Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Chemistry
versão On-line ISSN 1996-840X
versão impressa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.71 Durban 2018
http://dx.doi.org/10.17159/0379-4350/2018/v71a4
RESEARCH ARTICLE
Anti-corrosion Properties of Areca Palm Leaf Extract on Aluminium in 0.5 M HCl Environment
Narasimha Raghavendra*; Jathi Ishwara Bhat
Department of Chemistry, Mangalore University, Mangalagangotri, Karnataka 574199, India
ABSTRACT
The aluminium corrosion inhibition behaviour in the presence of Areca palm leaves (AL) extract in hydrochloric acid (0.5 M) medium was examined by chemical (mass loss), AC impedance spectroscopy, potentiodynamic polarization and surface (scanning electron microscopy) methods. Aluminium protection rates increased with an increase in the amount of AL extract to the 0.5 M HCl system and decreased with increasing in the contact time of aluminium metal and temperature of 0.5 M HCl solution. Arrhenius equation was applied in the determination of activation energy values. The other activation parameters such as activation entropy and enthalpy values were obtained from the transition state plot. The adsorption of AL extract species on the Al surface in 0.5 M HCl solution follows the Langmuir adsorption mechanism. Tafel curves reflect the mixed (both anodic and cath-odic) inhibition behaviour of green inhibitor (AL extract species) on electrode surface (Al) in 0.5 M system. Impedance method indicates that the Al dissolution in hydrochloric acid environment was fully hindered by charge transfer process. The Al surface morphology was examined by applying scanning electron microscopy (SEM) and atomic force microscopy (AFM) techniques.
Keywords: Aluminium, Areca palm leaves, weight loss, Arrhenius equation, Langmuir adsorption.
1. Introduction
Hydrochloric acid solutions are widely utilized in oil well acidification, removal of scale and rust in metallurgy, acid pickling, de-scaling and cleaning of boilers in industrial processes. During this time, Al surface is greatly affected by corrosion.1-4 To hinder the effect of hydrochloric acid solution on the electrode surface, corrosion researchers added inhibitors to the hydrochloric acid solution during the several industrial processes. Application of inhibitors as corrosion inhibitor is the simplest and viable technique to protect the Al surface in a hydrochloric acid environment. Al metal treatment with synthesized compounds has been planned to enhance the anticorrosion action.
In practice, copious synthesized species are being applied for the prevention and control of Al disintegration in hostile fluid systems. These synthesized species strongly defending the Al surface from the acid solution by forming the adsorption film on the metal surface via active centres existed in the species. The inhibition capacity mainly depends on the nature of synthesized species, nature corrosive environment and nature of the metal.
Several classes of inhibitors are toxic in nature and noxious to the both environment and living creatures. Hence, these specific reasons strongly hinder their utility as corrosion inhibitors in several industrial processes. Because of increased environmental risks and awareness of health, many scientists (especially corrosion researchers) have paid more attention towards the effective non-toxic inhibitors.5-12
Green species (natural products) are cheaper compared to synthetic compounds. The electron-rich species molecules in the plants were extracted by simple methods. Previously, many researchers reported the green corrosion inhibition property of natural extracts on Al metal in hydrochloric acid environ-ment.13-8
At present, the majority of Areca palm waste products (including Areca leaves) is disposed by burning, which generally results in loss of valuable plant nutrients and potential source of organic matter. The Areca leaves are a good source of organic dung. The use of Areca waste products, especially Areca leaves (dried) in the field of corrosion science has not realized in the world. Areca leaves extract mainly contains electron rich species molecules such as lignin, cellulose, ursolic acid, 3/3 acetyl ursolic acid and hemicelluloses.19,20 There is no report on potential corrosion inhibition property of Areca leaf extract for any central metal in any corrosive systems. Therefore, in the present case, we selected Areca leaf extract for the protection of Al surface from hydrochloric acid solution. The experimental methods such as gravimetric (mass loss), potentiodynamic polarization (Tafel curves) and impedance techniques are used to explain the Al corrosion inhibition mechanism. Further, scanning electron microscopy (SEM) and atomic force microscopy (AFM) tool was employed for the Al surface study in uninhibited (without Areca leaf extract) and inhibited (with Areca leaves extract) conditions.
2. Experimental Section
2.1. Material Preparation
Table 1 represents the chemical composition of Al-type.21 The Al surface was polished with sandpaper in order to get smooth and rust free surface. Dust particles on the Al surface were removed by treating the Al surface with acetone. Finally, wiped aluminium metals are carefully stored.
2.2. Preparation of Green Inhibitor
1gL-1,2gL-1,4gL-1 and6gL-1 of inhibitor concentration were prepared by extracting the 180 g of Areca leaves powder with 350 mL of acetone in Soxhlet extraction chamber for 7 h.
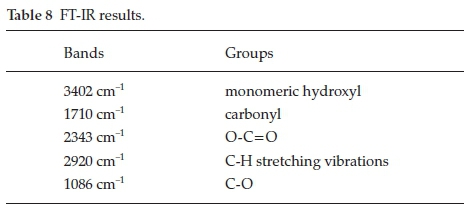
The presence of functional groups in the Areca leaf extract was examined by Fourier-transform infrared spectroscopy tool.
2.3. Weight Loss Measurements
The cleaned and pre-weighed Al surface was suspended in uninhibited and inhibited systems. The loss in the weight of Al metal with respect to different immersion time (1, 2, 3, 4, 5 and 10 h) and different solution (0.5 M HCl) temperatures (303, 308, 313, 318 and 323 K) were noted. The experiment was repeated three times in order to confirm the truth of the results until the concordant value was obtained. The Al corrosion rate in mild penetration per year was calculated by22,

where W=Alweight loss (mg), A = Al area (sq inch),T=Al immersion time (h) and D = Al density (g cm-3).
The Areca leaves extract protection efficiency (inhibition capacity) was calculated by using the following equation,
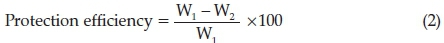
where W2= Al weight loss in protected state and W1 = Al weight loss in unprotected state.
The Al surface topography after treatment with 0.5 M HCl and 0.5 M HCl plus Areca leaf extract were examined through SEM and AFM technique.
2.4. Electrochemical Measurements
The electrochemical tests (CHI660C work station) were carried out by three electrode configurations (Al = working electrode, pt = auxiliary electrode and calomel = reference electrode). For potentiodynamic polarization technique, potential of ±200 mv was applied with scan rate 0.01 V/s. Impedance study was carried out with the frequency of the range in between 105 to 1 Hz using 0.01 V amplitude. The electrochemical experiment was repeated and concordant values were reported.
3. Results and Discussion
3.1. Weight Loss Technique
The loss of Al weight sample in 0.5 M HCl system with and without various Areca leaves extract concentrations at different immersion period and solution temperature was evaluated. Tables 2 and 3 represent the results of mass loss (gravimetric measurement) technique. The introduction of1gL1,2gL1, 4gL 1 and6gL1 of Areca leaves extract to the 0.5 M HCl solution greatly reduces the aluminium corrosion rate. This suggests that protection of Al metal occurs by adsorption of Areca leaves extract at active sites of Al surface. The minimum aluminium corrosion rate (maximum protection efficiency) was observed at 6gL 1 of Areca leaf extract. The number of species adsorbed on the Al surface was increased with increase in Areca leaf extract concentration. Hence, maximum protection was achieved at 6 g L-1 of Areca leaf extract.
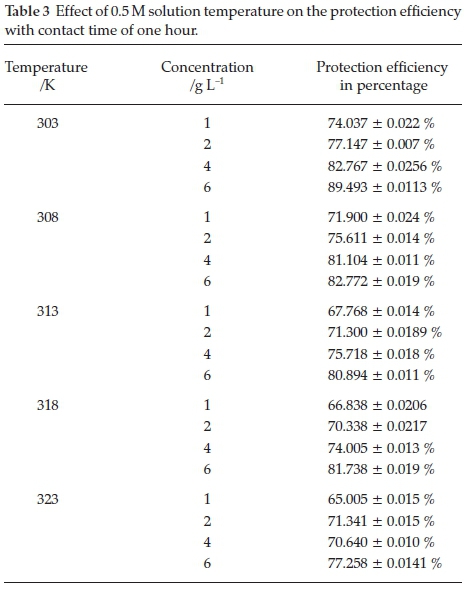
The increase in Al immersion time leads to increase in corrosion rate values (decrease in protection efficiency values), which is probably due to adsorbed Areca leaves extract species on the Al surface undergo degradation (desorption) and protective film loses its stability leaving the Al surface in unprotected condition. Hence, the greater Al surface is attacked by corrosive ions (hydrochloric acid). Therefore, high Al dissolution was observed with increasing the immersion time from one hour to ten hours. It is also observed that the Al corrosion rate in hydrochloric acid solution enhanced with an increase in temperature; this may be due to increase in desorption rate and decreased desorption rate of Areca leaves extract species on the surface of metal. The desorption process weakens the Al-Areca leaves extract interaction. As a result, protection efficiency decreases with increases in solution temperature.23
Activation energy (Ea) values can be identified from the Arrhenius plots of aluminium corrosion rate (Fig. 1). Transition state plot (Fig. 2) was used to determine the activation enthalpy (ΔΗ*) and activation entropy (AS*) values. The obtained activation parameters are placed in the Table 4.
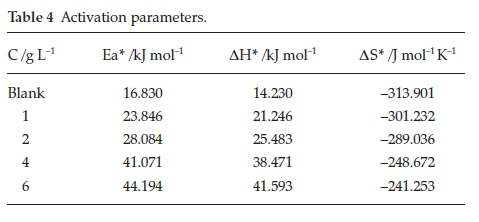
The greater activation energy values in the protected system as opposed to unprotected system recommended that adsorbed inhibitor species on the Al surface leads to increase the energy barrier of electrode (Al) dissolution, resulting in the reduction of Al dissolution in 0.5 M HCl. Hence, Al surface was protected by Areca leaf extract species. The obtained positive AH* values reflected the endothermic Al disintegration process in 0.5 M hydrochloric acid system.
The direction of AS* values directed towards the positive side is an indication of increase of disordeness of the system.
The basic thermodynamic information, knowledge between the Areca leaves extract and the Al surface can be studied by adsorption models. Surface coverage (0) values obtained from the gravimetric method were used to study the different adsorption model. In the present investigation, the surface coverage and inhibitor concentration values are fitted well to Langmuir adsorption model (Fig. 3). The various thermodynamic parameters obtained from this plot are shown in Table 5.
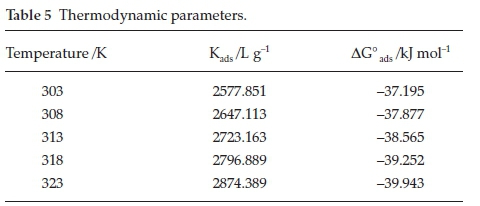
The equilibrium constant of the inhibitor adsorption (Kads) values increase with increasing solution temperature from 303 K to 323 K. The higher Kads values clearly proved the strong adsorption of Areca leaves extract molecules on Al surface. The introduction of Areca leaves extracts to the 0.5 M HCl solution caused -ve free energy of adsorption (ΔG°ads) values, which certified that chemical constituents of Areca leaves adsorb spontaneously on the Al surface in a hydrochloric acid environment. The ΔG°ads values obtained with the system were close to -40 kJ/mol, indicating that adsorption of Areca leaves extract species on the surface of the Al is more chemical phenomena than the physical phenomena.
3.2. Electrochemical Measurements
3.2.1. Potentiodynamic Polarization (Tafel Plot)
Figure 4 shows the Tafel curves24,25 (cathodic and anodic) for unprotected and protected system after the stabilization period in the 0.5 M HCl condition. The anodic (βa) and cathodic (βc) Tafel constants, corrosion current density (icorr) and corrosion potential (Ecorr) values are obtained Tafel plots. The ability of an Areca leaf extract toward Al corrosion inhibition (protection efficiency) was evaluated by following relation,

where i'corr = Al corrosion current density value in 0.5 M HCl plus inhibitor condition and icorr = Al corrosion current density value in bare condition.
All above data are presented in Table 6. The shape of potentiodynamic polarization curves (Tafel curves) is same in both unprotected and protected systems, but the plots are moves towards lower icorr values in the protected system (with the addition of different concentrations of Areca leaf extract), which confirming that the species of Areca leaves extract retard the Al corrosion rate by adsorbing on electrode surfaces. Hence, protection efficiency increases with increase in Areca leaves concentration. The molecules of Areca leaves extract causes marginal changes in the corrosion potential, anodic and cath-odic Tafel constant values with respect bare solution, implying that Areca leaves extract inhibit the both anodic and cathodic reactions (mixed inhibition property) of Al in 0.5 M HCl system through the adsorption mechanism.
3.2.2. Impedance Spectroscopy Technique
The Nyquist curves26,27 for Al obtained without and with Areca leave extract concentrations in 0.5 M HCl solution at laboratory temperature are represented in Fig. 5. The obtained plots are not semicircle due to roughness and non-homogeneity of Al surface. The charge transfer resistance (Rct), surface heterogeneity factor (n), frequency (fmax), chi-square (χ2) values and double layer capacitance (Cdl) values are obtained from Nyquist plot and are presented in Table 7.
From the charge transfer resistance values, the corrosion inhibition efficiency was calculated according to the following equation,

where, Rct = value of charge transfer resistance in inhibited free system, and Rct(inh) = value of charge transfer resistance in inhibiting system.
Quality of the fit was determined based on chi-square (χ2) values. In present study, the obtained chi-square (χ2) values are in between the range 0.001412-0.033260, which clearly indicates the proposed circuit is the best fit.
The data presented in Table 7 show that the value of Rct in 1gL-1,2gL-1,4gL-1 and6gL-1 of Areca leaves extract is high compared to bare (0.5 M) solution and maximum value is obtained at6gL-1 of Areca leaves extract is attributed to the existence of insulating adsorbed film at electrode (aluminium)-electrolyte (0.5 M hydrochloric acid) solution interface. Stability of insulating protective layer increases with improving the inhibitor concentration. Hence, maximum Al protection was successfully achieved at highest Areca leaf extract concentration. The decrease in Cdl value leads to enhance in double layer thickness, which shows that Areca leaves extract species retards the Al dissolution by adsorption at the Al/0.5 M HCl solution interface. The variation in the Cdl values confirms the substitution of H2O molecules by Areca leaf extract species. The obtained n values range 1-0.8975 showing that inhibition of Al corrosion in 0.5 M HCl solution was occurring through charge transfer phenomena.
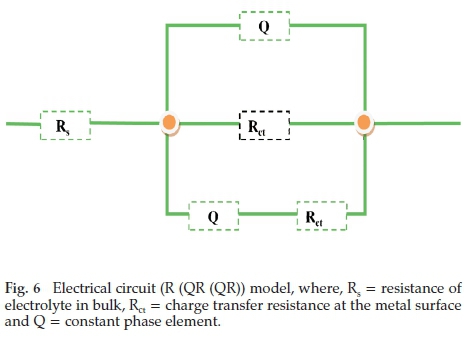
3.3. FT-IR Spectroscopy
Functional groups in organic molecules play very important role in the metal corrosion inhibition process. FT-IR spectros-copy was used in order to investigate the functional groups present in the Areca leaves extract (Fig. 7). The obtained results are shown in Table 8.
3.4. Surface Study
3.4.1. Scanning Electron Microscopy (SEM) Analysis
Figure 8a,b shows the SEM images of Al after 2 hours immersion in 0.5 M HCl environment without and with Areca leaves extract condition, respectively. With no inhibitor (without Areca leaf extract), the Al surface was highly damaged due to direct attack by corrosive ion metal surface resulting in the large of cracks in the Al surface. With inhibitor (Areca leaves extract), the Al surface was significantly improved with smooth surface, indicating the reduction of the Al corrosion rate.
3.4.2. Atomic Force Microscopy (AFM) Technique
It is observed that the reduction in the value of average roughness (Sa) and root mean square roughness (Sq) in inhibited system (0.5 M HCl + plant extract species) compared to bare system is an indication of lesser aluminium corrosion rate in inhibited state. Hence, the atomic force microscopy results (Fig. 9 a, b and Table 9) are fully favourable with the results of SEM studies.
4. Conclusions
Areca leaves extract acts as potential corrosion inhibitor for aluminium in a 0.5 M HCl environment. Areca leaves extract has potential efficiency of 89.493 % obtained by gravimetric measurements, 88.114 % by potentiodynamic polarization test and 97.035 % by impedance spectroscopy technique. The positive ΔH* values strongly reflect the endothermic Al dissolution process. The obtained negative ΔG°ads values indicate the spontaneous adsorption process of Areca leaves extract on the Al surface in 0.5 M HCl system. The SEM and AFM topography greatly favours the results of weight loss, potentiodynamic polarization and impedance studies.
Acknowledgement
The authors thankful to B.E. Kumaraswamy, Kuvempu University, for the CHI660C facility.
Conflicts of Interest
The authors declare no conflict of interest.
References
1 M.M. Fares, A.K. Maayta and M.M. Al-Qudah, Pectin as promising green corrosion inhibitor of aluminum in hydrochloric acid solution, Corros. Sci., 2012, 60, 112-117. [ Links ]
2 A. El-Aziz, E.-S. Fouda, H. Abu-El-Nader and M. Nor El-Din Moussa and I. Shehata, Efficiency of azodyes in retarding the dissolution of aluminium in hydrochloric acid, Bull. Chem. Soc. Jpn., 1988, 61, 4411-416. [ Links ]
3 A. Khadraoui, A. Khelifa, K. Hachama and R. Mehdaoui, Thymus algeriensis extract as a new eco-friendly corrosion inhibitor for 2024 aluminium alloy in 1 M HCl medium, J. Mol. Liquids, 2016, 214, 293-297. [ Links ]
4 K. Olusegun Abiola and Y. Tobunb, Cocos nucifera L. water as green corrosion inhibitor for acid corrosion of aluminium in HCl solution, Chinese Chem. Lett, 2010, 21, 1449-1452. [ Links ]
5 M. Abdallah, Antibacterial drugs as corrosion inhibitors for corrosion of aluminium in hydrochloric solution, Corros. Sci., 2004, 46, 1981-1996. [ Links ]
6 H. Ashassi-Sorkhabi, B. Shabani, B. Aligholipour and D. Seifzadeh, The effect of some Schiff bases on the corrosion of aluminum in hydrochloric acid solution, Appl. Surf. Sci, 2006, 252,4039-047. [ Links ]
7 S. Deng and X. Li, Inhibition by Jasminum nudiflorum Lindl. leaves extract of the corrosion of aluminium in HCl solution, Corros. Sci., 2012, 64, 253-262. [ Links ]
8 R. Xiao, K. Yan, J. Yan and J. Wang, Electrochemical etching model in aluminum foil for capacitor, Corros. Sci., 2008, 50, 1576-1583. [ Links ]
9 A.S. Fouda, A.A. Al-Sarawy, F.S. Ahmed and H.M. El-Abbasy, Corrosion inhibition of aluminum 6063 using some pharmaceutical compounds, Corros. Sci, 2009, 51, 485-92. [ Links ]
10 O.K. Abiola, J.O.E. Otaigbe and O.J. Kio, Gossipium hirsutum L. extracts as green corrosion inhibitor for aluminum in NaOH solution, Corros. Sci., 2009, 51, 1879-1881. [ Links ]
11 X. Li, S. Deng and X. Xie, Experimental and theoretical study on corrosion inhibition of oxime compounds for aluminium in HCl solution, Corros. Sci., 2014, 81, 162-175. [ Links ]
12 X. Li and S. Deng, Inhibition effect of Dendrocalamus brandisii leaves extract on aluminum in HCl, H3PO4 solutions, Corros. Sci., 2012, 65, 299-308. [ Links ]
13 L. Valek and S. Martinez, Copper corrosion inhibition by Azadirachta indica leaves extract in 0.5 M sulphuric acid, Mater. Lett., 2007, 61, 148-151. [ Links ]
14 H. Hussin M., A. Abdul Rahim, M. Nasir, M. Ibrahim and N. Brosse, The capability of ultrafiltrated alkaline and organosolv oil palm (Elaeis guineensis) fronds lignin as green corrosion inhibitor for mild steel in 0.5 M HCl solution, Measurement, 2016, 78, 90-103. [ Links ]
15 A.M. Abdel-Gaber, B.A. Abd-El-Nabey, I.M. Sidahmed, A.M. El-Zayady and M. Saadawy, Inhibitive action of some plant extracts on the corrosion of steel in acidic media, Corros. Sci., 2006, 48, 2765-2779. [ Links ]
16 O.K.Abiola and J.O.E. Otaigbe, The effects of Phyllanthus amarus extract on corrosion and kinetics of corrosion process of aluminum in alkaline solution, Corros. Sci., 2009, 51, 2790-2793. [ Links ]
17 E.E. Oguzie, Corrosion inhibition of aluminium in acidic and alkaline mediaby Sansevieria trifasciata extract, Corros. Sci., 2007,49,1527-1539. [ Links ]
18 A.Y. El-Etre, Inhibition of aluminum corrosion using Opuntia extract, Corros. Sci., 2003, 45, 2485-2495. [ Links ]
19 R. Nagaraja, B.R. Gurumurthy and M.B. Shivanna, Bio softening of arecanut waste areca husk, leaf and leaf sheath for value added compost, Int. J. Res. Appl. Nat. Soc. Sci, 2014, 2, 105-112. [ Links ]
20 W. Peng, Y.-J. Liu, N. Wu, T. Sun, X.-Y. He, Y.-X. Gao and C.-J. Wu, Areca catechu L. (Arecaceae): a review of its traditional uses, botany, phytochemistry, pharmacology and toxicology, J. Ethnopharmacol., 2015, 164, 340-356. [ Links ]
21 A. Nayar, The Metals Databook, Tata McGraw-Hill Publishing Ltd, New Delhi, ISBN 10:0070460884, 1997. [ Links ]
22 H.M. Tawancy, A. Ul-Hamid and N.M. Abbas, Practical Engineering Failure Analysis, CRC Press, Boca Raton, p. 352, 2004. [ Links ]
23 A.S. Fouda, K. Shalabi and A. E-Hossiany, Moxifloxacin antibiotic as green corrosion inhibitor for carbon steel in 1 M HCl, J. Bio. Tribo. Corros., 2016, 2, 8. [ Links ]
24 K.M. Shainy, P. Rugmini, Ammal and K.N. Unni, S. Benjamin and A. Joseph, Surface interaction and corrosion inhibition of mild steel in hydrochloric acid usingpyoverdine, an eco-friendly bio-molecule, J. Bio. Tribo. Corros, 2016, 2, 20. [ Links ]
25 D.C. Silverman and J.E. Carrico, Electrochemical impedance technique - A practical tool for corrosion prediction, Corrosion, 1988, 44, 280-287. [ Links ]
26 M. Tezeghdenti, L. Dhouibi and N. Etteyeb, Corrosion inhibition of carbon steel in 1 M sulphuric acid solution by extract of eucalyptus globulus leaves cultivated in Tunisia arid zones, J. Bio. Tribo. Corros., 2015, 1, 16. [ Links ]
27 N. Raghavendra and J. Ishwara Bhat, Natural products for material protection: an interesting and efficacious anticorrosive property of dry arecanut seed extract at electrode (aluminum)-electrolyte (hydrochloric acid) interface, J. Bio. Tribo. Corros., 2016, 2, 21. [ Links ]
Received 25 February 2017
Rrevised 16 November 2017
Accepted 1 February 2018
* To whom correspondence should be addressed E-mail: raghu.kyasambally@gmail.com
Supplementary Data
The supplementary data is available in pdf: [Supplementary data]





![Synthesis and characterization of new bis-symmetrical adipoyl, terepthaloyl, chiral diimido-di-L-alanine diesters and chiral phthaloyl-L-alanine ester of tripropoxy p-tert-butyl calix[4]arene and study of their hosting ability for alanine and Na+](/img/pt/next.gif)








