jelodari, M. Safarpoor, N. Langroodi and S. .Zamani, Introduction of a new bi-SO3H ionic liquid based on 2,2'-bipyridine as a novel catalyst for the synthesis of various xanthene derivatives, RSC Adv., 2014, 4, 63526-63532. [ Links ]
11 a) E. Ware, The chemistry of the hydantoins, Chem. Rev., 1950, 46, 403-476. [ Links ] b) B. Eftekhari-Sis andM. Zirak, Chemistry of á-oxoesters: a powerful tool for the synthesis of heterocycles, Chem. Rev., 2015,115, 156-264. [ Links ] c) B. Eftekhari-Sis, M. Zirak and A. Akbari, Arylglyoxals in synthesis of heterocyclic compounds, Chem. Rev., 2013, 113,2958-3043. [ Links ]
12 a) F. Shirini, M. Abedini, S. Zarrabzadeh and M. Seddighi, Efficient synthesis of 4H-pyran derivatives using a polymeric catalyst based on PVP, J. Iran. Chem. Soc., 2015,12,2105-2113. [ Links ] b) B. Eftekhari-Sis, M. Sarvari Karajabad and S. Haqverdi, Pyridylmethylaminoacetic acid functionalized Fe3O4 magnetic nanorods as an efficient catalyst for the synthesis of 2-aminochromene and 2-aminopyran derivatives, Sientia Iranica, 2014, 24, 3022-3031. [ Links ]
13 M. Seyyedhamzeh, P. Mirzaei and A. Bazgir, Solvent-free synthesis of aryl-14H-dibenzo [a, j] xanthenes and 1, 8-dioxo-octahydro-xan-thenes using silica sulfuric acid as catalyst, Dyes Pigm., 2008, 3, 836-839. [ Links ]
14 B.P. Bandgar, P.E. More and V.T. Kamble, J.V Totre, Synthesis of poly-hydroquinoline derivatives under aqueous media, Arkivoc, 2008, 15, 1-8. [ Links ]
15 A. Davoodnia, S. Allameh, S. Fazli and N. Tavakoli-Hoseini, One-pot synthesis of 2-amino-3-cyano-4-arylsubstituted tetrahydrobenzo-[b]pyranscatalysed by silica gel-supported polyphosphoric acid (PPA-SiO2) as an efficient and reusable catalyst, Chem. Pap., 2., 2011, 65, 714-720. [ Links ]
16 G.M. Ziarani, A. Abbasi, A. Badiei and Z. Aslani, An efficient synthesis of tetrahydrobenzo[b]pyran derivatives using sulfonic acid functio-nalized silica as an efficient catalyst, J. Chem., 2011, 8, 293-299. [ Links ]
17 F. Shirini, M. Abedini and R. Pourhasan, N-sulfonic acid poly (4-vinyl-pyridinium) chloride: a novel polymeric and reusable catalyst for the preparation of xanthenes derivatives, Dyes Pigm., 2013, 99,250-255. [ Links ]
18 F. Shirini, A. Yahyazadeh and K. Mohammadi, One-pot synthesis of various xanthene derivatives using ionic liquid 1,3-disulfonic acid imidazolium hydrogen sulfate as an efficient and reusable catalyst under solvent-free conditions, Chin. Chem. Lett., 2014, 25, 341-347. [ Links ]
19 M. Abedini, F. Shirini and M. Mousapour, Poly(vinylpyrrolidinium) perchlorate as a new and efficient catalyst for the promotion of the synthesis of polyhydroquinoline derivatives via Hantzsch condensation, Res. Chem. Intermed, 2016,42, 2303-2315. [ Links ]
20 M.V. Reddy and Y.T. Jeong, Polystyrene-supported p-toluenesulfonic acid: a new, highly efficient, and recyclable catalyst for the synthesis of hydropyridine derivatives under solvent-free conditions, Synlett., 2012, 23, 2985-2991. [ Links ]
21 Z. Zhou, Y. Zhang and X. Hu, Efficient one-pot synthesis of tetrahydrobenzo[b]pyrans by ethylenediamine diacetate-catalyzed multicomponent reaction under solvent-free conditions, Polycyclic Aromat. Compd, 2017, 37, 39^5. [ Links ]
22 A. Hasaninejad, M. Shekouhy, N. Golzar, A. Zare and M.M. Doroodmand, Silica bonded n-propyl-4-aza-1-azoniabicyclo-[2.2.2]octane chloride (SB-DABCO): a highly efficient, reusable and new heterogeneous catalyst for the synthesis of 4H-benzo[b]pyran derivatives, Appl. Catal. A: General, 2011,402, 11-22. [ Links ]
23 I. Devi and P.J. Bhuyan, Sodium bromide catalysed one-pot synthesis of tetrahydrobenzo[b]pyrans via a three-component cycloconden-sation under microwave irradiation and solvent free conditions, Tetrahedron Lett., 2004, 45, 8625-8627. [ Links ]
24 S. Rezayati, Z. Erfani and R. Hajinasiri, Phospho sulfonic acid as efficient heterogeneous Bronsted acidic catalyst for one-pot synthesis of 14H-dibenzo[a,j]xanthenes and 1, 8-dioxo-octahydro-xanthenes, Chem. Pap., 2015, 69, 536-543. [ Links ]
25 F. Shirini and N.G. Khaligh, Succinimide-N-sulfonic acid: an efficient catalyst for the synthesis of xanthene derivatives under solvent-free conditions, Dyes Pigm, 2012, 95, 789-794. [ Links ]
26 Hasaninejad, A. Silica-supported phosphorus-containing catalysts efficiently promoted synthesis of 1, 8-dioxo-octahydro-xanthenes under solvent-free conditions, Chem. Sci. Trans. 1, 2012, 2, 233-238. [ Links ]
27 B. Karami, Z. Zare and K. Eskandari, Molybdate sulfonic acid: preparation, characterization, and application as an effective and reusable catalystfor octahydroxanthene-1,8-dione synthesis, Chem. Pap. ,2013, 67, 145-154. [ Links ]
28 P. Sivaguru and A. Lalitha, Ceric ammonium nitrate supported HY-zeolite: an efficient catalyst for the synthesis of 1, 8-dioxo-octa-hydroxanthenes, Chin. Chem. Lett, 2014, 25, 321-323. [ Links ]
29 M. Dabiri, S. Azimi and A. Bazgir, One-pot synthesis of xanthene derivatives under solvent-free conditions, Chem. Pap., 2008, 62, 522526. [ Links ]
30 L.S. Gadekar, S.S. Katkar, S.R. Mane, B.R. Arbad, M.K. Lande, Scolecite catalyzed facile and efficient synthesis of polyhydro-quinoline derivatives through Hantzsch multi-component condensation, Bull. Korean Chem. Soc, 2009, 30, 2532-2534. [ Links ]
31 M. Abdollahi-Alibeik and S.S. Hoseinikhah, ClO4-/Zr-MCM-41 nanoparticles prepared at mild conditions: a novel solid acid catalyst for the synthesis of polyhydroquinolines, J. Iran. Chem. Soc., 2016,13, 1339-1347. [ Links ]
32 H. Khabazzadeh, E.T. Kermani, D. Afzali, A. Amiri and A. Jalaladini, Efficient one-pot synthesis of polyhydroquinoline derivatives using Cs25H05PW12O40 as a heterogeneous and reusable catalyst in molten salt media, Arab. J. Chem, 2012, 5, 167-172. [ Links ]
33 N.O. Mahmoodi and Z. Khodaee, Evaluating the one-pot synthesis of hydantoins, Arkivoc, 2007, 3, 29-36. [ Links ]
34 S.U. Tekale, V.P. Pagore, S.S. Kauthale and R P. Pawar, La2O3/TFE: an efficient system for room temperature synthesis of Hantzsch poly-hydroquinolines, Chin. Chem. Lett, 2014, 25,1149-1152. [ Links ]
35 G. Mohammadi Ziarani, A.R. Badiei, Y. Khaniania and M. Haddad-pour, One pot synthesis of polyhydroquinolines catalyzed by sulfonic acid functionalized SBA-15 as a new nanoporous acid catalyst under solvent free conditions, Iran. J. Chem. Chem. Eng., 2010,29, 1-10. [ Links ]
36 F. Nemati and S.G. Alizadeh, Bi-SO3H Functionalized ionic liquid based on DABCO: new and efficient catalyst for facile synthesis of dihydropyrimidinones, J. Chem, 2013,1, 2013-2017. [ Links ]
Received 28 August 2018
Revised 8 June 2019
Accepted 29 July 2019
* To whom correspondence should be addressed. E-mail: b.khalili@guilan.ac.ir
Supplementary Data
The supplementary data is available in pdf: [Supplementary data]














