Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.70 Durban 2017
http://dx.doi.org/10.17159/0379-4350/2017/v70a27
RESEARCH ARTICLE
Simultaneous determination of permethrin and deltamethrin in water samples by magnetic solid-phase extraction coupled with dispersive liquid-liquid microextraction combined with gas chromatography
Arezoo Hassan NooriI; Mohammad RezaeeII; Maryam KazemipourI; Hossein Ali MashayekhiIII, *
IDepartment of Chemistry, Kerman branch, Islamic Azad University, Kerman, Iran
IIMaterials and Nuclear Fuel Research School, Nuclear Science and Technology Research Institute, Atomic Energy Organization of Iran, P.O. Box 14395-836, Tehran, Iran
IIIDepartment of Chemistry, Tonekabon Branch, Islamic Azad University, Tonekabon, Iran
ABSTRACT
In this study, new and efficient method, magnetic solid phase extraction coupled with dispersive liquid-liquid microextracton (MSPE-DLLME) combined with gas chromatography-flame ionization detector (GC-FID) was developed for the preconcentration and determination of permethrin and deltamethrin in water samples. Several factors influencing the extraction efficiency including amount of sorbent, sorption time, type of extraction solvent and its volume, type of disperser solvent and its volume and elution time were investigated and optimized. Using optimum extraction conditions, dynamic linear range of 0.5-100 L-1 for both, limits of quantification (LOQs) of 0.6 L-1 for both and limits of detection (LODs) of 0.01 L-1 for both were obtained. Finally, the method was successfully applied for the extraction and determination of permethrin and deltamethrin in water samples in the range of micrograms per litre with RSDs < 4 %.
Keywords: Magnetic solid-phase extraction, dispersive liquid-liquid microextraction, permethrin, deltamethrin, gas chromatography, water samples
1. Introduction
Insecticides on the basis of pyrethrine were first introduced in the 1950s. Pyrethrines are a class of natural substances which originates from the blossom of the chrysanthemum. Pyrethrines were, and still are, used as natural insecticides against vermin in households. Nevertheless, the production of pyrethrine is expensive and the resources are limited. For these reasons synthetic pyrethroids were developed based on the basic structure of natural pyrethrines.1
Synthetic pyrethroids are utilized in households and greenhouses, as well as to control fleas and scabies. Moreover, these compounds are used for wood and textile protection.24 It has been firmly established that synthetic pyrethroids act as powerful neurotoxic agents.5 The molecular basis for the neurotoxicity of pyrethroids have been attributed to their actions on voltage-dependent sodium channels5,6 and on receptor-regulated channels, like the nicotinic7,8 and GABA-gated chloride channels.9,10 Effects on Ca2+,Mg2+-ATPases have also been reported.11-13
Determination of pesticides in different sample matrices is usually performed after pretreatment steps using either gas chromatography (GC)-mass spectrometry (MS),14 GC-electron capture and ion trap mass spectrometric detectors,15 GC-nitrogen-phosphorus detector,16 GC-flame photometric detec-tor,17 GC-flame ionization detector,18 or high performance liquid chromatography (HPLC) with different detectors (e.g. MS/MS spectrometry,19 diode array detector).20 The use of ultra-performance liquid chromatography in detection of pesticides has also been reported.21
Different preconcentration methods such as solid-phase extraction (SPE),22 solid-phase microextraction (SPME),20 liquidphase microextraction (LPME),17 single drop microextraction (SDME),23 headspace solid-phase microextraction (HSPME),14 dispersive liquid-liquid microextraction (DLLME),24 homogeneous liquid-liquid microextraction (HLLME),25 ultrasonic assisted headspace single drop microextraction (USA-HSDME),16 vortex-assisted liquid-liquid microextraction (VALLME),26 ultrasound-assisted solvent extraction followed by dispersive liquid-liquid microextraction,27 supercritical fluid extraction combined with dispersive liquid-liquid micro-extraction,18 microwave-assisted extraction-solid-phase extrac-tion,28 or SPE in combination with DLLME29 have been used for the preparation of water samples containing pesticides.
Solid phase extraction (SPE) has become a well-established sample preparation method to extract and preconcentrate desired components.30-33 Application of magnetic nanoparticles in SPE (MSPE) simplifies sample pretreatment and overcomes some limitations of conventional SPE.34 The sorbent does not need to be packed into cartridges (as in traditional SPE), and the separation steps can be carried out easily by applying an external magnetic field. Nanoparticles (NPs) possess large surface area, high adsorption capacity, and rapid adsorption rate; so, low amounts of sorbent and short equilibrium time are required to extract analytes from large volumes of samples.35-37
Magnetic nanoparticles (MNPs) are materials composed of magnetic elements, including iron, cobalt and nickel. MNPs also have certain characteristic properties such as super paramagnetism, high coercivity, high magnetic susceptibility and low curie temperature.38 Iron oxide MNPs may exist in different forms, such as magnetite, maghemite, hematite, and goethite or their combinations, depending on the Fe(22)/Fe(222) ratio, which influences size, composition, morphology, and magnetic properties of the particles.39-41 With the latest developments in nano-technology,42 MNPs can be applied for magnetophoretic separation of a wide range of materials41 as they provide several advantages over other available separation methods. The advantages include, recyclability for multiple usage, high-throughput process, low operational cost, high efficiency, flexible implementation, and scalability.43 These features make MNPs very attractive in many fields such as biotechnology/biomedicine,44 environmental remediation,45 data storage,46 and other emerging fields. However, unavoidable problems may occur, associated with particles in this nanosize range, such as their intrinsic instability/tendency to form aggregates and their interaction with the (often high ionic strength) media. The inherent magnetic forces contribute to the attractive forces among NPs which may lead to aggregation.47,48 Stabilization of MNPs may be achieved by coating the particles, either by chemical bonding or physical adsorption, with different capping agents. Among the different types of coatings used as sorbents for the extraction of organic analytes, composites of conductive polymers are of interest. This is due to their multifunctional properties including hydro-phobicity, acid-base character p-p interaction, polar functional groups, ion exchange property, hydrogen bonding and electro-activity.49-53
Dispersive liquid-liquid microextraction (DLLME) was introduced by Rezaee and co-workers in 2006.54 In this method, an appropriate mixture of extraction and disperser solvents are used. The method has attracted much attention due to its advantages such as short extraction time, low consumption of organic solvent, and simplicity.55-57
In this study, polypyrrole as a conductive polymer was coated on the surface of Fe3O4 nanoparticles. The samples were first extracted by magnetic solid phase extraction (MSPE), then the eluents were recovered, and subjected to dispersive liquid-liquid microextracton (DLLME) for further purification and enrichment of permethrin and deltamethrin compounds. The effect of principle factors such as sorbent amount, extraction time, extraction solvent, extraction solvent volume, disperser solvent, disperser solvent volume, and desorption time were studied.
2. Experimental
2.1. Chemical and Reagents
Permethrin (>98 %) and deltamethrin (99.7 %) were provided by Sigma-Aldrich (UK). Carbon tetrachloride (>99.5 %), 1,1,2-trichloroethane (>98 %), chloroform (>99.8 %), and chlorobenzene (>99 %), as extraction solvents and acetone (>99.8 %), acetonitrile (>99.9 %), ethanol (>99.5 %), and methanol (>99.9 %), as disperser solvents were obtained from Merck (Darmstadt, Germany). Double-distilled water was used for preparation of aqueous solutions. An amount of 0.001 g of permethrin and deltamethrin were dissolved in 10.0 mL of methanol to obtain standard stock solution with a concentration of 100 mg L-1. A fresh 10 mg L-1 standard solution containing permethrin and deltamethrin was prepared in methanol every week and stored at 4 °C. Ferric chloride (>98 %), ferrous chloride (99.5 %), sodium hydroxide (>99 %), reagent grade NaCl (>99.5 %) and pyrrole (>97 %) were purchased from Merck.
2.2. Instrumentation
The chromatographic analysis was performed on an Agilent GC-7890 system equipped with a split/splitless injector system and flame ionization detector for separation and determination of permethrin and deltamethrin. Highly pure helium gas (99.999 %, Air Products, UK) was passed through a molecular sieve and oxygen trap (Crs, USA) and was employed as a carrier gas with a flow rate of 1.5 mL min-1. The injection port was held at 290 °C and operated in the splitless mode for 1 min then split valve was opened and split ratio of 1:5 was applied. Separation was carried out on a DB5, 25 m x 0.32 mm i.d. and 0.25 μπι film thickness from SGE (Victoria, Australia) capillary column. The oven temperature was kept at 230 °C for 5 min and then increased to 285 °C at the rate of 10 °C min-1, and was held for 10 min. The FID oven temperature was maintained at 300 °C. Hydrogen was generated by hydrogen generator (OPGU-2200S, Shimadzu) for FID at a flow rate of 40 mL min-1. The flow of air (99.999 %, Air Products) for FID was 400 mL min-1. The model 2010 D Centurion Scientific centrifuge (Westsussex, UK) was used for separation of sediment phase from sample solution.
2.3. Preparation of Magnetic Nanoparticles of Fe3O4
The chemical co-precipitation method was used in the preparation of the Fe3O4 NPs [48]. First, a stock solution was prepared by mixing 10.4 gof FeCl3-6H20,4.0gofFeCl2-4H2O, and 1.7mLof HCl (12 mol L-1) in 50 mL of deionized water in a beaker. The solution was then degassed using nitrogen gas for 20 min before use. Simultaneously, 500 mL of 1.5 mol L-1 NaOH solution was degassed (for 15 min) and heated to 80 °C in a reactor. The solution of iron salts was then added dropwise using a dropping funnel for 30 min under nitrogen gas protection with vigorous stirring (1000 rpm) using a glassware stirrer. During the entire process, the solution temperature was maintained at 80 °C and nitrogen gas was used to prevent the intrusion of oxygen. After the reaction, the Fe3O4 NPs precipitate obtained was separated from the reaction medium using a magnetic field, and then washed four times with 500 mL of deionized water. Finally, the NPs obtained were resuspended in 500 mL of degassed deionized water.
2.4. Preparation of the PPy/Fe3O4 Nanocomposites
The PPy/Fe3O4 nanocomposites were synthesized by in situ polymerization of the pyrrole (Py) monomer in the presence of suspended Fe3O4 nanoparticles, using FeCl3 as oxidant at ambient temperature. In a typical polymerization technique, 0.2gofFe3O4 nanoparticles were added to 25 mL deionized water in a conical flask and ultrasonicated for 10 min for better dispersion of Fe3O4 into water. A quantity of3gofFeCl3, oxidant was added to the deionized water containing the Fe3O4 nanoparticles and was shaken for 10 min. To this mixture 0.8 mL of pyrrole was added to the mixture using a syringe. Then the reaction mixture was kept under constant shaking for3hat ambient temperature. Finally, to stop the reaction, acetone was added to the reaction mixture. The black powder obtained was filtered and washed with distilled water until the filtrate became colourless and finally washed with acetone. Then the composites were dried at 100 °C for 8 h.
2.5. MSPE-DLLME Procedure
Ten millilitres of the aqueous sample solution were transferred to a beaker and spiked at a given concentration of the target analytes. Fe3O4@PPy NPs (20 mg) was added into a beaker containing 10 mL aqueous solution spiked at the level of 100 μg L-1 of the analytes, and mechanically stirred for 10 min (Fig. 1a). The magnetic adsorbent was isolated from a solution using a magnet (Fig. 1b). Thereafter, the magnetic adsorbent was mixed with 800 methanol using a vortex mixer for 0.5 min (Fig. 1c) and separated using magnet (Fig. 1d), and thereafter the eluent was transferred into a vial for DLLME step. For the extraction process (DLLME), 40 of 1,1,2-trichloroethane was added to the eluent, and then the mixture was rapidly injected into a conical test tube containing 5 mL double-distilled water. After agitation for 0.5 min using a vortex mixer, a cloudy solution resulting from the dispersion of fine droplets of 1,1,2-trichloro-ethane in the aqueous solution was formed in the test tube (Fig. 1f). Then the solution was centrifuged for 5 min at 2000 rpm (Fig. 1g) to force the dispersed fine particles of 1,1,2-trichloro-ethane to sediment at the bottom of the test tube (Fig. 1h). Thereafter, 2 of the organic (1,1,2-trichloroehane) phase was injected into GC-FID for analysis.
3. Results and Discussion
3.1. Characterization of Fe3O4@ PPy Nanoparticles
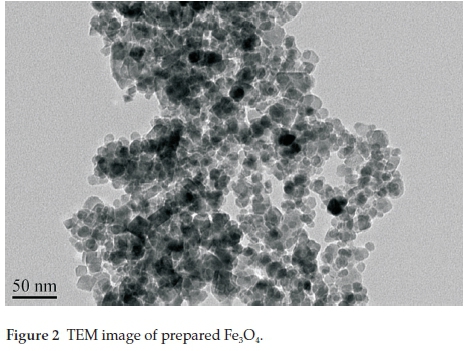
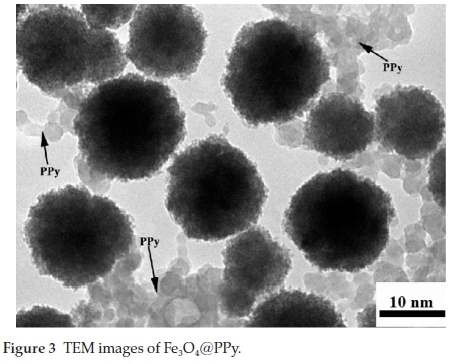
The obtained NPs were stable under these conditions for up to about one month and were characterized using a transition electron microscope (TEM). The obtained product is shown in Fig. 2. The shape, size and morphology of the synthesized Fe3O4@PPy NPs were determined by TEM and SEM. Furthermore, the coated PPy was characterized by FT-IR. The shape and size of the nanoparticles were observed by TEM (Fig. 3). The TEM images of Fe3O4@PPy particles show that an obvious coating of PPy is immobilized on the surface of Fe3O4NPs. The coated PPy layer is clearly seen due to the different electron densities of magnetic nanoparticles core (with dark colour) and PPy coating (with light colour) in TEM micrograph. The synthesized Fe3O4@PPy NPs showed a spherical shape with an average diameter of about 5-10 nm, however, the nanoparticles tended to aggregate.
In addition, the size and morphology of the resultant Fe3O4@PPy NPs were determined by SEM (Fig. 4). The Fe3O4@PPy NPs have a nearly spherical shape with a smooth and uniform surface morphology. Due to agglomeration of the particles and the lower resolution of SEM in comparison to TEM, size of the particles in SEM image is larger than that in TEM image.
The coated PPy was characterized by FT-IR in a range of 4000 and 400 cm-1. FT-IR spectra for bare and PPy-coated Fe3O4 NPs are illustrated in (Fig. 5). The characteristic absorption peaks of Fe3O4NPs, appeared in two spectrums (a and b), corresponding to the stretching vibrations of hydrogen-bonded surface water molecules and hydroxyl groups at 3400 cm-1 and the Fe-O transverse vibration at 580 cm-1 were observed. Coating of PPy onto Fe3O4 NPs was confirmed by the appearance of characteristic PPy bands in spectrum (b). The weak bands at 2800 and 2900 cm-1 were assigned to the stretching vibrations of C-H bonds. The absorption peak at 1050 and 1314 cm-1 were attributed to the bending vibration of C-H bond in the pyrrole ring and C-N stretching vibration. The absorption bands at 1549 and 1460 cm-1 belong to C-C asymmetric and symmetric stretching vibrations of the pyrrole ring, respectively. The absorption bands at 2358 and 909 cm-1 belong to C-H vibrations and the absorption bands at 3737 and 3820 cm-1 belong to N-H vibrations. These results indicate that PPy has been successfully coated on the surface of Fe3O4 NPs.
3.2. Effect of Sorbent Amount
To study the effect of sorbent quantity on the extraction efficiency of permethrin and deltamethrin, different amounts of sorbent in the range of 10-25 mg were added to the solution (Fig. 6). Finally, an amount of 20 mg of sorbent was found as the optimum value.
3.3. Effect of Extraction Time
The extraction recovery strongly depends on the mass transfer of the analytes from sample solution to the extraction media. To study the effect of this parameter, the extraction was performed in the range of 5-30 min. The extraction time profile for permethrin and deltamethrin showed that the equilibrium was reached quite rapidly. The developed method offers a short extraction time which could be due to the dispersion of sorbent throughout the sample solution during the extraction and the absence of internal diffusion resistance. Overall, a duration time of 20 min was found to be the optimum value for the extraction time (Fig. 7).
3.4. Effect of Elution Solvent Type and its Volume
In MSPE-DLLME procedure, the elution solvent used for the SPE step also plays a role as a disperser solvent during the DLLME stage. For this purpose, acetone, acetonitrile, ethanol and methanol, were selected and tested as elution solvents. The Fe3O4@PPy was eluted using 800 μL of each solvent. The results (Fig. 8) indicated that the extraction efficiency, when using methanol, was more effective than the other potential elution solvents. Therefore, methanol was selected as the elution solvent in further experiments.
To obtain the optimized volume of elution solvent, various experiments were carried out using different volumes of methanol (700-1000 μL). According to the results shown in Fig. 9, methanol volumes lower than 800 μL decreases the extraction efficiency, indicating lower volumes cannot elute the analytes effectively. Also, at lower volumes of methanol the emulsion does not form, thereby the recovery is low. At methanol volumes higher than 800 μL, the extraction efficiency decreases, due to the increasing solubility of the target analytes in the water phase. Therefore, a volume of 800was chosen as the optimum volume for the elution solvent.
3.5. Effect of Extraction Solvent Type and its Volume
The extraction solvent must possess certain properties, including higher density than water, high extraction capability of the analytes, and low solubility in water. To investigate the effect of the extraction solvent, chloroform, tetrachloroethylene, chloro-benzene, and 1,1,2- trichloroethane were tested. The results (Fig. 10) clearly indicate that the extraction efficiency obtained using 1,1,2-trichloroethane was higher than that of the other extraction solvents. It is probably because of the higher solubility of the analytes in 1,1,2-trichloroethane in comparison with the other tested solvents. Therefore, 1,1,2-trichloroethane was selected as the extraction solvent in further experiments.
To examine the effect of the volume of the extraction solvent, additional experiments were carried out using 800 methanol (elution solvent) and different volumes of the extraction solvent (20-90 μL), and the results as presented in Fig. 11. It was found that with an increase in the volume of 1,1,2-trichloroethane from 20.0 to 40.0 μL, extraction efficiency increases and after that decreases, because of increasing the volume of the organic (sediment) phase. According to the results, 40.0 of 1,1,2-trichloro-ethane was selected as the optimum extraction solventvolume.
3.6. Desorption Time
The influence of desorption time was also investigated. It was observed (Fig. 12) that after 5 min, no notable changes occurred in the extraction efficiencies. Therefore, 5 min was considered as the optimal desorption time of the analytes in subsequent experiments.
3.7. Quantitative Aspects
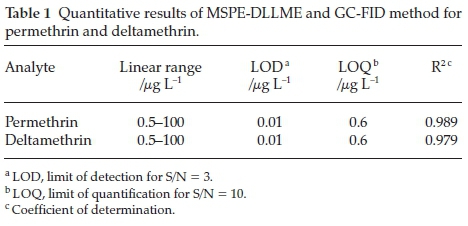
The analytical characteristics of the proposed method was validated under the optimized conditions in terms of linearity, precision and limit of detection to estimate the efficiency and feasibility of the method for its application in analysis of environmental samples. The results are listed in Table 1 under optimum conditions. The analytes demonstrated good linearity in the range of 0.5-100 μgL-1 with good correlation coefficients r2 = 0.989 and r2 = 0.979 for permethrin and deltamethrin, respectively. The limits of detection (LODs), based on signal-to-noise ratio (S/N) of 3, was 0.01 L-1 for both. The limits of quantification (LOQs), based on signal-to-noise ratio (S/N) of 10, was 0.6 μg L-1 for both.
The precision of the method was evaluated by carrying out five independent measurements of the studied compounds at three concentration levels. The results are shown in the Table 2.

Table 3 compares the proposed method with other extraction methods for the determination of the target analytes in water samples. The quantitative results of the proposed method are better than those of homogeneous liquid-liquid microextraction via flotation assistance (HLLME-FA),59 or micro liquid-liquid extraction.60 Comparison of the proposed method with micro liquid-liquid extraction and competitive enzyme-linked immunosorbent assays (C-ELISAs)61 for the extraction and determination of the analytes indicates that this novel method has a short extraction time for determination of the analytes. C-ELISAs is expensive and requires more organic solvents and time. Also, micro liquid-liquid extraction requires more toxic organic solvent and time. Moreover, the proposed method has potential for the determination of the target analytes in complex matrices such as waste water due to clean up of the MSPE before DLLME method. In addition, it can be used in the large volumes of sample in contrast to the DLLME method. The recovery of the proposed method are comparable with other extraction methods59-61. Finally, the proposed method has exciting potential to determine the selected analytes at trace levels in water samples.
3.8. Analysis of Real Samples
During the present investigation, matrix effects on the extraction were also evaluated by investigating the applicability of the proposed method to determine permethrin and deltamethrin concentrations in waste water, river, tap and well water samples. These samples were extracted using MSPE-DLLME method and analyzed by GC-FID. The results from waste water, tap, river and well water samples showed that they were free of permethrin and deltamethrin contamination. These samples were spiked with permethrin and deltamethrin standard solution (10.0 ,ug L-1 concentration level) to assess matrix effects. The results of relative recoveries were between 88 to 95 %. These results (Table 4) show that the waste water, tap, well and river water matrices, in our present context, had negligible effect on MSPE-DLLME method.
4. Conclusion
This paper describes the application of the MSPE-DLLME method combined with GC-FID for determination of trace amounts of permethrin and deltamethrin in water samples. The relative recoveries were in the range of 88-95 % and showed that waste water, tap, well and river waters matrices had negligible effect on the MSPE-DLLME. The method is precise, reproducible and linear over a wide range and require small volumes of organic extractant. Moreover, the proposed method is promising for trace analysis of permethrin and deltamethrin in natural water samples.
References
1 BUI, Verein f. Umwelt- und Arbeitsschutz e.V., Bremer Umwelt Institut e.V., 1995. [ Links ]
2 A. Tippe, Zentralblatt f. Hygiene u. Umweltmedizin, 1993,194,342-359. [ Links ]
3 Bundesgesundheitsblatt, 1994,11, 468-470 [ Links ]
4 G.F. Pang, Y.Z. Chao, X.S. Lin and C.L. Fan, Modification of AOAC multiresidue method for determination of synthetic pyrethroid residues in fruits, vegetables, and grains. Part II: Acetone extraction system, J. AOAC Int., 1995, 78, 1474-1480. [ Links ]
5 T. Narahashi, Nerve membrane Na+ channels as targets of insecticides, Trends Pharmacol. Sci., 1992,13, 236-241 [ Links ]
6 H.P.M. Vijverberg and J.R. de Weille, The interaction of pyrethroids with voltage-dependent Na+ channels, Neurotoxicology, 1985, 6, 23-34. [ Links ]
7 S.M. Sherby, A.T. Eldefrawi, S.S. Deshpande, E.X. Albuquerque and M.E. Eldefrawi, Effects of pyrethroids on nicotinic acetylcholine receptor binding and function, Pestic. Biochem. Physiol., 1986, 26, 107-115. [ Links ]
8 M.A. Abbassy, M.E. Eldefrawi and A.T. Eldefrawi, Pyrethroid action on the nicotin acetylcholine receptor/channel, Pestic. Biochem. Physiol., 1983,19, 299-308. [ Links ]
9 J.R. Bloomquist and D.M. Soderlund, Neurotoxic insecticides inhibit GABA-dependent chloride uptake by mouse brain vesicles. Biochem. Biophys. Res. Commun., 1985,133, 37-43. [ Links ]
10 L.J. Lawrence and J.E. Casida, Stereospecific action of pyrethroid insecticides on the gamma-aminobutyric acid receptor-ionophore complex, Science, 1983, 221, 1399-1401. [ Links ]
11 O.T. Jones and A.G. Lee, Effects of pyrethroids on the activity of a purified (Ca2+-Mg2+) - ATPase, Pestic. Biochem. Physiol., 1986, 25, 420-430. [ Links ]
12 J.M. Clark and F. Mastsumura, Two different types of inhibitory effects of pyrethroids on nerve Ca- and Ca + Mg -ATPase activity in the squid Loligo pealei, Pestic. Biochem. Physiol., 1982,18, 180-190. [ Links ]
13 F. Michelangeli, M.J. Robson, J.M. East and A.G. Lee, Fluorescence and kinetic studies of the interactions of pyrethroids with the (Ca2+- Mg2+)-ATPase. Biochem. Biophs. Acta, 1990,1028, 58-66. [ Links ]
14 F.D.M. Rourigues, P.R.R. Mesquita, L.S. De Oliveira, F.S. Filho, A.M. Pereira, P.A. de P. and J.B. De Andrade, Development of a headspace solid-phase microextraction/gas chromatography-mass spectrome-try method for determination of organophosphorus pesticide residues in cow milk, Microchem. J., 2011, 98, 56-61. [ Links ]
15 I.S. Jeong, B.M. Kwak, and J.H. Ahn, Determination of pesticide residues in milk using a QuEChERS-based method developed by response surface methodology, Food Chem., 2012,133, 473-481. [ Links ]
16 A. Salemi, R. Rasolzadeh, M. Mohebbi Nejad and M. Vosough, Ultrasonic assisted headspace single drop micro-extraction and gas chro-matography with nitrogen-phosphorus detector for determination of organophosphorus pesticides in soil, Anal. Chim. Acta, 2013, 769, 121-126. [ Links ]
17 M.R. Khalili-Zanjani, Y. Yamini, N. Yazdanfar and S. Shariati, Extraction and determination of organophosphorus pesticides in water samples by a new liquid phase microextraction-gas chromatogra-phy-flame photometric detection. Anal. Chim. Acta, 2008, 606, 202-208. [ Links ]
18 M.H. Naeeni, Y. Yamini and M. Rezaee, Combination of supercritical fluid extraction with dispersive liquid-liquid microextraction for extraction of organophosphorus pesticides from soil and marine sediment samples. J. Supercrit. Fluids, 2011, 57, 219-226. [ Links ]
19 H. Tian, Determination of chloramphenicol, enrofloxacin and 29 pesticides residues in bovine milk by liquid chromatography -tandem mass spectrometry, Chemosphere, 2011, 83, 349-355. [ Links ]
20 A. Melo, A. Aguiar, C. Mansilha, O. Pinho and I.M.P.L.V.O. Ferreira, Optimization of a solid-phase microextraction - HPLC-diode array method for multiple pesticide screening in lettuce, Food Chem., 2012, 130, 1090-1097. [ Links ]
21 F. Galan-Cano, R. Lucena, S. Cardenas and M. Valcarcel, Dispersive micro-solid phase extraction with ionic liquid-modified silica for determination of organophosphate in water by ultra performance liquid chromatography, Microchem. J., 2013,106, 311-317. [ Links ]
22 B. Cavaliere, M. Monteleone, A. Naccarato, G. Sindona, and A. Tagarelli, A solid-phase microextraction-gas chromatographic approach combined with triple quadruple mass spectrometry for the assay of carbamate pesticides in water samples, J. Chromatogr. A, 2012, 1257, 149-157. [ Links ]
23 N.G. Tsiropoulos and E.G. Amvrazi, Determination of pesticide residues in honey by single-drop microextraction and gas chromatography. J. AOAC Int., 2011, 94, 634-644. [ Links ]
24 C.K. Zacharis, I.R. Petros, G. Zachariadis and A. Zotos, Dispersive liquid-liquid microextraction for the determination of organo-chlorine pesticides residues in honey by gas chromatography-elec-tron capture and ion trap mass spectrometric detection. Food Chem., 2012, 134, 1665-1672. [ Links ]
25 N. Yazdanfar, Y. Yamini and M. Ghambarian, Homogenous liquidliquid microextraction for determination of organochlorine pesticides in water and fruit samples. Chromatographia, 2014, 77, 329-336 [ Links ]
26 C. Jia, X. Zhu, J. Wang, E. Zhao, M. He and L. Chen, Extraction of pesticides in water samples using vortex-assisted liquid-liquid microextraction. J. Chromatogr. A, 2010, 1217, 5868-5871. [ Links ]
27 A. Bidari, M.R. Ganjali, P. Norouzi, M.R. Milani Hosseini and Y. Assadi, Sample preparation method for the analysis of some organo-phosphorus pesticides residues in tomato by ultrasound-assisted solvent extraction followed by dispersive liquid-liquid microextraction, Food Chem., 2011,126, 1840-1844. [ Links ]
28 D. Mutavdzic, A.J.M. Horvat, S. Babic and M. Kastelan-Macan, SPE-Microwave-assisted extraction coupled system for the extraction of pesticides from water samples, J. Sep. Sci., 2005, 28, 1485-1492. [ Links ]
29 A.C. Alves, M.M. Goncalves, M.M. Bernardo and B.S. Mendes, Determination of organophosphorous pesticides in the ppq range using a simple solid-phase extraction method combined with dispersive liquid-liquid microextraction, J. Sep. Sci., 2011, 34, 2475-2481. [ Links ]
30 B. Ebrahimpour, Y. Yamini and A. Esrafili, Extraction of azole anti-fungal drugs from milk and biological fluids using a new hollow fiber liquid-phase microextraction and analysis by GC-FID, Chromato-graphia, 2011, 74, 281-289. [ Links ]
31 J.A. Lopez-Lopez, C. Mendiguchia, J.J. Pinto and C. Moreno, Liquid membranes for quantification and speciation of trace metals in natural waters, Trends Anal. Chem., 2010, 29 645-653. [ Links ]
32 S. Pedersen-Bjergaard, K.E. Rasmussen and T. Gronhaug, Liquidliquid extraction procedures for sample enrichment in capillary zone electrophoresis, J. Chromatogr. A, 2000, 902, 91-105. [ Links ]
33 C.F. Poole, New trends in solid-phase extraction, Trends Anal. Chem., 2003, 22, 362-373. [ Links ]
34 X. Jiang, K. Huang, D. Deg, H. Xia, X. Hou and C. Zheng, Nanomaterials in analytical atomic spectrometry, Trends Anal. Chem., 2012, 39, 38-59. [ Links ]
35 F. Pena-Pereira, R.M.B.O. Duarte, T. Trindade and A.C. Duarte, Determination of anionic surface active agents using silica coated magnetite nanoparticles modified with cationic surfactant aggregates, J. Chromatogr. A, 2013, 1299, 25-32. [ Links ]
36 Y. Yamini, E. Tahmasebi and L. Ranjbar, Magnetic nanoparticle-based solid-phase extraction of vitamin B12 from pharmaceutical formulations, Biol. Trace Elem. Res., 2012,147, 378-385. [ Links ]
37 Q. Liu, J. Shi, T. Wang, F. Guo, L. Liu and G. Jiang, Hemimicelles/ admicelles supported on magnetic graphene sheets for enhanced magnetic solid-phase extraction, J. Chromatogr. A, 2012,1257, 1-8. [ Links ]
38 W. Wu, Q. He and C. Jiang, Magnetic iron oxide nanoparticles: synthesis and surface functionalization strategies, Nanoscale. Res. Lett., 2008, 3, 394-415 [ Links ]
39 S. Laurent, D. Forge, M. Port, A. Roch and C. Robic, Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physico-chemical characterizations, and biological applications, Chem. Rev., 2008, 108, 2064-2110. [ Links ]
40 D. Ho, X. Sun and S. Sun, Monodisperse magnetic nanoparticles for theranostic applications, Acc. Chem. Res., 2011,44, 875-882. [ Links ]
41 Y.G. Li, H.S. Gao, W.L. Li, J.M. Xing and H.Z. Liu, In situ magnetic separation and immobilization of dibenzothiophene-desulfurizing bacteria, Bioresour Technol, 2009,100, 5092-5096. [ Links ]
42 J.A. Dahl, L.S. Maddux and J.E. Hutchinson, Toward greener nano- synthesis, Chem. Rev., 2007,107, 2228-2268. [ Links ]
43 J.H. Kang and J.K. Park, Technical paper on microfluidic devices-cell separation technology, APBN, 2005, 9, 1136-1146. [ Links ]
44 A.K. Gupta and M. Gupta, Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials, 2005, 26, 3995-4021. [ Links ]
45 J. Li, Y. Zho, M. Li, N. Xia, Q. Huang and H. Do, Carboxymethylated dextran-coated magnetic iron oxide nanoparticles for regenerable bioseparation, J. Nanosci. Nanotech., 2011, 11, 10187-10192. [ Links ]
46 E. Kadar, G.A. Tarran, A.N. Jha and S.N. Al-Subiai, Stabilization of engineered zero-valent nanoiron with Na-acrylic copolymer enhances spermiotoxicity, Environ. Sci. Technol., 2011,45, 3245-3325. [ Links ]
47 D. Rosick and J. Sembera, Influence of structure of iron nanoparticles in aggregation their magnetic properties, Nanoscale. Res. Lett., 2011,6, 527-536. [ Links ]
48 E. Kadar, S.N. Al-Subiai, O. Dyson and R.D. Handy, Are reproduction impairments of free spawning marine invertebrates exposed to zero-valent nano iron associated with dissolution of nanoparticles, Nanotoxicology, 2013, 7, 135-143. [ Links ]
49 H. Bagheri, A. Mohammadi and A. Salemi, On-line trace enrichment of phenolic compounds from water using a pyrrole-based polymer as the solid-phase extraction sorbent coupled with high-performance liquid chromatography, Anal. Chim. Acta, 2004, 513, 445-449. [ Links ]
50 H. Bagheri, Z. Ayazi and M. Naderi, Conductive polymer-based microextraction methods: areview, Anal. Chim. Acta, 2013,767,1-13. [ Links ]
51 H. Bagheri, R. Daliri and A. Roostaie, A novel magnetic poly (aniline-naphthylamine)-based nanocomposite for micro solid phase extraction of rhodamine B, Anal. Chim. Acta, 2013, 794, 38-46. [ Links ]
52 T.M. Wu and S.H. Lin, Synthesis, characterization and electrical properties of polypyrrole/multiwalled carbon nanotube composites, J. Polymer Sci. Part A: Polymer Chem., 2006,44, 6449-6457. [ Links ]
53 G. Han, J. Yuan, G. Shi and F. Wei, Electrodeposition of polypyrrole/ multiwalled carbon nanotube composite films, Thin Solid Films, 2005, 474, 64-69. [ Links ]
54. M. Rezaee, Y. Assadi, E. Aghaee, F. Ahmadi and S. Berijani, Determination of organic compounds in water using dispersive liquid-liquid microextraction, J. Chromatogr. A, 2006,1116, 1-9. [ Links ]
55 M. Rezaee, Y. Yamini, S. Shariati, A. Esrafili and M. Shamsipur, Dispersive liquid-liquid microextraction combined with high-performance liquid chromatography-UV detection as a very simple, rapid and sensitive method for the determination of bisphenol A in water samples, J. Chromatogr. A, 2009, 1216, 1511-1514. [ Links ]
56 M. Rezaee, Y. Yamini and M. Faraji, Evolution of dispersive liquid-liquid microextraction, J. Chromatogr. A, 2010, 1217, 2342-2357. [ Links ]
57 H.A. Mashayekhi, P. Abroomand-Azar, M. Saber-Tehrani and S.H. Waqif, Rapid determination of carbamazepine in human urine, plasma samples and water using DLLME followed by RP-LC, Chromatographia, 2010, 12, 517-521. [ Links ]
58 J.W. Wong, M.G. Webster, C.A. Halverson, M.J. Hengel, K.K. Ngim and S.E. Ebeler, Multiresidue pesticide analysis in wines by solidphase extraction and capillary gas chromatography - mass spectro-metric detection with selective ion monitoring. J. Agric. Food Chem., 2003, 51, 1148-1161. [ Links ]
59 H. Haddadi, M. Shirani, A. Semnani, M. Rezaee, H.A. Mashayekhi and A. Hosseinian, Simultaneous determination of deltamethrin and permethrin in water samples using homogeneous liquid-liquid microextraction via flotation assistance and GC-FID, Chromatographia, 2014, 77, 715-721. [ Links ]
60 A. Femandez-Gutierrez, J.L. Martinez-Vidal, F.J. Arrebola-Liebanas, A. Gonzalez-Casado and J.L. Vilchez, Determination of endosulfan and some pyrethroids in waters by micro liquid-liquid extraction and GC-MS, Fresenius J. Anal. Chem., 1998, 360, 568-572. [ Links ]
61 H.J. Lee, G. Shan, T. Watanabe, D.W. Stoutamire, S.J. Gee and B.D. Hammock, Enzyme-linked immunosorbent assay for the pyrethroid deltamethrin, J. Agric. Food Chem., 2002, 50, 5526-5532. [ Links ]
Received 14 March
Revised 7 October 2017
Accepted 25 October 2017
* To whom correspondence should be addressed. E-mail: chem.mashayekhi@toniau.ac.ir














