Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Chemistry
versión On-line ISSN 1996-840X
versión impresa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.71 Durban 2018
http://dx.doi.org/10.17159/0379-4350/2018/v71a2
RESEARCH ARTICLE
Performance of Mn2+ modified Bentonite Clay for the Removal of Fluoride from Aqueous Solution
Rabelani MudzielwanaI, §; Mugera W. GitariI, *; Segun A. AkinyemiI; Titus A.M. MsagatiII
IEnvironmental Remediation and Water Pollution Chemistry Research Group, Department of Ecology and Resources Management, School of Environmental Sciences, University of Venda, Private bag X5050, Thohoyandou, 0950, Limpopo, South Africa
IIUniversity of South Africa, College of Science Engineering and Technology, The Science Campus, Florida Park, Roodepoort, Private Bag X6, Florida, 1710, Johannesburg, South Africa
ABSTRACT
A low-cost adsorbent produced from Mn2+-modified bentonite clay was evaluated for groundwater defluoridation. Batch experiments were used to evaluate the effect of contact time at various adsorbent dosages, adsorption isotherms and the effect of pH on fluoride removal. The results showed that the optimum F- uptake occurred within the first 30 min contact time and the percentage removal increased with increasing adsorbent dosage. The data fitted better to pseudo-second-order reaction indicating that Fadsorption occurred via chemisorption. The Weber-Morris model of intra-particle diffusion revealed that both surface and intra-particle diffusion processes were involved during the F- adsorption process. Furthermore, the batch results showed that pH of the solution governed the percentage of fluoride removal with the optimum of 75.2 % fluoride removal achieved at pH 4. The adsorbent chemical stability assessment showed that chemical species were leached at trace concentrations which are within the South African National Standards (SANS) limits. Electrostatic attraction and ion-exchange were established as the major mechanisms responsible for fluoride adsorption at acidic pH and at moderate to alkaline pH levels, respectively. The study demonstrated that Mn2+ intercalated bentonite clay has potential for application in defluoridation of groundwater.
Keywords: Adsorption, defluoridation, ion exchange, ligand exchange, intra-particle diffusion.
1. Introduction
The presence of fluoride in water has both beneficial and detrimental implications on human health depending on concentrations. At concentrations below 1 mg L-1 fluoride helps in prevention of dental caries and development of bones particularly for children below the age of 10.1,2 Conversely, at concentrations above the World Health Organization (WHO) permissible limit of 1.5 mg L-1 fluoride is linked to dental and skeletal fluorosis.3 Fluorosis is an incurable disease and it is now regarded as a global health problem with countries such as India, China, Mexico, Pakistan, Ethiopia and Egypt largely affected.4,5
Defluoridation is the viable option for areas that have ground-water containing fluoride concentration greater than World Health Organization standard of 1.5 mg L-1.3 Several authors have identified adsorption as suitable method for defluoridation in rural areas because it uses materials that are available at little or no cost and it is easy to operate.6,7 Furthermore, adsorption has proved to be the sustainable method since it uses materials that can be regenerated. Some authors have also identified number of promising adsorbent including acid-activated kaolinite clay soils,8 La3+-modified bentonite,9 organosmectite,10 smectite-rich clay soils,11 Al/Fe oxide-coated diatomaceous earth,12 mixed Mukondeni clay soils,13 and MnO2-coated bentonite clay.14
Clay minerals are promising materials for defluoridation of groundwater due to their abundance and cost-effectiveness. Nonetheless, clays have good adsorptive properties such as: larger chemically active surface area, higher cation exchange capacity as well as chemical and mechanical stability. Bentonite clay belongs to the class of alumino-silicates. It has a permanent negative charge caused by the isomorphous substitution of Al3+ for Si4+ in the tetrahedral layer and Mg2+ for Al3+ in the octahedral layer.15 The negatively charged surface allows bentonite to weakly adsorb anionic pollutants. This is due to repulsion forces between the anions and the permanent negative charge on the edge of the bentonite sheet. The clay can be modified to improve its binding capacity for the anions. Bentonite clay modified with Mg2+,Fe3+ and Al3+ have been observed to provide improved fluoride adsorption capacity.16-18 Mn2+ is a high charge density polycation that is stable at extreme pH levels and its use in de-fluoridation has not been exploited. This study aimed at evaluating the applicability of Mn2+-modified bentonite clay in defluoridation of groundwater. Physicochemical properties of the raw and modified bentonite clay were also evaluated. The adsorption data were modelled through the pseudo-reaction kinetics models, intra-particle diffusion model and Langmuir adsorption isotherm model. The chemical stability of the adsorbent was also evaluated.
2. Experimental
2.1. Sample Preparation
Raw bentonite clay with a chemical composition of: SiO2 (65.2 %), Al2O3 (15.30 %), CaO (1.50 %), Fe2O3 (3.41 %), K2O (0.98 %), MgO (3.20 %), MnO (0.1 %) and Na2O (2.12 %) was collected from ECCA Pty. (Ltd.) in Cape Town, South Africa. All reagents and Total Ionic Solution Buffer (TISAB-III) were purchased from Rochelle Chemicals & Lab. Equipment CC, South Africa Ltd. and they were of analytical grade. A stock solution of 1000 mg L-1 fluoride was prepared by dissolving 2.21 g NaF inlLof Milli-Q water (18.2 Ω cm-1) while Mn2+ stock solution containing 1000 mg L-1 was prepared by dissolving 2.29 g MnLl2 in 1 L. Working solutions for batch experiments were prepared by appropriate dilutions.
2.2. Synthesis of Mn2+ Bentonite Clay
Raw bentonite clay was soaked in Milli-Q water at a ratio of 1:5 in a 1000 mL beaker, the mixture was stirred for 5 min and the procedure was repeated twice. Samples were then dried in an oven for 12 h at 110 °L and then milled to pass through <250 μηη sieve. To synthesize Mn2+-modified bentonite, 200 mL of 50 mg L-1 Mn2+ solution was mixed with8gofraw bentonite in 1 L Erlenmeyer flask, the pH of the mixture was then adjusted to 8 using 0.1 M NaOH and 0.1 M HCl. The mixture was agitated for 60 min at 250 rpm on a Stuart reciprocating table shaker (SSL2, UK) and filtered through 0.45 μπι filter membrane. The solid residues were dried for 12 h. at 110 °L in the oven. The modified clay was then milled to pass through <250 μΓΐι sieve. The experiments were repeated five times to generate enough Mn2+-modi-fied bentonite for subsequent experiments.
2.3. Physicochemical and Mineralogical Characterization of Mn2+-modified Bentonite
The mineralogical composition of Mn2+ bentonite clay was examined using D8 advance X-ray diffractometer (XRD) (Bruker, Germany) and Lu-Ka radiation source. The pore size distribution, pore volume, and pore diameter were determined by Barrett Joyner Halenda (BJH) sorption model using a specific surface area analyzer (Autosorb-iQ & Quadrasorb SI, USA). Nitrogen adsorption-desorption isotherms were used to determine specific surface area of the adsorbent according to Brunauer Emmett Teller (BET) model. Cation exchange capacity was determined using ammonium acetate buffer at pH 5.4 and 7.4 as described by Gitari et al.17The surface charge and point of zero charge was determined using solid addition method as described by Kumar et al.19
2.4. Batch Defluoridation Experiments
Batch experiments were conducted to examine the effect of contact time at various adsorbent dosages, the effect of pH and leaching of chemical species from the adsorbent during adsorption of F-. The effect of contact time was evaluated by varying contact time from 5 to 270 min at 0.1,0.3 and 0.5 g 100 mL-1 adsorbent dosage. To evaluate the adsorption isotherms at various contact times, initial concentration was varied from 3 to 25 mg L-1,1 g 100 mg-1 adsorbent dosage was used. To evaluate the effect of pH, initial pH of the F- solution was adjusted from 4 to 12 using 0.1 M NaOH and 0.1 M HCl. The metal leaching and chemical stability assessment of the adsorbent was conducted by analyzing concentration of metals in the filtrates obtained from effect of pH experiment using Inductively Coupled Plasma-Mass Spectrometer (Agilent 7900ICP-MS, US). All mixtures were agitated for 30 min except for the effect of contact time. After agitation samples were filtered through 0.45 μΓη pore membrane. The residual fluoride concentration in the treated samples was measured using an ion selective electrode 9609 BNWP Orion (USA) attached to Thermo Scientific Orion Star ISE/pH/EC meter. Similar meter was used to measure the pH. For residual fluoride, ion selective electrode was calibrated with four standards containing 1 mL of TISAB III per 10 mL of solution. The same ratio was maintained for the sample. All experiments were conducted in triplicate for better accuracy and the mean values were reported. Equations 1 and 2 were used to calculate the percentage removal and adsorption capacity, respectively.13
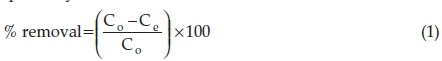
where Q =adsorption capacity (mg g-1), Co = initial F- concentration (mg L-1), Ce = F- concentrations at equilibrium (mg L-1), V = volume of solution (L) and m = mass of the adsorbent (g).

3. Results and Discussion
3.1. Physicochemical and Mineralogical Characterization
3.1.1. Mineralogical Composition
Figure 1 present the XRD spectra of raw and Mn2+-modified bentonite clay. No significant change was observed in the XRD spectra of the bentonite after modification. However, a slight decrease in the montmorillonite peak was observed. Quantitative XRD analysis results indicated montmorillonite (i.e. 70.24 %) and quartz (i.e. 21.24 %) as the major minerals. Nevertheless, muscovite (i.e. 2.0 %) which belongs to the mica group of mineral occurred in minor quantities in the bentonite clay. However, after modification, the quantities of montmorillonite and quartz decreased from 70.24 to 66.34 % and 21.24 to 17.32 %, respectively. While muscovite increased from 2.0 to 4.75 %. The decrease could be attributed to the exchange of Mg2+,Na+,K+ and Ca2+ cations for Mn2+ ions during modification.
3.1.2. Cation Exchange Capacity (CEC)
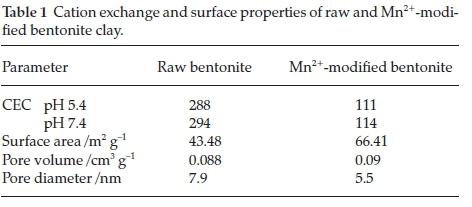
The results of CEC are presented in Table 1. Cation exchange capacity for raw bentonite was found to be higher than the CEC of Mn2+-modified bentonite. pH of the buffering media does not seem to have significant influence on the exchangeable cation concentration and CEC. Similar results on CEC have been reported by Gitari et al.17
3.1.3. Determination of pHpzc
Figure 2 presents the pHpzc of raw bentonite (a) and Mn2+-modified bentonite (b). The pHpzc values for raw benton-ite and Mn2+ bentonite were found to be 8.4 and 8.8, respectively. At pH above the pHpzc the clay surface is negatively charged while at pH below pHpzc the clay surface is positively charged. Both raw and Mn2+-modified bentonite clay have high pHpzc indicative of clay dominated by alumino-silicate materials and manganese oxides. Modification of the raw bentonite clay with Mn2+ increases the pHpzc and which extends the pH range for adsorption of anions. This trend indicated that the Mn2+-modi-fied bentonite clay will have high adsorption capacity for anions than the raw bentonite clay. An increase in pHpzc of bentonite clay after modification has also been reported for Al3+-modified bentonite by Masindi et al.16
3.1.4. Surface Properties Analysis
Surface properties of raw bentonite and Mn2+-modified ben-tonite are presented in Table 1. The results showed an increase in total surface area of the bentonite after modification from 43.48 to 66.41 m2 g-1. Furthermore, pore volume decreased to 0.09 cm3 g-1 (Table 1). This could be attributed to swelling or hydration of the bentonite clay during intercalation of Mn2+ ions. The average pore diameter of raw bentonite is relatively higher when compared to that of Mn2+-modified bentonite clay (Table 1). This suggests that Mn2+ ions were incorporated onto the surface of the raw clay, including adsorption on the edges. Figure 3 depicts pore distribution curve of raw and Mn2+-modi-fied bentonite. It is observed that majority of the pores lies within 2 nm and 50 nm indicating that both raw and Mn2+-modi-fied bentonite are mesoporous in nature.
3.2. Batch Experiments
3.2.1. Effect of Contact Time and Adsorption Kinetics
Figure 4 depicts the relationship between contact time and percentage F- removal. It is evident that F- removal was rapid during the first 30 min of the experiment where optimum uptake of F- was achieved. Thereafter, slight change in the percentage F-removal was observed which indicate that the adsorbent was getting saturated and system has reached equilibrium. The increase in F- removal is attributed to the availability of sorption sites in the adsorbent. The similar trend was observed at both adsorbent dosages and the percentage F- removal was increasing with adsorbent dosage. Contact time of 30 min was taken as the optimum time and was applied in subsequent experiments.
Pseudo-first and second-order reaction based models were employed to determine the rate limiting steps of fluoride adsorption onto Mn2+-modified bentonite as well as the potential rate controlling steps. The pseudo first order is shown in the Equation 3 and it is used to describe liquid-solid phase adsorption systems, and it is the earliest known kinetic model describing the adsorption rate based on the adsorption capacity.20
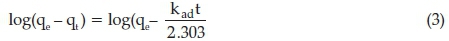
Pseudo second order is shown in the linear Equation 4 and it is used to describe chemisorption, as well as cation exchange reactions.14

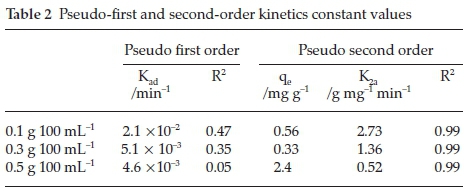
where qe and qt (both in mg g-1) are the amount adsorbed per unit mass at a time, t (in min). Kad and K2ads are first- and second-order rate constant (g mg-1 min-1). The value of Kad is determined from the slope and intercepts of log (qe -qt) vs. t (min) and the value of K2ads is determined from the slope and intercepts of t/qt vs. qe Adsorption of fluoride ion onto Mn2+ bentonite did not follow the pseudo-first-order process since it did not yield a straight line (figure no presented). Figure 5 shows the plot of t/q with time which indicate high correlation coefficient at both adsorbent doses. This implies that fluoride sorption onto Mn2+-modified bentonite clay followed pseudo second order and is chemisorption. The constant values of pseudo first and second order are presented in Table 2.
During the process of fluoride adsorption, fluoride ions also diffuses into the interior of the adsorbent. Intra-particle diffusion model based on the hypothesis proposed by Weber Morris was tested to identify the diffusion mechanism.21 The linear equation of the intra-particle diffusion is given by the Equation 5 below.

where qt is the amount adsorbed (mg g-1) at a given time t (min), K (mg g-1 min-1) is the intra particle diffusion rate constant and it is determined from the slope of t0.5vs. qt.
This indicates that if the plot of qt against t0.5 gives straight line passing through the origin, then the adsorption is solely controlled by intra-particle diffusion, while a bilinear plot indicates that adsorption occurred via film and intra-particle diffusion mechanism.1
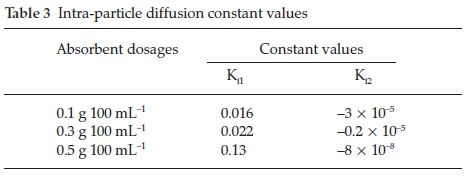
Figure 6 shows the plot of intra-particle diffusion model. Adsorption of fluoride onto Mn2+ bentonite showed bilinear plot with the initial plot (phase 1) indicating external diffusion and the second plot (Phase 2) indicates intra-particle diffusion. The intra-particle rate constants Ki1 and Ki2 obtained from the plot are shown in Table 3. At both adsorbent dosages, Ki1 value is greater than the Ki2 value, indicating that the initial step is faster than the final step where equilibrium was established. Furthermore, the Ki1 value increases with increasing adsorbent dosage. This could be attributed to difference in the rate of mass transfer in the initial and final stages of adsorption. This observed trend is connected to a mechanism consisting of an external mass transfer followed by diffusion into micro- and mesoporous surfaces. In conclusion, fluoride adsorption onto Mn2+-modified benton-ite is a complex process involving external and internal diffusion of Fions.
3.2.2. Adsorption Isotherms
Figure 7 shows the effect of adsorbate concentration on the percentage fluoride removal at various contact times. It is observed that the percentage F- removal decreased with an increase in the initial concentration. Similar trend was observed at all the contact times. This could be due to availability of more fluoride ions in the solution at higher adsorbate concentration. Besides, it could also indicate that fluoride binding sites of the adsorbent was getting exhausted.
Langmuir isotherm was used to describe the data (Langmuir; 1997). It is applicable to homogeneous adsorption where adsorption process has equal activation energy. It is assumed that the adsorbent surface is uniform, i.e. all the adsorption sites are equivalent and adsorption molecules do not interact (Zhang et al. 2011; Gosh et al. 2015). The linear equation for Langmuir isotherm model is expressed in the Equation 6:

where, Ce is the equilibrium concentration (mg L-1). Qe is the adsorption capacity (mg L-1). Qm is theoretical maximum adsorption capacity (mg g-1). b is the Langmuir constant related to enthalpy of adsorption (L mg-1). Qm and b are determined from the slope and intercept of the plot of 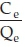 . Ce. Calculated
. Ce. Calculated
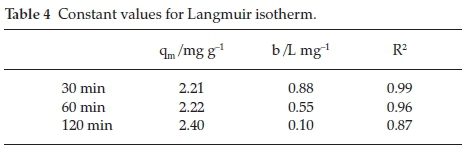
Langmuir constants are presented in Table 4 and the Langmuir plots are shown in Fig. 8.
Furthermore, to reveal the feasibility of Langmuir adsorption isotherm, the dimensionless parameter of the equilibrium or adsorption intensity (RL) was used for further analysis of Langmuir equation. The values of RL were calculated using Equation 7:

where Cois the initial concentration (mg L-1)andb is the Langmuir constant (L mg-1).
The value of RL less than 1 generally indicate favourable adsorption while greater than 1 indicate unfavourable adsorption. Figure 9 shows calculated RL values which ranged between 0 and 1 indicating adsorption process was favourable at room temperature for all the adsorbate concentrations tested.
3.2.3. Effect of pH and Fluoride Removal Mechanism
Figure 10 shows the effect of initial pH on percentage fluoride removal. The removal of F- removal decreases from 75.2 to 24.33 % at pH 4 and pH 6, respectively. Thereafter, the F- removal increased to 30 % at pH 10 and this was followed by significant decrease at pH greater than 10. This observed trend could be due to the abundance of OH- ions in alkaline pH that compete with F- for adsorption sites. The equilibrium pH at initial pH of 4 was found to be 8.96 which is suitable for human consumption. The pHpzc of the Mn2+-modified bentonite was found to be at pH 8.8. Therefore, at pH <8.8 the surface of the clay will be positively charged (Equation 8) and at pH > 8.8 the surface of the adsorbent will be negatively charged. At pH less than the pHpzc, fluoride was removed through electrostatic attraction mechanism (Equations 9 & 10), whereas at moderate pH, fluoride adsorption occurs through ion exchange (Equation 11). At pH higher than the pHpzc where the surface of the clay is negatively charged fluoride adsorption also occurs through ion exchange mechanism (Equation 12).
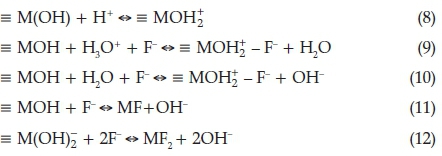
where= M represent metal in the adsorbent surface (Mn, Si and Al)
3.3. Leaching Assessment of the Mn2+-modified Bentonite
Concentrations of chemical species leached are shown in Fig. 11a,b. From the results, it is observed that the concentration of Na+ was very high at both pH levels. This could be attributed to the use of NaOH for pH adjustment. High Si4+ and Al3+ concentration was observed at higher pH levels. This observed
trend could have been influenced by chemical dissolution of silica at higher pH. Metals such as Mn2+,Fe2+,Al3+ and Cu2+ were released at minor concentrations and were found to be within the SANS permissible limits.20 The final F- concentration in the solution at pH 4,6,8,10 and 12 were found to be 1.26,2.27,2.19, 2.12 and 3.95 mg L-1, respectively. Table 5 shows the comparison between concentration of metal species in treated water and the SANS-241 permissible limits. Therefore, based on the obtained data, Mn2+-modified bentonite is a promising material for defluoridation of groundwater since it does not give rise to secondary pollutants in the treated water.
3.4. Comparison with Other Adsorbent
Table 6 present the comparison of the adsorption capacities between Mn2+-modified bentonite and other poly-cation-modified bentonite reported in the literature. Mn2+-modified bentonite reported in this study showed higher adsorption capacity compared to Mg2+ and La2+-modified bentonite. Unlike Fe3+ and Al3+-modified bentonite clays which exhibited highest adsorption capacity at pH 2, Mn2+ bentonite exhibited its maximum adsorption capacity at initial pH 4.
4. Conclusions
The physicochemical properties and fluoride removal efficiency of Mn2+-modified bentonite were successfully evaluated.
The mineralogical characterization results by XRD showed that modification of bentonite clay by Mn2+ polycations does not alter the parent mineralogy of the clay. However, it decreases the content of the major minerals. The modified bentonite showed higher surface area and pHpzc compared to the raw bentonite clay. Conversely, the CEC decreased after modification by Mn2+. A maximum of 75.2 % F- removal was achieved from the initial 5mgL-1 using 1 g 100 mL-1 adsorbent dosage at a contact time of 30 min and initial pH of 4. The adsorption data fitted better to pseudo-second-order reaction kinetics model indicating that Fadsorption occurred via chemisorption. Langmuir isotherm model described the data successfully indicating that adsorption occurred in a monolayer surface. Fluoride adsorption onto Mn2+ bentonite occurred through electrostatic attraction and ion exchange adsorption mechanism at lower and higher pH levels, respectively. Leaching assessment showed that the released chemical species from the adsorbent at various pH levels fall within SANS-241 permissible limits. The results proved that Mn2+ bentonite has the potential for use in fluoride removal in areas where groundwater has maximum fluoride concentration of 3 mg L-1.
Acknowledgements
The authors hereby acknowledge the financial support from WRC ProjectNo. K5/2363/3, NRF Project No. CSUR13092849176, Grant No. 90288, THRIP Project No. TP12082610644 and Research & Innovation Directorate, University of Venda, South Africa.
§ORCID iDs
R. Mudzielwana: orcid.org/0000-0002-7744-4561
M.W. Gitari: orcid.org/0000-0002-6387-0682
References
1 N. Chen, Z. Zhang, C. Feng, M. Li, D. Zhu and N. Sugiura, Studies on fluoride adsorption of iron-impregnated granular ceramics from aqueous solution, J. Mater. Chem. Phys., 2011,125, 293-298. [ Links ]
2 T. Nur, P. Loganathan, T.C. Nguyen, S. Vigneswaran, G. Singh and J. Kandasamy, Batch and column adsorption and desorption of fluoride using hydrous ferric oxide: solution chemistry and modelling, J. Chem. Eng., 2014, 247, 93-102. [ Links ]
3 World Health Organization, Guidelines for Drinking-water Quality, 4th edn., Geneva, Switzerland, 2011. [ Links ]
4 S.V Jadhav, E. Bringas, G.D. Yadav, VK. Rathod, I. Ortiz and K.V Marathe, Arsenic and fluoride contaminated groundwaters: a review of current technologies for contaminants removal, J. Environ. Manage., 2015,162, 306-325. [ Links ]
5 K.M. Kut, A. Sarswat, A. Srivastava, C.U. Pittman, U. Mohan, A review offluoride in African groundwater and local remediation methods, Groundw. Sust. Dev., 2016, 2,190-212. [ Links ]
6 R. Tovar-Gómez, M.R. Moreno-Virgen, J.A. Dena-Aguilar, V. Hernández-Montoya, A. Bonilla-Petriciolet and M.S. Montes-Morán, Modeling of fixed-bed adsorption of fluoride on bone char using a hybrid neural network approach, Chem., Eng., 2013, 228, 1098-1109. [ Links ]
7 Y. Yu, L. Yu and J.P. Chen, Adsorption of fluoride by Fe-Mg-La triple-metal composite: adsorbent preparation, illustration of performance and study of mechanisms, Chem. Eng., 2015, 262, 839-846. [ Links ]
8 P.K. Gogoi and R. Baruah, Fluoride removal from water by adsorption on acid activated kaolinite clay, Indian J. Chem. Technol,. 2008, 15, 500-503. [ Links ]
9 S. Kamble, P. Dixit, S.S. Rayalu and N.K. Labhsetwar, Defluoridation of drinking water using chemically modified bentonite clay, Desal. Water Treat., 2009, 249, 687-693. [ Links ]
10 S. Gammoudi, N. Frini-Srasra, E. Srasra, Preparation, characterization of organo-smectites and fluoride ion removal, Int. J. Miner. Process., 2013,125, 10-17. [ Links ]
11 R. Mudzielwana, W.M. Gitari, T.A.M. Msagati, Characterization of smectite-rich clay soil: implication for groundwater defluoridation, S. Afr. J. Sci., 2016,112 (11-12), 41-8. [ Links ]
12 A.A. Izuagie, WM. Gitari and J.R. Gumbo, Synthesis and performance evaluation of Al/Fe oxide coated diatomaceous earth in groundwater defluoridation: towards fluorosis mitigation, J. Environ. Sci. Heal. A., 2016, 1-15. [ Links ]
13 T. Ngulube, M.W. Gitari, H. Tutu, Defluoridation of groundwater using mixed Mukondeni clay soils, WST: Water Supply, 2017, 17(2), 480-492. [ Links ]
14 R. Mudzielwana, M.W. Gitari, S.A. Akinyemi, T.A.M. Msagati. Synthesis and physicochemical characterization of MnO2 coated Na-bentonite for groundwater defluoridation: adsorption modelling and mechanistic aspect, Appl. Surf. Sci., 2017, 422, 745-753. [ Links ]
15 Y. Ma, S.G. Wang, M. Fan, W.X. Gong and B.Y. Gao, Characteristics and defluoridation performance of granular activated carbons coated with manganese oxides, J. Hazard. Mater., 2012,168,1140-1146. [ Links ]
16 D. Thakre, S. Rayalu, R. Kawade, S. Meshram, J. Subrt and N. Labhsetwar, Magnesium incorporated Bentonite clay for defluori-dation of drinking water, J. Hazard. Mater., 2010,180, 122-130. [ Links ]
17 W.M. Gitari, T. Ngulube, V. Masindi and J.R. Gumbo, Defluoridation of groundwater using Fe3+-modified bentonite clay: optimization of adsorption condition, J. Desalin. Water Treat., 2013, 1-13. [ Links ]
18 V. Masindi, W.M. Gitari and T. Ngulube, Defluoridation of drinking water using Al3+-modified bentonite clay: optimization of fluoride adsorption conditions, Toxicol. Environ. Chem., 2014, 96(9), 1-16. [ Links ]
19 E. Kumar, A. Bhatnagar, U. Kumar and M. Sillanpaa, Defluoridation from aqueous solution by nano-alumina: characterization and sorption studies, J. Hazard. Mater., 2011,186, 1042-1049. [ Links ]
20 N.A. Oladoja, B. Helmreich, H.A. Bello, Towards the development of a reactive filter from green resource for groundwater defluoridation, Chem. Eng. J., 2016, 301, 166-177. [ Links ]
21 Y. Jia, B.S. Zhu, K.S. Zhang, Z. Jin T. Luo, X.Y. Yu, L.T. Kong. and J.H. Liu. Porous 2-line ferrihydrite/byerite composite (LFBC): fluoride removal performance and mechanism, Chem. Eng. J., 2015, 268, 325-336. [ Links ]
22 South African National Standard (SANS) 241 Drinking Water Specification, South African Bureau of Standards (SABS), Pretoria, South Africa, 2006. [ Links ]
23 Z. Yi, X. Ying, C. Hao, L. Bingjie, G. Xiang, W. Dongfeng and L. Peng, La (III)-loaded bentonite/chitosan beads for defluoridation from aqueous solution, J. Rare Earth., 2014, 32(5), 458-69. [ Links ]
Received 14 March 2017
Revised 12 December 2017
Accepted 13 December 2017
* To whom correspondence should be addressed. E-mail: mugera.gitari@univen.ac.za














