Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Chemistry
versión On-line ISSN 1996-840X
versión impresa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.71 Durban 2018
http://dx.doi.org/10.17159/0379-4350/2018/v71a25
RESEARCH ARTICLE
Synthesis, in vitro Cytotoxicity and Trypanocidal Evaluation of Novel 1,3,6-Substituted Non-fluoroquinolones
Richard M. BeteckI, *; Michelle IsaacsIII; Heinrich C. HoppeII, III; Setshaba D. KhanyeI, III, IV, *
IDepartment of Chemistry, Rhodes University, Grahamstown, 6140, South Africa
IIDepartment of Biochemistry and Microbiology, Rhodes University, Grahamstown, 6140, South Africa
IIICentre for Chemico- and Biomedical Research, Rhodes University, Grahamstown, 6140, South Africa
IVFaculty of Pharmacy, Rhodes University, Grahamstown, 6140, South Africa
ABSTRACT
Sleeping sickness (trypanosomiasis) is a neglected tropical disease that affects mostly the poorest communities in sub-Saharan Africa. Toxic side effects associated with the use of current anti-trypanosomal drugs, which in some cases kill faster than the disease itself, necessitate the search for new drugs with better safety margins. To this effect, a small library bearing different substituents at position -1, -3, and -6 of the quinolone nucleus were synthesized and evaluated in vitro against HeLa cell lines and Trypanosoma brucei brucei for cytotoxicity and trypanocidal potentials, respectively. While most of these compounds showed no cytotoxic effect, they exhibited moderate to weak anti-trypanosomal activities. The SAR studies of this series provide new information worth considering in future exploration of the quinolone scaffold in search of more potent and safe trypanocidal agents.
Keywords: Sleeping sickness, trypanosomiasis, quinolones, non-fluoroquinolones.
1. Introduction
Human African trypanosomiasis (HAT), also referred to as sleeping sickness,1 is among the WHO's list of neglected tropical diseases (NTDs).2 It mostly affects people living in rural areas of sub-Saharan Africa,3 where medical facilities are scarce and drug purchasing power of inhabitants is very low.4 At least 2184 new cases of the disease were reported in 2016,5 and approximately 60 million people living in 36 different countries are presently at risk of contracting the disease.6
HAT takes two forms caused by two different subspecies of Trypanosoma brucei.7 T. ". gambiense is the pathogenic subspecies causing the form of HAT commonly found in central and western Africa,8,9 whereas T. ". rhodesiense is the subspecies responsible for the form of HAT prevalent in eastern and southern Africa.10 It has been noted that the two sub-pathogenic species coexist in Uganda.11 These pathogens are transmitted between humans following the bite of an infected tsetse fly.12 The disease exists in two stages:13,14 haemolymphatic stage - wherein the parasites are localized in blood and lymphatic systems,15 and an encephalitic stage - wherein the parasites have invaded the central nervous system.16,17
Current treatment options are limited to just four drugs.18 In any case of HAT, the drug to be used is dictated by the form and stage of the disease. Pentamidine is the drug of choice for treating the haemolymphatic stage of HAT caused by T.". gambiense,19 while a combination of nifurtimox and eflornithine is used to treat the encephalitic stage.20 In cases of T ". rhodesiense, suramin is the recommended drug for treating the haemolymphatic stage,21 while malarsoprol is used to treat the encephalitic stage.22 Besides limited treatment options, the foregoing drugs are far from ideal. They all have poor oral bioavailability (and hence are administered intravenously), and considerable toxic side effects.23 For example, pentamidine causes hyper or hypoglycae-mia and hypotension,24 suramin causes renal failure, eflor-nithine causes alopecia and seizures,25 while at least 5.9 % of patients on malarsoprol die from its toxicity,26 creating a scenario wherein patients either die from an acute illness or die faster from a pill.
The overwhelming life-threatening side effects of existing drugs used to treat sleeping sickness create a dire need for new compounds with better drug properties such as high oral bioavailability, and a high safety margin. The therapeutic potentials of quinolones cannot be over emphasized. Compounds containing this scaffold are currently in use as drugs to treat bacterial and viral infections as well as other conditions such as cancers.27 Fluoroquinolones (Fig. 1a) in clinical use have been extensively screened against trypanosomes and their activity profiles established as moderate to poor.28,29,30 A hit optimization study by Hiltensperger and co-workers generated a potent library of fluoroquinolones characterized by benzylamides, (a)cyclic amines, and aliphatic chains at positions -3, -7, and -1 of the quinolone nucleus. However, the lead compound (Fig. 1b) in this series suffers from poor solubility,31 necessitating further work on this class of compounds. Unlike fluoroquinolones, the anti-trypanosomal potentials of non-fluorinated quinolones have not been extensively investigated, with just one study on non-fluorinated quinolones bearing substituents at position -1 and -2, respectively, being reported32 (Fig. 1c). To further expand the SAR around the non-fluoquinolone scaffold, we conceptualized and synthesized a library of 18 non-fluorinated quinolones bearing unique concurrent substituents at position -1, -3, and -6. Target compounds were subjected to in vitro cytotoxicity and anti-trypanosomal evaluation. At 20 μΜ concentration, most of the compounds exhibited less than 50 % parasite viability while having little effect on the viability of HeLa cell lines (see Supplementary information). This suggests that the quinolone scaffold can be tailored via chemical synthesis to generate safe and potent drug substrates to treat trypanosomiasis.
2. Results and Discussion
We conceptualized and synthesized a set of 18 novel compounds that bear different substituents at positions -1, -3 and -6 of the quinolone scaffold. Conceptualized compounds were synthesized as depicted in Scheme 1. Briefly, 4'-nitroaceto-phenone was reduced to 4'-aminoacetophenone using reduced iron powder and acetic acid.33 4'-Aminoacetophenone and 4-chloroaniline were each treated with diethyl ethoxy-methylenemalonate in refluxing acetonitrile to form condensed methylenemalonate esters, which underwent cyclization in boiling diphenylether at 245-250 °C for 5 min to form compound 2. Deprotonation of 2 using K2CO3, followed by N-alkylation with alkylhalides afforded target compounds 3a-h in yields ranging between 40 and 70 %. Ester functionality in compounds 3d-h underwent selective aminolysis in the presence of a ketone to afford target compounds 4a-j in 30-50 % yields. This transformation was realized using DBU as a base. All targeted compounds were characterized using proton and carbon NMR, HRMS and IR. The carbon NMR spectra of all compounds show a peak at c. 174 ppm, which is indicative of an oxo carbon within the quinolone nucleus (C-4) and the peak at c. 164 ppm is assigned to ester or amide carbonyl carbon (C-3a). With the exception of compounds 3a-c, the proton NMR spectra of all compounds show a singlet signal at ~2.3-2.6 ppm, which is assigned to a methyl attached to a carbonyl carbon (CH3C=O). The carbonyl carbon is also evident in the respective carbon spectra at ~197ppm. The presence of a triplet peak (J = 5.9 Hz) at c. 9.9 ppm in the proton NMR spectra of compounds 4a-j, which is absent in compounds 3d-h, suggests the successful conversion of ester to amide. It is also worth noting that the acquisition of NMR spectroscopic data was at times hindered by compounds crystallizing out of the solutions. This sometimes necessitated the use of hot solvents to encourage compounds to remain in solution long enough to obtain 1H and 13C NMR spectroscopic data (see Supplementary information). The absorption band at 1654/cm on the IR spectrum further confirms the presence of amide.
This focused library was screened in vitro against human cervix adenocarcinoma (HeLa) cell lines to investigate potential cytotoxicity effects. The compounds were incubated at 20 μΜ in 96-well plates containing HeLa cells for 48 h. The numbers of cells surviving upon drug exposure were determined using resazurin reduction to resorufin by live cells and reading resorufin fluorescence in a multiwell plate reader. Compounds were tested in duplicate, and a standard deviation (S.D.) calculated. Results are expressed as % viability based on fluorescence readings in treated wells versus untreated control wells. Emetine (which induces cell apoptosis) was used as positive control. With the exception of compound 4f (% viability, -19 %), and 4j (% viability, -13 %), which strongly inhibited HeLa cell lines, the rest of the series had little to no effect on HeLa cell viability. This observation suggests that this series with necessary optimization could serve as templates for the development of non-toxic anti-trypanosomal agents.
The compounds were further evaluated in vitro for anti-trypanosomal activities by screening against the 427 strain of T.b. brucei. Compounds were added to in vitro cultures of T b. brucei in 96-well plates at a 20 μΜ concentration. After an incubation period of 48 h, the numbers of parasites surviving drug exposure were determined by adding resazurin. As in HeLa cells, resazurin is reduced to resorufin by living parasites. Resorufin is a fluorophore (Exc560/Em590) and can thus be quantified in a multiwell fluorescence plate reader. Compounds were tested in duplicate wells, and a standard deviation (S.D.) calculated. Results are expressed as % viability - the resorufin fluorescence in compound-treated wells relative to untreated controls. Pentamidine (an existing drug for treatment of trypanosomiasis) was used as a positive control. At 20 μΜ concentration, 11 compounds inhibited parasite growth below 50 % (Table 1); however, only compounds inhibiting parasite viability below 25 % with little or no effect on HeLa cell line were considered for IC50 determination. The IC50 values for the selected compounds are summarized in Fig. 2.
Structure-activity relationship analysis across this series suggests that modifications at position -1, -3, and -6 of the quinolone scaffold influence anti-trypanosomal activities. For example, comparing the activity profiles of compounds 3b (IC50, 19 μΜ) and 3g (IC50, 24 μΜ), all having the same substituents at position -1 and -3 and differing in substitution pattern only at position -6 suggests that a chloride substituent at this position leads to increase anti-trypanosomal activity than a ketone. Also, comparing compounds 3d (IC50,32 μΜ) bearing an alkyl chain at position -1 and 3g (IC50, 24 μΜ) bearing a substituted-benzyl moiety at position -1, indicates that the presence of a substituted-benzyl moiety at position -1 seems to promote antitrypanosomal activities over alkyl chains. We also observed that the substituent on the benzyl moiety at position -1 also influences anti-trypanosomal activities. This is evident when comparing the effects of compounds 3a, 3b and 3c on parasite viability at a concentration of 20 μΜ. Compounds 3a and 3b bearing -NO2, and -Br, respectively, exhibited more than 75 % parasite growth inhibition, while compound 3c having -CF3 substituent exhibited less than 5 % parasite growth inhibition. These results suggest that electron-withdrawing units promote activity while electron-donating unit leads to poor activity. The substitution pattern at position -3 also seems to greatly influence activity. Comparing the activity of compounds 3g (ΚΖ50, 24μΜ) and 4g (IC50,7μΜ), both of which differ only in the substituent at position -3, suggests that an amide moiety at position -3 seems to enhance activity over ethyl ester.
3. Conclusion
We have synthesized a series of novel quinolones with varied substituents at position -1, -3 and -6 of the quinolone scaffold. While most compounds in this series showed no promising cytotoxicity potentials, compounds 4g emerged as potent anti-trypanosomal hit with IC50 value of 7 μΜ. Although this series exhibited moderate to weak activities profiles, the comprehensive structure-activity relationship analyses of this series will undoubtedly serve as a resource for further optimization of the quinolone scaffold in search of new and potent anti-trypano-somal agents.
4. Experimental
4.1. General Method
All the chemicals and solvents used were purchased from various chemical suppliers and were used without further purification. Melting points were determined using a Reichert hot stage microscope and are uncorrected. The progress of the reactions was monitored by thin layer chromatography (TLC) using Merck F254 silica gel plates supported on aluminium. The crude products were purified by a silica gel column chromatog-raphy using Merck Kieselgel 60 A: 70-230 (0.068-0.2 mm) silica gel mesh. 1H and 13C NMR spectra were recorded on Bruker Biospin 300 MHz, or 400 MHz spectrometers, and the chemical shifts are given in d values referenced to solvents and are reported in parts per million (ppm). The high-resolution mass spectrometric data of final compounds was recorded on a Waters Synapt G2 quadrupole time-of-flight (QTOF) mass spectrometer operated with an electrospray ionization probe in the positive mode (University of Stellenbosch). The instrument was operated with an electrospray ionization probe in the positive mode. The starting quinolones 2 were synthesized from the synthetically accessible compounds 1 as previously described in literature.34
4.2. Synthesis of Compounds
4.2.1. General Method for the Preparation of N-alkylated Compounds 3a-h
A mixture of 2 (3.86 mmol, 1 g, 1 eq), K2CO3 (5.0 mmol, 0.53 g), alkyl halide (5 eq.) in acetone (50 mL) was refluxed for 15 h. Upon reaction completion as indicated by TLC, the mixture was filtered, and the filtrate evaporated to dryness to obtain a crude N-alkylated product which was purified through silica gel column chromatography using CH2Cl2/MeOH (10:1) as the mobile phase. Compounds 3a-h were obtained in 40-70 % yield following this procedure.
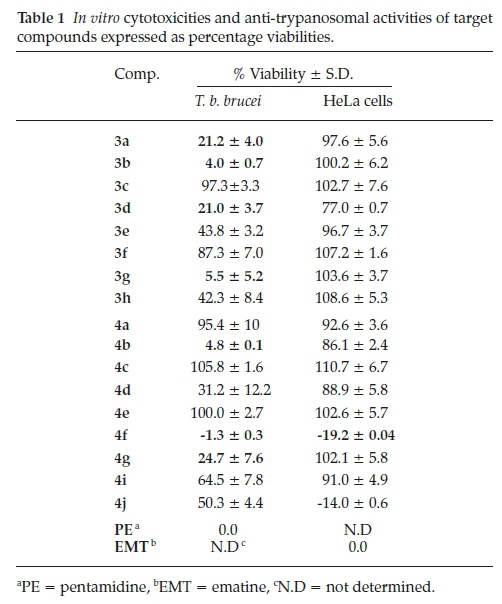
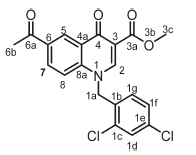
Methyl 6-chloro-1-(4-nitrobenzyl)-4-oxo-1,4-dihydroquinoline-3-carboxylate, 3a
Brown powder, 0.515 g (48 %), R = 0.81 (DCM/MeOH 10:1) m.p. 153-155 °C; 1H NMR (300 MHz, DMSO) d 9.00 (s, 1H, H-2), 8.16 (d, J = 2.5 Hz, 1H, H-5), 7.89-7.57 (m, 3H, H-1c, H-8), 7.48 -7.05 (m, 3H, H-7, H-1d), 5.82 (s, 2H, H-1a), 4.24 (s, 3H, H-3c). 13C NMR (75 MHz, DMSO) d 172.2 (C-4), 164.1 (C-3a), 151.3 (C-2), 141.1 (C-3), 138.2 (C-6), 133.2 (C-1d), 131.3 (C-1c), 130.5 (C-5), 130.1 (C-7), 127.6 (C-1b), 126.3 (C-4a), 125.9 (C-8), 120.7 (C-8a), 110.9 (C-1e), 57.3 (C-3c), 55.7 (C-1a). IR (neat, cm-1): 3093, 2972, 2931,1702,1686; ESI-HRMS m/z [M+H]+ calcd for C18H14ClN2O5 373.0586, found 373.0594.
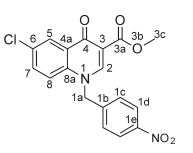
Ethyl 1-(4-bromobenzyl)-6-chloro-4-oxo-1,4-dihydroquinoline-3-carboxylate, 3b
White powder, 0.015 g (46 %), R = 0.8 (DCM/MeOH 10:1), m.p. 196-198 °C; 1H NMR (400 MHz, DMSO) d 8.94 (s, 1H, H-2), 8.16 (d, J = 2.5 Hz, 1H, H-5), 7.81-7.48 (m, 4H, H-7, H-8, H-1c), 7.21 (d, J = 8.4 Hz, 2H, H-1d), 5.68 (s, 2H, H-1a), 4.25 (q, J = 7.1 Hz, 2H, H-3c), 1.30 (t, J = 7.1 Hz, 3H, H-3d). 13C NMR (101 MHz, DMSO) d 172.0 (C-4), 165.0 (C-3a), 151.0 (C-2), 138.5 (C-3), 135.5 (C-6), 132.9 (C-5), 132.2 (C-1d), 130.6 (C-1b), 129.9 (C-1c), 128.9 (C-4a), 125.9 (C-7), 121.8 (C-1e), 120.6 (C-8), 111.1 (C-8a), 60.3 (C-3c), 55.6 (C-1a), 14.2 (C-3d). IR (neat, cm-1): 3096, 2978, 2941, 1702, 1680. ESI-HRMS m/z [M+H]+ calcd for C19H16BrClNO3 419.997, found 419.9998.
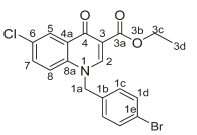
Ethyl 6-chloro-4-oxo-1-(4-(trifluoromethyl)benzyl)-1,4-dihydroquino-line-3-carboxylate, 3c
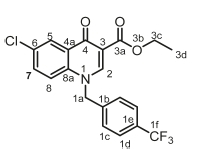
Brown powder, 0.5 g (70 %), R = 0.81 (DCM/MeOH 10:1), m.p. 203-205 °C; 1H NMR (400 MHz, Pyridine) d 9.19 (s, 1H, H-2), 7.65 (d, J = 8.4 Hz, 2H, H-1c), 7.56-7.49 (m, 3H, H-5, H-7, H-8), 7.43 (d, J = 8.4 Hz, 2H, H-1d), 5.00 (s, 2H, H-1a), 4.36 (q, J = 6.9 Hz, 2H, H-3c), 1.23 (t, J = 6.9 Hz, 3H, H-3d).13C NMR (101 MHz, Pyridine) d 173.0 (C-4), 164.7 (C-3a), 150.64 (C-2), 140.6 (C-3), 140.2 (C-1b), 137.9 (C-6), 135.5 (C-1d), 132.9 (C-1c), 130.9 (C-5), 130.2 (C-7), 127.2 (C-1e), 126.4 (C-4a), 125.9 (C-8), 120.7 (C-8a), 119.5 (C-1f), 112.2 (C-1f), 60.3 (C-3c), 56.0 (C-1a), 14.2 (C-3d). IR (neat, cm-1): 3003, 2962, 2921, 1705, 1684, ESI-HRMS m/z [M+H]+ calcd for C20H16ClF3NO3 410.0765, found 410.0766.
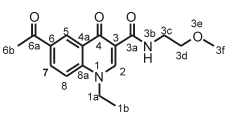
Ethyl 6-acetyl-1-ethyl-4-oxo-1,4-dihydroquinoline-3-carboxylate, 3d
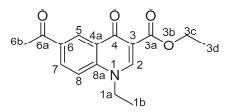
Red powder, 0.65 g (70 %), Rf= 0.71 (DCM/MeOH 10:1); m.p. 143-147 °C; 1H NMR (300 MHz, CDCl3) d 8.97 (s, 1H, H-2), 8.44 (d, J = 2.1 Hz, 1H, H-5), 8.22 (dd, J = 8.9,2.1 Hz, 1H, H-7), 7.46 (d, J = 9.0 Hz, 1H, H-8), 4.39-4.31 (m, 2H, H-1a), 4.23 (q, J = 7.2 Hz, 2H, H-3c), 2.63 (s, 3H, H-6b), 1.50 (t, J = 7.3 Hz, 3H, H-3d), 1.37-1.20 (m, 3H, H-1b). 13C NMR (75 MHz, CDCl3) d 196.9 (6a), 173.9 (4), 165.4 (3a), 149.1 (2), 141.6 (3), 133.3 (6), 131.4 (5), 129.5 (7), 128.7 (4a), 116.2 (8), 112.4 (8a), 61.1 (3c), 49.1 (1a), 26.6 (6b), 14.5 (1b), 14.4 (3d). IR (neat, cm-1): 3053, 2982, 2921,1712,1685, ESI-HRMS m/z [M+H]+ calcd for C16H18NO4 288.1230, found 288.1234.
Methyl 6-acetyl-4-oxo-1-(4-(trifluoromethyl)benzyl)-1,4-dihydro-quinoline-3-carboxylate, 3e
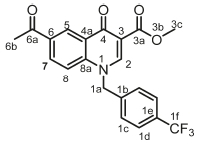
Brown powder, 0.680 g (68 %), R = 0.81 (DCM/MeOH 10:1), m.p. 133-136 °C; 1H NMR (300 MHz, DMSO) d 9.02 (s, 1H, H-2), 8.76 (d, J = 3.4 Hz, 1H, H-5) 8.15 (dd, J = 9.1,3.4 Hz, 1H, H-7), 7.73 (d, J = 8.0, Hz, 2H, H-1c), 7.60 (d, J = 7.1 Hz, 2H, H-1d), 7.43 (d, J = 9.1 Hz, 1H, H-8), 5.82 (s, 2H, H-1a), 3.89 (s, 3H, H-3c), 2.49 (s, 3H, H-6b). 13C NMR (75 MHz, DMSO) d 197.1 (C-6a), 173.9 (4), 164.4 (3a), 151.4 (C-2), 142.2 (C-3), 140.2 (C-1b), 135.7 (C-1d), 133.3 (C-6), 132.3 (C-1c), 132.0 (C-5), 129.2 (C-7), 128.2 (C-1e), 127.6 (C-4a), 121.5 (C-8), 118.8 (C-8a), 111.6 (C-1f), 55.3 (C-3c), 53.7 (C-1a), 26.6 (6b). IR (neat, cm-1): 3100, 2970, 2921, 1700, 1680. ESI-HRMS m/z [M+H]+ calcd for C21H16F3NO4 404.1104, found 404.1107.
Ethyl 6-acetyl-1-benzyl-4-oxo-1,4-dihydroquinoline-3-carboxylate, 3f
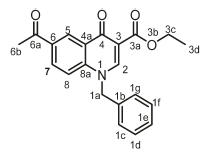
White powder, 0.80 g (58 %), R = 0.81 (DCM/MeOH 10:1) m.p. 142-144 °C; 1H NMR (300 MHz, DMSO) d 9.00 (s, 1H, H-2), 8.81 (s, 1H, H-5), 8.19 (d, J = 8.8 Hz, 1H, H-8), 7.77 (d, J = 8.9 Hz, 1H, H-7), 7.49-7.21 (m, 5H, H-1c/H-1g), 5.77 (s, 2H, H-1a), 4.31 (q, J= 7.0 Hz, 2H, H-3c), 2.55 (s, 3H, H-6b), 1.40-1.26 (m, 3H, H-3d). 13C NMR (75 MHz, DMSO) d 197.1 (C-6a), 173.3 (C-4), 164.7 (C-3a), 151.1 (C-2), 142.4 (C-3), 136.2 (C-1b), 133.2 (C-6), 131.8 (C-5), 129.4 (C-1g), 128.4 (C-4a), 128.3 (C-1f), 127.7 (C-7), 126.9 (C-1e), 118.8 (C-8), 111.9 (C-8a), 60.5 (C-3c), 56.2 (C-1a), 27.2 (C-6a), 14.7 (C-3d). IR (neat, cm-1): 3083, 2970, 2921, 1705, 1681, ESI-HRMS m/z [M+H]+ calcd for C21H20NO4 350.1387, found 350.1391.
Brown powder, 0.58 g (62 %), R = 0.81 (DCM/MeOH 10:1), m.p. 173-175 °C; 1H NMR (300 MHz, DMSO) d 8.99 (s, 1H, H-2), 8.75 (s, 1H, H-5), 8.16 (d, J = 8.6 Hz, 1H, H-7), 7.81-7.52 (m, 3H, H-1c, H-8), 7.19 (d, J = 7.6 Hz, 2H, H-1d), 5.72 (s, 2H, H-1a), 3.79 (s, 3H, H-3c), 2.50 (s, 3H, H-6b). 13C NMR (75 MHz, DMSO) d 197.4 (C-6a), 173.1 (C-4), 166.4 (C-3a), 151.4 (C-2), 142.2 (C-3), 135.7 (C-1d), 133.3 (C-6), 132.3 (C-1c), 132.0 (C-5), 129.2 (C-7), 128.3 (C-1b), 127.6 (C-4a), 127.5 (C-8), 119.8 (C-8a), 111.6 (C-1e), 55.3 (C-3c), 53.7 (C-1a), 26.6 (6b). IR (neat, cm-1): 3048,2972,2931,1702, 1682. ESI-HRMS m/z [M+H]+ calcd for C20H17BrNO4 414.0335, found 414.0333.
Methyl 6-acetyl-1-(2,4-dichlorobenzyl)-4-oxo-1,4-dihydroquino-line-3-carboxylate, 3h
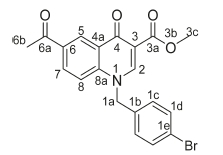
Brown powder, 0.28 g (48 %), R = 0.81 (DCM/MeOH 10:1), m.p. 197-1199 °C; 1H NMR (300 MHz, DMSO) d 9.16 (s, 1H, H-2), 8.92 (d, J = 2.2 Hz, 1H, H-5), 8.26 (dd, J = 8.9,2.2 Hz, 1H, H-7), 7.85 (d, J = 9.0 Hz, 1H, H-8), 7.72-7.59 (m, 2H, H-1d, H-1g), 7.21 (dd, J = 8.3,2.2 Hz, 1H, H-1f), 5.87 (s, 2H, H-1a), 3.77 (s, 3H, H-3c), 2.70 (s, 3H, H-6b). 13C NMR (75 MHz, DMSO) d 197.1 (C-6a), 176.3 (C-4), 164.0 (C-3a), 150.2 (C-2), 142.1 (C-3), 137.4 (C-6), 133.4 (C-1b), 132.1 (C-5), 131.9 (C-1g), 131.6 (C-1c), 131.1 (C-1e), 129.4 (C-1d), 127.7 (C-7), 127.4 (C-1f), 127.2 (C-4a), 118.7 (C-8), 112.8 (C-8a), 55.5 (C-1a), 52.3 (C-3c), 27.2 (C-6b). IR (neat, cm-1): 3073, 2972, 2941, 1702, 1683. ESI-HRMS m/z [M + H]+ calcd for C20H16Cl2NO4 404.0451, found 404.0452.
4.2.2. General Method for the Preparation of Amides 4a-j
A mixture of 3 (1 g, 1 eq.), DBU (320 //L, 0.33 g, 2.1 mmol), an appropriate amine (5 eq.), and chloroform (15 mL) in a 100 mL round-bottom flask was stirred under reflux for 24-30 h.34 Upon reaction completion as indicated by TLC, the mixture was evaporated to dryness and resultant crude subjected to silica gel column chromatography eluting with CH2Cl2/MeOH (10:1). Fractions containing the desired product were combined, evaporated to dryness and recrystallized from ethanol. Compounds 4a-j were obtained in 30-50 % yield following this procedure.
6-Acetyl-1-ethyl-N-(2-methoxyethyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide, 4a
Orange powder, 0.028 g (32 %), R = 0.48 (DCM/MeOH 10:1), m.p. 163-165 °C; 1H NMR (300 MHz, DMSO) d 9.93 (s, 1H, Nh), 8.88 (s, 1H, H-2), 8.83 (d, J = 1.5 Hz, 1H, H-5), 8.32-8.22 (m, 1H, H-7), 7.97 (d, J = 9.0 Hz, 1H, H-8), 4.55 (q, J = 7.0 Hz, 2H, H-1a), 3.49 (s, 3H, H-3f), 3.42-3.04 (m, 4H, H-3c, H-3d), 2.68 (s, 3H, H-6b), 1.39 (t, J = 7.0 Hz, 3H, H-1b). 13C NMR (75 MHz, DMSO) d 197.6 (C-6a), 175.9 (C-4), 164.2 (C-3a), 148.8 (C-2), 142.1 (C-3), 132.9 (C-6), 132.1 (C-5), 127.9 (C-7), 127.1 (C-4a), 118.4 (C-8), 112.6 (C-8a), 71.3 (C-3d), 58.3 (C-3f), 49.1 (C-1a), 38.8 (C-3c), 27.5 (C-6b), 14.9 (C-1b). IR (neat, cm-1): 3393, 3041, 2970, 2929, 1682, 1656. ESI-HRMS m/z [M+H]+ calcd for C17H21N2O4 317.1496, found 317.1497.
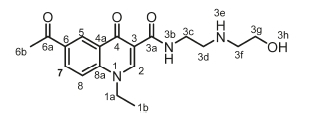
6-Acetyl-1-ethyl-N-(2-(2-hydroxyethoxy)ethyl)-4-oxo-1,4-dihydro-quinoline-3-carboxamide, 4b
White powder, 0.235 g (33 %), R = 0.39 (DCM/MeOH 10:1), m.p. 127-129 °C; 1H NMR (400 MHz, CDCl3) d 10.12 (s, 1H, NH), 8.97 (s, 1H, H-2), 8.75 (s, 1H, H-5), 8.27 (d, J = 7.9 Hz, 1H, H-7), 7.54 (d, J =7.9Hz,1H,H-8),4.31 (q, J =7.1Hz,2H,H-1a),3.74-3.61 (m, 8H, H-3c, H-3d, H-3f, H-3g), 2.67 (s, 3H, H-6b), 1.53 (t, J = 7.2 Hz, 3H, H-1b). 13C NMR (101 MHz, CDCl3) d 196.8 (C-6a), 176.7 (C-4), 164.7 (C-3a), 147.9 (C-2), 141.5 (C-3), 133.6 (C-6), 131.9 (C-5), 129.2 (C-7), 127.9 (C-4a), 116.2 (C-8), 113.5 (C-8a), 72.7 (C-3g), 69.7 (C-3f), 61.7 (C-3d), 49.3 (C-1a), 39.0 (C-3c), 26.6 (C-6b), 14.6 (C-1b). IR (neat, cm-1): 3333,3252,3001,2970,2929,1682,1654. ESI-HRMS m/z [M+H]+ calcd for C18H23N2O5 347.1601, found 347.1604.
6-Acetyl-1-ethyl-N-(2-((2-hydroxyethyl)amino)ethyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide, 4c
White powder, 0.475 g (48 %), R = 0.11 (DCM/MeOH 10:1), m.p. 157-159 °C; 1H NMR (300 MHz, DMSO) d 9.97 (t, J = 5.9 Hz, 1H, NH-3b), 8.91 (s, 1H, H-2), 8.84 (d, J = 2.2 Hz, 1H, H-5), 8.30 (dd, J = 9.0,2.2 Hz, 1H, H-7), 8.01 (d, J = 9.0 Hz, 1H, H-8), 5.27 (s, 1H, H-3h), 4.56 (q, J = 7.1 Hz, 2H, H-1a), 3.67 (t, J = 6.3 Hz, 4H, H-3d, H-3f), 3.15 (t, J = 8.8 Hz, 2H, H-3g), 3.03 (t, J = 5.4 Hz, 2H, H-3c), 2.68 (s, 3H, H-6b), 1.39 (t, J = 7.1 Hz, 3H, H-1b). 13C NMR (75 MHz, DMSO) d 197.2 (C-6a), 175.8 (C-4), 165.1 (C-3a), 149.2 (C-2), 141.8 (C-3), 133.2 (C-6), 132.2 (C-5), 127.6 (C-7), 127.2 (C-4a), 118.5 (C-8), 112.3 (C-8a), 56.9 (C-3g), 49.7 (C-1a), 49.0 (C-3c), 47.1 (C-3f), 35.9 (C-3d), 27.2 (C-6b), 14.9 (C-1b). IR (neat, cm-1): 3313, 3243, 3081,2879,2819,1687,1656. ESI-HRMS m/z [M+H]+ calcd for C18H24N3O4 346.1761, found 346.1762.
N-(3-(1H-imidazol-1-yl)propyl)-6-acetyl-1-ethyl-4-oxo-1,4-dihydro-quinoline-3-carboxamide, 4d
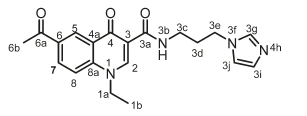
White powder, 0.521 g (57 %), R = 0.4 (DCM/MeOH 10:1), m.p. 207-209 °C; 1H NMR (300 MHz, CDCl3) d 9.98 (s, 1H, NH), 8.99 (s, 1H, H-2), 8.76 (d, J = 0.9 Hz, 1H, H-5), 8.28 (dd, J = 9.0,0.9 Hz, 1H, H-7), 7.57 (d, J = 9.0 Hz, 1H, H-8), 7.49 (s, 1H, H-3g), 6.99 (d, J = 9.2 Hz, 1H, H-3j), 6.93 (d, J = 9.2 Hz, 1H, H-3i), 4.32 (q, J = 7.2 Hz, 2H, H-1a), 4.01 (t, J = 7.0 Hz, 2H, H-3e), 3.43 (q, J = 6.3 Hz, 2H, H-3c), 2.66 (s, 3H, H-6b), 2.05 (dt, J = 6.8, 6.3 Hz, 2H, H-3d), 1.54-1.49 (t, J = 7.2 Hz, 3H, H-1b). 13C NMR (75 MHz, CDCl3) d 196.6 (C-6a), 176.5 (C-4), 164.8 (C-3a), 147.9 (C-2), 141.6 (C-3), 137.2 (C-3g), 133.4 (C-6), 131.7 (C-5), 129.5 (C-3j), 128.9 (C-7), 127.4 (C-4a), 118.9 (C-3i), 116.4 (C-8), 112.9 (C-8a), 49.4 (C-1a), 44.5 (C-3c), 36.1 (C-3e), 31.3 (C-3d), 26.6 (C-6b), 14.6 (C-1b). IR (neat, cm-1): 3303, 3087, 2960, 2912, 1687, 1655. ESI-HRMS m/z [M+H]+ calcd for C20H23N4O3 367.1765, found 367.1767.
6-Acetyl-1-(4-bromobenzyl)-N-(2-methoxyethyl)-4-oxo-1,4-dihydro-quinoline-3-carboxamide, 4e
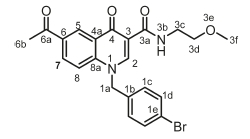
White powder, 0.432 g (43 %), R = 0.4 (DCM/MeOH 10:1), m.p. 136-138 °C; 1H NMR (300 MHz, DMSO) d 9.94 (t, J = 5.0 Hz, 1H, NH), 9.10 (s, 1H, H-2), 8.86 (d, J = 2.1 Hz, 1H, H-5), 8.19 (dd, J = 9.0, 2.2 Hz, 1H, H-7), 7.79 (d, J = 9.0 Hz, 1H, H-8), 7.62-7.45 (m, 2H, H-1d), 7.29-7.16 (m, 2H, H-1c), 5.79 (s, 2H, H-1a), 3.51 (t, J = 4.6 Hz, 2H, H-3c), 3.48-3.06 (m, 5H, H-3d, H-3f), 2.65 (s, 3H, H-6b). 13C NMR (75 MHz, DMSO) d 197.1 (C-6a), 176.2 (C-4), 164.1 (C-3a), 150.2 (C-2), 142.2 (C-3), 135.7 (C-1b), 133.3 (C-6), 132.3 (C-5), 132.0 (C-1c), 129.2 (C-1d), 127.7 (C-7), 127.3 (C-1e), 121.5 (C-4a), 118.9 (C-8), 112.7 (C-8a), 71.2 (C-3d), 58.5 (C-3f), 55.8 (C-1a), 38.8 (C-3c), 27.2 (C-6b). IR (neat, cm-1): 3317, 3051, 2960, 2900,1686,1657. ESI-HRMS m/z [M+H]+ calcd for C22H22BrN2O4 459.0763, found 459.0751.
6-Acetyl-1-(4-bromobenzyl)-N-(2-((2-hydroxyethyl)amino)ethyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide, 4f
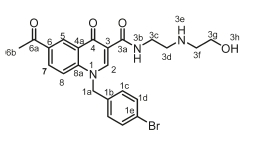
White powder, 0.20 g (48 %), R = 0.11 (DCM/MeOH 10:1), m.p. 178-180 °C; 1H NMR (300 MHz, DMSO) d 9.93 (t, J = 4.8 Hz, 1H, NH), 9.10 (s, 1H, H-2), 8.85 (d, J = 1.7 Hz, 1H, H-5), 8.19 (dd, J = 8.9,1.7 Hz, 1H, H-7), 7.79 (d, J = 9.0 Hz, 1H, H-8), 7.54 (d, J = 8.3 Hz, 2H, H-1d), 7.20 (d, J = 8.3 Hz, 2H, H-1c), 5.79 (s, 2H, H-1a), 4.62 (t, J = 5.2 Hz, 2H, H-3c), 3.60-3.49 (m, 6H, H-3d, H-3f, H-3g), 2.43 (s, 3H, H-6b). 13C NMR (75 MHz, DMSO) d 197.1 (C-6a), 176.2 (C-4), 164.1 (C-3a), 150.2 (C-2), 142.2 (C-3), 135.7 (C-1d), 133.4 (C-6), 132.2 (C-1c), 132.0 (C-5), 129.3 (C-7), 127.4 (C-1b), 121.6 (C-4a), 118.9 (C-8), 112.7 (C-8a), 111.9 (C-1e), 58.7 (C-3g), 55.8 (C-1a), 49.4 (C-3f), 48.9 (C-3d), 36.9 (C-3c), 27.2 (C-6b). IR (neat, cm-1): 3320, 3202, 3000, 2970, 2929, 1682, 1657. ESI-HRMS m/z [M+H]+ calcd for C23H25BrN3O4 486.1023, found 486.1021.
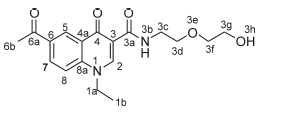
6-Acetyl-1-(4-bromobenzyl)-N-[2-(2-hydroxyethoxy)ethyl]-4-oxo-1,4-dihydroquinoline-3-carboxamide, 4g
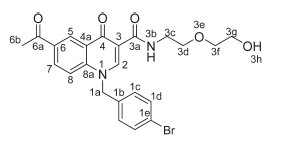
White powder, 0.20 g (48 %), R = 0.4 (DCM/MeOH 10:1), m.p. 183-185 °C; 1H NMR (300 MHz, DMSO) d 9.93 (t, J = 4.8 Hz, 1H, NH), 9.10 (s, 1H, H-2), 8.85 (d, J = 1.7 Hz, 1H, H-5), 8.19 (dd, J = 8.9,1.7 Hz, 1H, H-7), 7.79 (d, J = 9.0 Hz, 1H, H-8), 7.54 (d, J = 8.3 Hz, 2H, H-1d), 7.20 (d, J = 8.3 Hz, 2H, H-1c), 5.79 (s, 2H, H-1a), 4.62 (t, J = 5.2 Hz, 2H, H-3c), 3.60-3.49 (m, 6H, H-3d, H-3f, H-3g), 2.43 (s, 3H, H-6b). 13C NMR (75 MHz, DMSO) d 197.1 (C-6a), 176.2 (C-4), 1- 64.1 (C-3a), 150.2 (C-2), 142.2 (C-3), 135.7 (C-1d), 133.4 (C-6), 132.2 (C-1c), 132.0 (C-5), 129.3 (C-7), 127.4 (C-1b), 121.6 (C-4a), 118.9 (C-8), 112.7 (C-8a), 111.9 (C-1e), 72.7 (C-3d), 69.7 (C-3f), 60.7 (C-3g), 55.0 (C-1a), 39.7 (C-3c), 27.2 (C-6b). IR (neat, cm-1): 3397, 3252, 3041, 2950, 2861, 1686, 1654. ESI-HRMS m/z calcd for C23H24BrN2O5 487.0863, found 487.0665 [M+H]+. HPLC purity > 96 %, retention time = 9.89 min.
6-Acetyl-1-benzyl-N-(2-((2-hydroxyethyl)amino)ethyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide, 4h
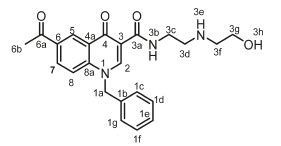
Orange powder, 0.320 g (48 %), R = 0.11 (DCM/MeOH 10:1), m.p. 169-171 °C; 1H NMR (300 MHz, DMSO) d 9.93 (t, J = 4.8 Hz, 1H, NH), 9.10 (s, 1H, H-2), 8.85 (d, J =1.7 Hz, 1H, H-5), 8.19 (dd, J = 8.9,1.7 Hz, 1H, H-7), 7.79 (d, J = 9.0 Hz, 1H, H-8), 7.54-7.20 (m, 5H, H-1c, H-1d, H-1f, H-1g), 5.79 (s, 2H, H-1a), 4.62 (t, J = 5.2 Hz, 2H, H-3c), 3.60-3.49 (m, 6H, H-3d, H-3f, H-3g), 2.43 (s, 3H, H-6b). 13C NMR (75 MHz, DMSO) d 197.1 (C-6a), 176.2 (C-4), 164.1 (C-3a), 150.2 (C-2), 142.2 (C-3), 135.7 (C-1d), 133.4 (C-6), 132.2 (C-1c), 132.0 (C-5), 129.3 (C-7), 127.4 (C-1b), 121.6 (C-4a), 118.9 (C-8), 112.7 (C-8a), 111.9 (C-1e), 72.7 (C-3d), 69.7 (C-3f), 60.7 (C-3g), 55.02 (C-1a), 39.7 (C-3c), 27.2 (C-6b). IR (neat, cm-1): 3328, 3212, 3038,2971,2921,1682,1659. ESI-HRMS m/z [M+H]+ calcd for C23H26N3O4 408.1918, found 408.1923.
6-Acetyl-1-(2,4-dichlorobenzyl)-N-(2-methoxyethyl)-4-oxo-1,4-dihy-droquinoline-3-carboxamide, 4i
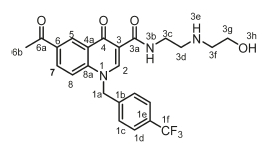
Brown powder, 0.370 g (44 %), R = 0.4 (DCM/MeOH 10:1). m.p. 203-205 °C; 1H NMR (300 MHz, DMSO-d6) d 9.99 (t, J = 5.1 Hz, 1H, NH), 9.16 (s, 1H, H-2), 8.92 (d, J = 2.2 Hz, 1H, H-5), 8.26 (dd, J = 8.9, 2.2 Hz, 1H, H-7), 7.85 (d, J = 9.0 Hz, 1H, H-8), 7.72-7.59 (m, 2H, H-1d, H-1f), 7.21 (d, J = 8.3, Hz, 1H, H-1g), 5.87 (s, 2H, H-1a), 3.56-3.13 (m, 4H, H-3c, H-3d), 3.37 (s, 3H, H-3f), 2.70 (s, 3H, H-6b). 13C NMR (75 MHz, DMSO) d 197.1 (C-6a), 176.3 (C-4), 164.0 (C-3a), 150.2 (C-2), 142.1 (C-3), 137.4 (C-6), 133.4 (C-1b), 132.1 (C-5), 131.9 (C-1g), 131.6 (C-1c), 131.1 (C-1e), 129.4 (C-1d), 127.7 (C-7), 127.4 (C-1f), 127.2 (C-4a), 118.7 (C-8), 112.8 (C-8a), 71.2 (C-3d), 58.5 (C-3f), 55.3 (C-1a), 39.0 (C-3c), 27.2 (C-6b). IR (neat, cm-1): 3298,3071,2971,2929,1682,1655. ESI-HRMS m/z [M+H]+ calcd for C22H21Cl2N2O4 447.0873, found 447.0878.
6-Acetyl-N-(2-((2-hydroxyethyl)amino)ethyl)-4-oxo-1-(4-(trifluoro-methyl)benzyl)-1,4-dihydroquinoline-3-carboxamide, 4j
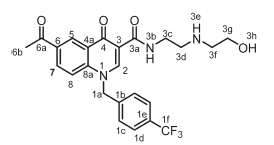
Orange powder, 0.367 g (44 %), R = 0.11 (DCM/MeOH 10:1), m.p. 193-195 °C; 1H NMR (300 MHz, DMSO) d 10.03 (t, J = 5.9 Hz, 1H, NH), 9.22 (s, 1H, H-2), 8.92 (d, J = 2.1 Hz, 1H, H-5), 8.28 (dd, J = 8.9, 2.2 Hz, 1H, H-7), 7.87 (d, J = 9.0 Hz, 1H, H-8), 7.79 (d, J = 8.1 Hz, 2H, H-1c), 7.51 (d, J = 8.0 Hz, 2H, H-1d), 6.03 (s, 2H, H-1a), 5.36 (t, J = 5.1 Hz, 1H, OH), 3.83-3.70 (m, 4H, H-3d, H-3f), 3.22 (t, J = 6.3 Hz, 2H, H-3c), 3.11 (t, J = 5.3 Hz, 2H, H-3g), 2.70 (s, 3H, H-6b). 13C NMR (75 MHz, DMSO) d 197.1 (C-6a), 176.1 (C-4), 165.0 (C-3a), 150.4 (C-2), 142.2 (C-3), 141.1 (C-1b), 133.5 (C-6), 132.4 (C-5), 129.1 (C-1e), 127.7 (C-7), 127.4 (C-1c), 127.3 (C-1d), 126.3 (C-1f), 126.3 (C-4a), 118.9 (C-8), 112.6 (C-8a), 56.9 (C-3g), 55.9 (C-1a), 49.6 (C-3f), 47.0 (C-3d), 35.9 (C-3c), 27.2 (C-6b). IR (neat, cm-1): 3293, 3252, 3081, 2974, 2926, 1682, 1654. ESI-HRMS m/z [M+H]+ calcd for C24H25F3N3O4 476.1792, found 476.1797.
4.3. In vitro Anti-trypanosomal Assay
Trypanosoma brucei brucei 427 trypomastigotes were cultured in Iscove's Modified Dulbecco's Medium (IMDM; Lonza) supplemented with 10 % fetal calf serum,35 HMI-9 supplement, hypoxanthine and penicillin/streptomycin at 37 °C ina5%CO2 incubator. Serial dilutions of test compounds were incubated with the parasites in 96-well plates for 24 h and residual parasite viability in the wells determined by adding 20 of 0.54 mM resazurin in phosphate buffered saline (PBS) and incubating for an additional 2-4 h. Reduction of resazurin to resorufin by viable parasites was assessed by fluorescence readings (excitation 560 nm, emission 590 nm) in a Spectramax M3 plate reader. Fluorescence readings were converted to % parasite viability relative to the average readings obtained from untreated control wells. IC50 values were determined by plotting % viability vs. log[compound] and performing non-linear regression using GraphPad Prism (v. 5.02) software.
4.4. In vitro Cytotoxicity Assay
HeLa cells (Cellonex) were cultured in Dulbecco's modified Eagle's medium (DMEM; Lonza) supplemented with 10 % fetal calf serum and antibiotics (penicillin/streptomycin/ampho-tericin B) at 37 °C ina5%CO2 incubator. Cells were plated in 96-well plates at a cell density of2x104 cells per well and grown overnight. Serial dilutions of test compounds were incubated with the cells for an additional 24 h, and cell viability in the wells assessed by adding 20 0.54 mM resazurin in PBS for an additional 2-4 h. Fluorescence readings (excitation 560 nm, emission 590 nm) obtained for the individual wells were converted to % cell viability relative to the average readings obtained from untreated control wells. Plots of % cell viability vs. log[com-pound] were used to determine IC50 values by non-linear regression using GraphPad Prism (v. 5.02).
Supplementary Material
Supplementary information is provided in the online supplement.
Acknowledgements
The authors acknowledge the financial support by the National Research Foundation (SDK), Rhodes University for a Postdoctoral fellowship (RMB) and Rhodes University Sandisa Imbewu (SDK) towards this research. The bioassay component of the project was funded by the South African Medical Research Council (MRC) with funds from National Treasury under its Economic Competitiveness and Support Package awarded to HCH.
ORCID iDs
S.D. Khanye: © orcid.org/0000-0003-0725-5738 R.M. Beteck: © orcid.org/0000-0002-6282-043X
References
1 R. Brun, R. Schumacher, C. Schmid, C. Kunz and C. Burri, The phenomenon of treatment failures in human African trypanosomiasis, Trop. Med. Int. Health., 2001, 6, 906-914. [ Links ]
2 WHO. Neglected tropical diseases http://www.who.int/neglected_diseases/diseases/en/ accessed on 14 August 2017. [ Links ]
3 M. Kaiser, M. Bray, M. Cal, B. Trunz, E. Torreele and R. Brun, Anti-trypanosomal activity of fexinidazole, a new oral nitroimidazole drug candidate for treatment of sleeping sickness, Antimicro". Agents Chemother., 2011, 55, 5602-5608. [ Links ]
4 M. Berninger, I. Schmidt, A. Ponte-Sucreb and U. Holzgrabe, Novel lead compounds in pre clinical development against African sleeping sickness, Med. Chem. Commun., 2017, 8, 1872-1890. [ Links ]
5 WHO. Trypanosomiasis, human African (Sleeping sickness), http://www.who.int/mediacentre/factsheets/fs259/en/ accessed on 14 August 2017. [ Links ]
6 H.-H. Tran, Z. Zheng, X. Wen, S. Manivannan, A. Pastor, M. Kaiser, R. Brun, F. Snyder and T. Back, Synthesis and activity of nucleoside-based antiprotozoan compounds, Bioorg. Med. Chem., 2017, 25, 2091-2104. [ Links ]
7 X. Wang, D. Inaoka, T. Shiba, E. Balogun, S. Allmann, Y. Watanabe, M. Boshart, K. Kita and S. Harada, Expression, purification, and crystallization of type 1 isocitrate dehydrogenase from Trypanosoma brucei brucei, Protein Expr. Purif., 2017,138, 56-62. [ Links ]
8 H. Gordhan, S. Patrick, M. Swasy, A. Hackler, M. Anayee, J. Golden, J. Morris and D. Whitehead, Evaluation of substituted ebselen derivatives as potential trypanocidal agents, Bioorg. Med. Chem. Lett., 2017, 27, 537-541. [ Links ]
9 M. Beig, F. Oellien, L. Garoff, S. Noack, L. Krauth-Siegel and P. Selzer, Trypanothione reductase: a target protein for a combined in vitro and in silico screening approach, PLOS Negl. Trop., 2015, 9, e0003773. [ Links ]
10 I. Kuepfer, E. Hhary, M. Allan, A. Edielu, C. Burri and J. Blum, Clinical presentation of T b. rhodesiense sleeping sickness in second stage patients from Tanzania and Uganda, PLOS Negl. Trop. Dis., 2011, 5, e968. [ Links ]
11 F. Wamwiri and R. Changasi, Tsetse flies (Glossina) as vectors of human African trypanosomiasis: a review, BioMed. Res. Int., 2016, 2016, 1-8. [ Links ]
12 D. Steverding, The history of African trypanosomiasis, Parasites & Vectors, 2008, 1, 3. [ Links ]
13 M. Banerjee, D. Paraia, P. Dhar, M. Roy, R. Barik, S. Chattopadhyay and S. Mukherje, Andrographolide induces oxidative stress-dependent cell death in unicellular protozoan parasite Trypanosoma brucei, Acta Trop., 2017,176, 58-67. [ Links ]
14 F. Ranjbarian, M. Vodnala, K. Alzahrani, G. Ebiloma, H. Koning and A. Hofer, 9-(2'-Deoxy-2'-fluoro-/?-D-Arabinofuransosyl)adenine is a potent antitrypanosomal adenosine analogue that circumvents transport-related drug resistance, Antimicrob. Agents Chemother., 2017, 61, e02719-16. [ Links ]
15 L. MacLean, H. Reiber, P. Kennedy and J. Sternberg, Stage progression and neurological symptoms in Trypanosoma brucei rhodesience sleeping sickness: role of the CNS inflammatory response, PLOS Negl. Trop. Dis., 2012, 6, e1857. [ Links ]
16 P. Kennedy, The continuing problem of human African trypano-somiasis (sleeping sickness), Ann. Neurol., 2008, 64, 116-126. [ Links ]
17 N. Tiberti, A, Hainard and J.-C. Sanchez, Translation of human African trypanosomiasis biomarkers towards field application, Transl. Prot., 2013,12, 12-24. [ Links ]
18 P. Babokhov, A. Sanyaolu, W. Oyibo, A. Fagbenro-Beyioku and N. Iriemenam, A current analysis of chemotherapy strategies for the treatment of human African trypanosomiasis, Pathog. Glob. Health, 2013,107, 242-252. [ Links ]
19 J. Song, N. Baker, M. Rothert, B. Henke, L. Jeacock, D. Horn and E. Beitz, Pentamidine is not a permeant but a nanomolar inhibitor of the Trypanosoma brucei aquaglyceroporin-2, PLOS Pathog., 2016, 12, e1005436. [ Links ]
20 E. Alirol, D. Schrumpf, J. Amici Heradi, A. Riedel, C. de Patoul, M. Quere and F. Chappuis, Nifurtimox-eflornithine combination therapy for second-stage gambiense human African trypanosomiasis: Médecins San Frontières experience in the Democratic Republic of Congo, Clin. Infect. Dis., 2013, 56, 195-203. [ Links ]
21 R. Jacobs, B. Nare and M. Phillips, State of the art in African trypano-some drug discovery, Curr. Top. Med. Chem., 2011,11, 1255-1274. [ Links ]
22 M. Barrett, D. Boykin, R. Brun and R. Tidwe, Human African trypanosomiasis: pharmacological re-engagement with a neglected disease, Br. J. Pharmacol., 2007,152, 1155-1171. [ Links ]
23 D. Malvy and F. Chappuis, Sleeping sickness, Clin. Microbiol. Infect., 2011,17, 986-995. [ Links ]
24 G. Pohlig, S.C. Bernhard, J. Blum, C. Burri, A. Mpanya, J.-P. Fina Lubaki, A. Mpoto, B. Munungu, M. Bilenge, V. Mesu, J. Franco, N. Dituvanga, R. Tidwell and C. Olson, Efficacy and safety of pafura-midine versus pentamidine maleate for treatment of first stage sleeping sickness in a randomized, comparator-controlled, international phase 3 clinical trial, PLOS Negl. Trop. Dis., 2016,10, e0004363. [ Links ]
25 P. Kennedy, Clinical features, diagnosis, and treatment of human African tripanosomiasis (sleeping sickness), Lancet Neurol., 2013, 12, 186-194. [ Links ]
26 C. Burri, Chemotherapy against human African tripanosomiasis: Is there a road to success?, Parasitol., 2010, 137, 1987-1994. [ Links ]
27 R. Beteck, F. Smit, R. Haynes and D. N'Da, Recent progress in the development of anti-malarial quinolones, Mal. J., 2014, 13, 339. [ Links ]
28 E. Nenortas, C. Burri and T. Shapiro, Antitrypanosomal activity of fluoroquinolones, Antimicrob. Agents Chemother., 1999,43,2066-2068. [ Links ]
29 J. Keiser and C. Burri, Antitrypanosomal activities of fluoroquinolones with pyrrolidinyl substitutions, Trop. Med. Inter. Health., 2001,6,369-389. [ Links ]
30 E. Nenortas, C. Burri, T. Kulikowicz and T. Shapiro, Antitrypano-somal activities of fluoroquinolones with pyrrolidinyl substitutions, Antimicrob. Agents Chemother., 2003, 47, 3015-3017. [ Links ]
31 G. Hiltensperger, N. Jones, S. Niedermeier, A. Stich, M. Kaiser, J. Jung, S. Puhl, A. Damme, H. Braunschweig, L. Meinel, M. Engstler and U. Holzgrabe, Synthesis and structure-activity relationships of new quinolone-type molecules against Trypanosoma brucei, J. Med. Chem., 2012, 55, 2538-2548. [ Links ]
32 A. Wube, A. Hüfner, W. Seebacher, M. Kaiser, R. Brun, R. Bauer and F. Bucar, 1,2-Substituted 4-(1H)-quinolones: synthesis, antimalarial and antitrypanosomal activities in vitro, Molecules, 2014, 19, 14204-142020. [ Links ]
33 A. Gamble, J. Garner, C. Gordon, S. Conner and P. Keller, Aryl nitro reduction with iron powder or stannous chloride under ultrasonic irradiation, Syn. Comm., 2007, 37, 2777-2786. [ Links ]
34 Richard M. Beteck, D. Coertzen, F.J. Smit, L.-M. Birkholtz, R.K. Haynes and D.D. N'Da, Straightforward conversion of decoquinate into inexpensive tractable new derivatives with significant anti-malarial activities, Boorg. Med. Chem. Lett., 2016, 26, 3006-3009. [ Links ]
35 H. Hirumi and K. Hirumi, Continous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers, J. Parasitol., 1989, 75, 985-989. [ Links ]
Received 7 March 2018
Revised 13 November 2018
Accepted 15 November 2018
* To whom correspondence should be addressed. E-mail: r.beteck@ru.ac.za/s.khanye@ru.ac.za
Supplementary Data
The supplementary data is available in pdf: [Supplementary data]














