Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.71 Durban 2018
http://dx.doi.org/10.17159/0379-4350/2018/v71a20
RESEARCH ARTICLE
Synthesis, Characterization and Antibacterial Properties of N,N'-Bis(4-dimethylaminobenzylidene)benzene-1,3-diamine as New Schiff Base Ligand and its Binuclear Zn(II), Cd(II) Complexes
Leila Kafi-AhmadiI, *, §; Ahmad Poursattar MarjaniII; Mohammadreza Pakdaman-AzariI
IDepartment of Inorganic Chemistry, Faculty of Chemistry, Urmia University, Urmia, Iran
IIDepartment of Organic Chemistry, Faculty of Chemistry, Urmia University, Urmia, Iran
ABSTRACT
We synthesized a new Schiff base ligand by condensation reaction of 4-dimethylaminobenzaldehyde and 1,3-phenylenediamine. Treatment of this Schiff base ligand with Zinc(II) nitrate and Cadmium(II) nitrate in ethanol medium afforded the corresponding metal complexes. The synthesized ligand and complexes were characterized by their UV-Vis, FT-IR and 1H-NMR, 13C-NMR spectral data and elemental analysis. The spectral data suggest an octahedral geometry for these complexes. Antibacterial activities of both synthesized free ligand and complexes were investigated against Escherichia coli, Staphylococcus aureus, and Bacillus subtilis bacteria. The complexes showed better antibacterial activity in comparison with that of the free ligand against selective bacteria.
Keywords: New Schiff base ligand, 4-dimethylaminobenzaldehyde, Zn(II), Cd(II) complexes, antibacterial activity.
1. Introduction
A significant increase in the rate of mortality of people exposed to infectious diseases is very important and a concerning issue. The main reason of such diseases is bacteria that are extremely resistant to different antibiotics. The development of resistance of microorganism to antibiotics may be due to the following reasons: a) intrinsic or natural whereby there is no site for the drugs to bind there or the permeability against the drug is low, so the drug cannot affect the microorganism, b) the microorganism acquire resistance in order to not to be affected by the drug.1-3 The lack of effective treatments is the main cause of this issue. Hence, development of new antibacterial agents with novel and more efficient mechanisms of action is definitely an urgent medical need.
Metal chelaters are among materials with antimicrobial activity that is based on chelation theory. The biochemical activity of bioactive species can be improved by chelation. Chelation reduces the polarity of the metal ion because of overlapping of the ligand orbital and partial sharing of the positive charge of the metal ion.4,5 Consequently, the complex may penetrate into the lipid membranes which may disable the enzymes of microorganisms by blocking their metal binding sites. Moreover, such complexes frustrate the respiration of the cell and as a result the growth of the organism is restricted by disturbing the protein synthesis.6 The effect mechanism of antimicrobial agents is dependent on two factors: a) the bacteria's structure; b) the function affected by the agent which these two factors are as follow:
1) Obstruction of ribosome function folate metabolism and nucleic acid synthesis.
2) Blockage of cell membrane function, and cell wall synthesis. Schiff base complexes are effective metal chelates that can be used as antibacterial agents against Staphylococcus aureus, Escherichia coli, and Bacillus subtilis. Condensation of a primary amine with a ketone or an aldehyde, leads to the synthesis of Schiff bases. Structurally, when the carbonyl group of an aldehyde or ketone is replaced with an imine or azomethine group, the compound is called a Schiff base.7 Schiff base ligands are readily synthesized and they form complexes with some metal ions that are used as catalysts in different reactions such as polymerization, oxidation reactions, reduction of ketones, aldol, Diels-Alder and Henry reactions, epoxidation of alkenes and hydrosilylation of ketones.8,9 The characteristics, quantity and the position of donor atoms of a Schiff base ligand play a critical role on controlling the number of the metal ions within homo and heteropoly nuclear complexes and also stereochemistry of the metallic centres.10 Schiff base complexes are used in oxygen storage devices,11 molecular architectures,12 lasers,13 OLED applications,14 transistors15 and fluorescent sensors.16 Furthermore, their biological applications such as antibacterial,17 antifungal,18 anticancer,19 antioxidant,20 anti-inflammatory,21 antimalarial,22 antiviral activity,23 are reported recently. Biological activity of Schiff bases are due to the azomethine linkage. Complexes with metal ions such as Cu(II), Co(II), Fe(II) and Zn(II) with octahedral coordination have exhibited significant physicochemical and biological properties.24 Zinc metal with Schiff base complexes show good photoluminescent (PL) and electroluminescent properties.25
Luminescence properties of Zn(II) complexes are related to the organic ligand rather than ligand metal charge transfer since the shell of central ion is completely filled.16 Molecular structures, degree of conjugation, and substitutes of ligands have a large effect on PL characteristics of Zn-Schiff base complexes.25
Herein, we have reported the synthesis of new bidentate ligands by a simple condensation of 4-dimethylaminobenz-aldehyde and 1,3-phenylenediamine to yield N,N'-bis(4-dimethylamino benzylidene)benzene-1,3-diamine as new Schiff base ligand and the corresponding complex with Zn(II), Cd(II) ions. The ligand and complexes were characterized by their FT-IR, UV-Vis and 1H-NMR spectral data and elemental analysis. The antibacterial activities of both Schiff base and its complexes were measured.
2. Experimental
2.1. Materials and Methods
All materials were used in high purification purchased from Merck Co. The FT-IR analyses were conducted with Nexus 670, Thermo Nicolet (USA model) FT-IR spectrophotometer. The UV-Vis spectra were obtained with a UK model UV-Vis spectro-photometer. UV-Vis electronic absorption spectrum was measured in the 200-800 nm wavelength range. The melting point of compounds was measured by electrothermal melting point apparatus model BÜCHI 510. The conductometric measurement was carried out with a SELECTA LAB 901 conductometer. CHNS analysis was performed with an 'ElementaryVario EL III' elemental analyzer. 1H and 13C NMR spectra were recorded on a Bruker Avance AQC (Bruker Crop., Billerica, MA) 300 MHz spectrometer and 75.5 MHz, respectively. Chemical shifts were measured in DMSO-d6 as solvent with reference to TMS as the internal standard. Mass spectra of all compounds were recorded using Shimadzu LCMS-2010A.
2.2. Synthesis of N,N'-bis(4-dimethylaminobenzylidene) benzene-1,3-diamine as New Schiff Base Ligand
108 mg (1 mmol) of 1,3-phenylenediamine in ethanol (20 mL) was added to a stirring solution of 4-dimethylaminobenz-aldehyde (298 mg, 2 mmol) in hot ethanol (20 mL). The resultant mixture was heated under reflux for 24 h with continuous stirring and consequently, a yellow precipitate was formed (Scheme 1). The purity of the ligand was confirmed by TLC (using Ethylacetate:hexane / 3:2 as eluents, Rf = 0.57). The obtained precipitate was filtered, rinsed with distilled water, ethanol and dried at room temperature.
The physical properties of ligand are shown in Table 1 which are closely matching with the amounts calculated by the suggested formula. Moreover, the melting points calculated are sharp which indicate the purity of the synthesized ligand.
2.3. Synthesis of Schiff Base Complexes with Zn(II), Cd(II) Ions
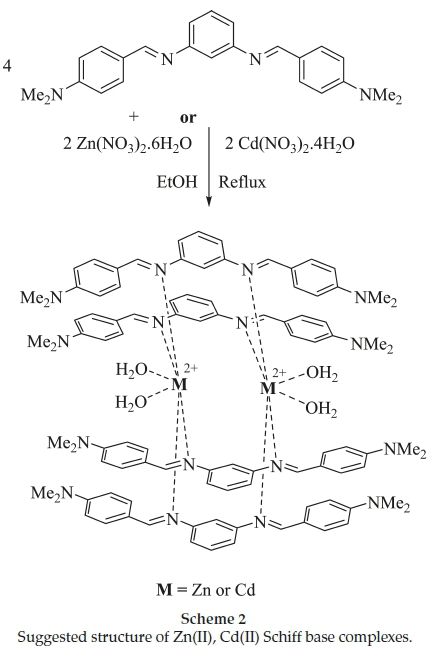
A mixture of the Schiff base (2 mmol) under investigation in 25 mL ethanol and the same amount of the same solvent of metal salt (Zn(NO3)2.6H2O and Cd(NO3)2.4H20,1 mmol) were refluxed for 3h on a waterbath. The purity of the complex was confirmed by TLC. The obtained solution was left to cool to room temperature, then the precipitate was filtered, washed many times with ethanol and then left to get dry at room temperature (Scheme 2). The physical properties of complexes is tabulated in Table 2. Endeavours to grow appropriate crystals for single crystal diffraction investigation were not successful.
2.4. Investigation of Antibacterial Activity
The diffusion method is a simple method that is commonly used in hospital laboratories to determine the antibacterial activity of various components that was used in this paper as well. In this method, some disks in the agar medium containing 2 % of glucose are used which the diameter of inhibited zone is visually read 18 h after incubation at temperature of 37 °C. Consequently, antibacterial activity is estimated by investigating the size of the zone of inhibition formed around the paper disks on the seeded agar plates. Ampicillin was used as a positive control.26
3. Results and Discussion
3.1. Conductance Measurements
Geometrical structures of the complexes were investigated with molar conductivity. Molar conductance (LM)of10-3 M ethanol solution of the complexes were determined. The values for the Zn(II), Cd(II) were 119.9 and 130.6 Ω-1 cm2 mol-1, respectively, consistent with 1:2 electrolytes.27,28
3.2. FT-IR Spectra
In the absence of a powerful technique such as X-ray crystallography, IR spectrum is the most suitable technique to obtain information in order to be able to elucidate the nature of bonding of the ligand to the metal ion. In the FT-IR spectra of ligand, a band at 1600 cm-1 is due to the C=N stretching vibration.29There is no peak related to unreacted primary amines or carbonyl groups. On complex formation, the IR band due to azomethine group shifts to the lower wave number (1580 cm-1 for Zn(II) complex and 1590 cm-1 for Cd(II) complex) which indicates that the nitrogen atom of azomethine groups are coordinated to the metal atom.30 The broad bands at approximately 3400 cm-1 for the complexes, along with a new band at 900 cm-1, indicate the presence of coordinated water.31 However, the band appearing at 475 and 483 cm-1 are due to M-N vibration in Zn(II), Cd(II) complexes, respectively.32
3.3. Electronic Spectra
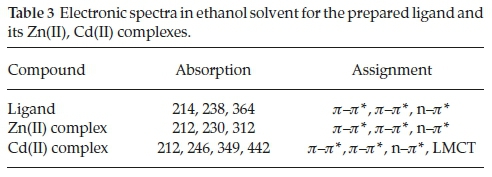
The electronic spectra of the Schiff base ligand and Zn(II), Cd(II) complexes in5x10-5 methanol solution at room temperature and resulting data are shown in Table 3. The UV-Visible spectrum of ligand showed three bands at 214,238 and 364 nm, of which the former two bands are because of the p-p* transitions within the aromatic ring and the third band is due to the n-p* transitions of azomethine group and also observed in the spectra of complex with a shift to lower wavelength, indicating that the ligand is coordinated to metal centre.33 Zn(II), Cd(II) complexes were diamagnetic as expected for d10 ions, so that no (d-d) transition can be expected in the visible region.34
3.4.1H NMR Spectral Studies
The data of 1H-NMR spectra of ligand and Zn(II), Cd(II) complexes are shown in Table 4 were measured in DMSO-d6 using TMS as an internal reference. The DMSO-d6 shows two impurities signals, one due to non-deuterated d-LC = 2.49 signals and water present in the solvent d-LC = 3.31 ppm.35,36
A singlet at d-LC = 8.46 ppm is assigned to the presence of azomethine proton.37 The peak at d-LC = 3.03 ppm attributed to NMe2 group in Schiff base ligand.38 Also, the 1H-NMR spectrum of ligand displayed signals located at d-LC = 6.75-7.76 ppm attributed to chemical shifts of aromatic protons.30
(Also 13C-NMR spectrum of ligand shown in supplementary information). In the Zn(II), Cd(II) complexes, the azomethine proton signal of ligand is shifted slightly to low field during the chelation process confirming the bonding of this group with metal ions through azomethine nitrogen. Also, the new peaks observed at d-LC = 2.03 and d-LC = 2.08 ppm (for Zn(II) complex) and d-LC = 2.04 and d-LC = 2.08 ppm (for Cd(II) complex) may be specified to coordinated water molecules.4,39
3.5. Mass Spectra of the Compounds
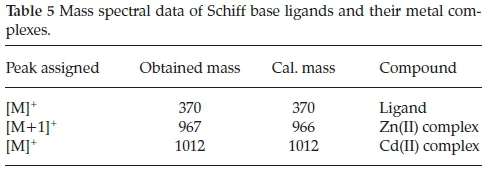
The ESI-mass spectra were measured to confirm the composition and the purity of the complexes and ligands under investigation (Table 5). The obtained mass spectra showed molecular ion peaks which were matching with the expected values.
3.6. Antibacterial Activity of Ligand and Complex in Agar Medium
Ampicillin26was used as a standard to study the antibacterial activity of prepared complex. The results are shown in Table 6.
The Schiff base and the complexes exhibited different antibacterial effects on the growth of the bacterial species. The activities of the metal complexes are found to be more as compared to the ligand that it may be related to the effect of the metal ion in the normal cellular process. Furthermore, the data show that Escherichia coli and Staphylococcus aureus were inhibited in a greater degree by the Zn(II) and Cd(II) complexes, respectively. Therefore, the complexes may be used as potential drugs with antibacterial activity after carrying out further researches on them.
4. Conclusions
In this paper, new Schiff base ligand obtained by condensation reaction of 4-dimethylaminobenzaldehyde and 1,3-phenylene-diamine give new N,N'-bis(4-dimethylamino benzylidene)ben-zene-1,3-diamine which is characterized by the spectral analysis. The reaction of the ligand with hydrated nitrate salts of Zn(II) and Cd(II) give the respective metal complexes in almost quantitative yields. From the analytical and spectral data, the ligand is found to coordinate to the metal ions through nitrogen atoms and acts as a bidentate ligand. According to the elemental analysis and analytical data, an octahedral geometry has been proposed for the complexes. Also, the ligand and complexes were tested in order to investigate their antibacterial effect on some hazardous bacterial such as Escherichia coli, Staphylococcus aureus, and Bacillus subtilis. The complexes exhibited sufficient biological activity which confirms that these compounds possess antibacterial effects.
Supplementary Material
Supplementary information is provided in the online supplement.
Acknowledgement
The authors are grateful to Urmia University for financial support.
References
1 E. Yousif, A. Majeed, Kh. Al-Sammarrae, N. Salih, J. Salimon and B. Abdullah, Metal complexes of Schiff base: preparation, characterization and antibacterial activity, Arab. J. Chem., 2017,10, S1639-S1644. [ Links ]
2 E. Dun, Antifungal resistance in yeast vaginitis. J. Biol. Med.,1999, 72, 281-285. [ Links ]
3 M.R. Yeaman and N.Y. Yount, Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev., 2003, 55, 27-55. [ Links ]
4 A.J. Abdulghani and R.K. Hussain, Synthesis and characterization of Schiff base metal complexes derived from cefotaxime with 1H-indole-2,3-dione (Isatin) and 4-N,N-dimethyl-aminobenzaldehyde, Open J. Inorg. Chem., 2015, 5, 83-101. [ Links ]
5 L.H. Abdel-Rahman, R.M. El-Khatib, L.A.E. Nassr, A.M. Abu-Dief, M. Ismail and A.A. Seleem, Metal based pharmacologically active agents: synthesis, structural characterization, molecular modeling, CT-DNA binding studies and in vitro antimicrobial screening of iron(II) bromosalicylidene amino acid chelates, Spectrochim Acta A., 2014,117, 366-378. [ Links ]
6 M.N. Ibrahim, S.A.I. Sharif, A.N. El-Tajory and A.A. Elamari, Synthesis and antibacterial activities of some Schiff bases, E-J. Chem., 2011,8, 212-216. [ Links ]
7 C.M. da Silva, D.L. da Silva, L.V. Modolo, R.B. Alves, M.A. de Resende, C. Martins and Â. de Fátima, Schiff bases: a short review of their antimicrobial activities, J. Adv. Res., 2011, 2, 1-8. [ Links ]
8 A.M. Abu-Dief and I.M.A. Mohamed, A review on versatile applications of transition metal complexes incorporating Schiff bases, J. Basic Appl. Sci., 2015,4, 119-133. [ Links ]
9 L.H. Abdel-Rahman, A.M. Abu-Dief, M.S.S. Adam and S.K. Hamdan, Some new nano-sized mononuclear Cu(II) Schiff base complexes: design, characterization, molecular modeling and catalyticpotentials in benzyl alcohol oxidation, Catal. Lett., 2016,146, 1373-1396. [ Links ]
10 C. Maxim, TD. Pasatoiu, V.C. Kravtsov, S. Shova, C.A. Muryn, R.E.P Winpenny, F. Tuna and M. Andruh, Copper(II) and zinc(II) complexes with Schiff-base ligands derived from salicylaldehyde and 3-methoxysalicylaldehyde: synthesis, crystal structures, magnetic andluminescence properties, Inorg. Chim. Acta., 2008,361,3903-3911. [ Links ]
11 N. Chantarasiri, T. Tuntulani, P. Tongraung, R. Seangprasertkit-Magee and W. Wannatong, New metal-containing epoxy polymers from diglycidyl ether of bisphenol A and tetradentate Schiff base metal complexes, Eur. Poly. J., 2000, 36, 695-702. [ Links ]
12 I.P. Oliveri, S. Failla, G. Malandrino and S. Di Bella, New molecular architectures by aggregation of tailored zinc(II) Schiff-base complexes, New J. Chem., 2011, 35, 2826-2831. [ Links ]
13 S.Gh. Musharraf, A. Bibi, N. Shahid, M. Najam-ul-Haq, M. Khan, M. Taha, U.R. Mughal and Kh. M. Khan, Acylhydrazide and isatin Schiff bases as alternate UV-laser desorption ionization (LDI) matrices for low molecular weight (LMW) peptides analysis, Am. J. Analyt. Chem., 2012, 3, 779-789. [ Links ]
14 P. Kathirgamanathan, S. Surendrakumar, J. Antipan-Lara, S. Ravic-handran, Y.F. Chan, V. Arkley, S. Ganeshamurugan, M. Kumara-verl, G. Paramswara, A. Partheepan, V.R. Reddy, D. Bailey and A.J. Blake, Novel lithium Schiff-base cluster complexes as electron injectors: synthesis, crystal structure, thin film characterisation and their performance in OLEDs, J. Mater. Chem., 2012, 22, 6104-6116. [ Links ]
15 A. Wachi, Y. Kudo, A. Kanesaka, H. Nishikawa, T. Shiga, H. Oshio, M.Chikamatsu and R. Azumi, Organic field-effect transistor based on paramagnetic Cu(II) neutral complexes coordinated by Schiff base-type TTF ligands, Polyhedron., 2017,136, 70-73. [ Links ]
16 V. Nishal, D. Singh, R.K. Saini, V Tanwar, S. Kadyan, R. Srivasta-vaand, and P.S. Kadyan, Characterization and luminescent properties of zinc-Schiff base complexes for organic white light emitting devices, Cogent Chem, 2015,1, 1079291. [ Links ]
17 N. Mahlooji, M. Behzad, H.A. Rudbari, G. Bruno and B. Ghanbari, Unique examples of copper(II)/sodium(I) and nickel(II)/sodium(I) Schiff base complexes with bridging bis-bidentate Salen type ligand: synthesis, crystal structures and antibacterial studies, Inorg. Chim. Acta., 2016,445, 124-128. [ Links ]
18 A.P. Sangamesh, T.P. Chetan, M.H. Bhimashankar, S.T. Shivakumar and S.B. Prema, DNA cleavage, antibacterial, antifungal and anthel-mintic studies of Co(II), Ni(II) and Cu(II) complexes of coumarin Schiff bases: synthesis and spectral approach. Spectrochim Acta A., 2015,137, 641-651. [ Links ]
19 M.A. Mokhles, A.L. Ammar, A.M. Hanan, A.M. Samia, M.A. Mamdouh and A.E. Ahmed, Synthesis, anticancer activity and molecular docking study of Schiff base complexes containing thiazole moiety, Beni-Seuf Univ. J. Appl. Sci., 2016, 5, 85-96. [ Links ]
20 M. Kumar, T. Padmini and K. Ponnuvel, Synthesis, characterization and antioxidant activities of Schiff bases are of cholesterol, J. Saudi. Chem. Soc, 2017, 21, S322-S328. [ Links ]
21 L. Jia, J. Xu, X. Zhao, Sh. Shen, T. Zhou, Z. Xu, T. Zhu, R. Chen, T. Ma, J. Xie, K. Dong and J. Huang, Synthesis, characterization, and antitumor activity of three ternary dinuclear copper(II) complexes with a reduced Schiff base ligand and diimine coligands in vitro and in vivo, J. Inorg. Biochem, 2016,159, 107-119. [ Links ]
22 J.F. Adediji, E.T. Olayinka, M.A. Adebayo and O. Babatunde, Antimalarial mixed ligand metal complexes: synthesis, physico-chemical and biological activities, Int. J. Phys. Sci., 2009, 4, 529-534. [ Links ]
23 E.L. Chang, C. Simmers and D.A. Knight, Cobalt complexes as antiviral and antibacterial agents, Pharmaceuticals, 2010, 3, 1711-1728. [ Links ]
24 I. Sheikhshoaie, Z. Ranjbar and H. Sohaleh, Sono chemical syntheses of a new nano-sized Zn(II)Schiff base complex as a precursor for the preparation of Zn(II) oxide nanoparticles by calcination process, Chem. Xpress.,2014, 3, 167-172. [ Links ]
25 A. Gusev, E. Braga, V. Shul'gin, K. Lyssenko, I. Eremenko, L. Samsonova, K. Degtyarenko, T. Kopylova and W. Linert, Luminescent properties of Zn and Mg complexes on N-(2-carboxy-phenyl)salicylidenimine basis, Materials, 2017,10, 897-906. [ Links ]
26 A.G. Awale, S.B. Gholse and P.S. Utale, Synthesis, characterization and antimicrobial activity of 2-amino-1,3-benzothiazole, Schiff bases and azo dyes of2-amino-1,3-benzothiazole. IOSR-JPBS, 2013,6,1-7. [ Links ]
27 M.B. Hallil, R.S. Malipatil and R.B. Sumathi, Preparation and characterization of Ni(II), Co(II), Cu(II), Zn(II), Cd(II) and Hg(II) complexes with Schiff base derived from benzofurane-2-carbohydrazide and p-chloroacetophenone, J. Chem. Pharm. Res., 2012,4, 62-66. [ Links ]
28 N.B. Ndosiri, M.O. Agwara, A.G. Paboudam, P.T. Ndifon, D.M. Yufanyi and C. Amah, Synthesis and characterization and antifungal activities of Mn(II), Co(II), Cu(II) and Zn(II) mixed-ligand complexes containing 1,10-phenanthroline and 2,2-bipyridine, Res. J. Pharm. Bio. Chem., 2013,4, 386-397. [ Links ]
29 K. Krishnankutty, M.B.Ummathur and P. Sayudevi, Metal complexes of Schiff bases derived from dicinnamoylmethane and aromatic amines, J. Argent. Chem. Soc., 2008, 96, 13-21. [ Links ]
30 T.P. Devi and R.K.H. Singh, Complexes of nickel(II) with the schiff bases derived from condensation of salicylaldehyde and bis-Ni (AMUH)2Cl2, Rasayan J. Chem., 2010, 3, 266-270. [ Links ]
31 N. Raman, S. Ravichandran and C. Thangaraja, Copper(II), cobalt(II), nickel(II) and zinc(II) complexes of Schiff base derived from benzil-2,4-dinitrophenylhydrazone with aniline. J. Chem. Sci., 2004, 116, 215-219. [ Links ]
32 P.S. Deshmukh, A.R. Yaul, J.N. Bhojane and A.S. Aswar, Synthesis, characterization and thermogravimetric studies of some metal complexes with N2O2 Schiff base ligand, World Appl. Sci. J., 2010, 9, 1301-1305. [ Links ]
33 J. Anacona, J. Rodriguez and J. Camus, Synthesis, characterization and antibacterial activity of a Schiff base derived from cephalexin and sulphathiazole and its transition metal complexes, Spectrochim Acta A.,2014,129, 96-102. [ Links ]
34 Z. Chohan, Antibacterial dimeric Copper(II) complexes with chro-mone-derived compounds, Trans. Met. Chem., 2009, 34, 153-161. [ Links ]
35 S.M. Methaq, Some transition metal complexes with new Schiff base ligand hexadentate, Acta Chim. Pharm. Indica, 2013, 3, 140-148. [ Links ]
36 K. Raj and T. Sheetal, Syntheses and biological screening of Schiff base complexes of Titanium(IV), Chem. Eng. Trans., 2013, 32, 18011806. [ Links ]
37 R. Radfard and A. Abedi, Synthesis and characterization of new Schiff bases of ethylenediamine and benzaldehyde derivatives, along with their iron complexes, J. App. Chem. Res., 2015, 9, 59-65. [ Links ]
38 L.H. Abdel-Rahman, R.M. El-Khatib, A.M. Abu-Dief, S.M. Abdel-Fatah and A.A. Sleem, Nano structure Iron(II) and Copper(II) Schiff base complexes of a NNO-tridentate ligand as new antibiotic agents: spectral, thermal behaviors and DNA binding ability, Int. J. Nano. Chem., 2016, 2, 83-91. [ Links ]
39 S.G. Yiase, S.O. Adejo, J.A. Gbertyo and J. Edeh, Synthesis, characterization and antimicrobial studies of salicylic acid complexes of some transition metals, IOSR J. Appl. Chem., 2014, 7, 4-10. [ Links ]
Received 3 August 2017
Revised 9 August 2018
Accepted 10 October 2018
* To whom correspondence should be addressed. E-mail: l.kafiahmadi@urmia.ac.ir
§ ORCID iD: L. Kafi-Ahmadi: orcid.org/0000-0001-8947-2706
Supplementary Data
The supplementary data is available in pdf: [Supplementary data]














