Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Chemistry
versão On-line ISSN 1996-840X
versão impressa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.70 Durban 2017
http://dx.doi.org/10.17159/0379-4350/2017/v70a23
RESEARCH ARTICLE
Pedospheric sorption investigation of sulfonyl urea herbicide Triasulfuron via regression correlation analysis in selected soils
Khuram Shahzad Ahmad*
Department of Environmental Sciences, Fatima Jinnah Women University, The Mall, Rawalpindi, 46000, Pakistan
ABSTRACT
Assessing pesticide fate in agricultural soils requires a detailed understanding of their interaction the with decomposing soil component in the environment. Sulfonyl urea herbicide, Triasulfuron, has been evaluated for its sorption behaviour in selected soils via batch equilibration method run in duplicate involving UV spectrophotometry. Selected soils possessed a variable degree of physicochemical make-up. Sorption of Triasulfuron fitted well with linear and Freundlich models, yielding C-type isotherms. Kd span over a range of 4.2-11.9 mL"1. Correlation and regression analysis proved that pH, organic matter, total organic content and textural properties of soils govern the adsorption and leaching of Triasulfuron. pH and sand expressed negative correlation with Kd (r = -0.997 and r = -0.987) while organic matter, organic content and clay positively impacted Kd (r = 0.987, r = 0.987 and r = 0.980). Negative values of AG vividly proved physical and exothermic adsorption processes. Lower values of AG £ -40 kJ mol-1 exhibited physiosorption of Triasulfuron in selected soils via weak Van der Waal's forces or hydrogen bonding. However, all kinetic values were dependent on the soil physicochemical parameters and demonstrated less adsorption of Triasulfuron for selected soils. Results have been statistically evaluated by ANOVA and the accuracy of fit has been calculated by plotting residual graphs in Minitab. Present study can be further extended to investigation of degradation patterns of Triasulfuron.
Keywords: Triasulfuron, adsorption, organic matter, correlation, linear.
1. Introduction
Weed control is an imperative factor for an effective crop production and therefore the prevention of weed-crop competition at an early stage plays a very paramount role.1 Sulfonylurea (SU) herbicides are a group of herbicides, recently manufactured and the preeminent qualities of these weedicides include their rather immensely active nature and very low application rates.2 Long-term application of sulfonylurea herbicides often cause curtailment in crop yield annually as well as emergence of resistant weed species to herbicide applications.3,4
Triasulfuron (TS) is readily applied to eradicate broad-leafed weeds in wheat and maize crops.5,6,7,8 Apart from controlling weeds, Sulfonylurea herbicides may even adversely affect the chemical content of crops.9 Triasulfuron was found to completely reduce the growth of fennel with tissue damage.10 Triasulfuron (TS) (1-[2-(2-chloroethoxy)phenyl]sulfonyl-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl)urea) is a member of the group sulfonylurea herbicides which were initially introduced in 1982. TS herbicides are generally used in many countries for its higher specificity for weed control, lower mammalian toxicity and low application rate.11 Oat, barley, wheat and rye are the main crops which are being protected from weeds by applying TS. Triasulfuron blocks the biosynthetic pathways and halt the synthesis of amino acid pathway, i.e. valine, leucine and isoleucine. At the low rate of about 10-30 g TS 10 000 m-2,TSis efficient enough to remove weeds on a large scale. TS being a weak acid readily dissolves in water and is eventually more mobile in soil and hydrolyze in acidic medium.12,13 TS degrades in soil with half-life of 5.8-6 days. This dissipation rate may be longer in soil laboratory conditions. Degradation of TS may be enhanced by lowering pH and increased microbial activity resulting in biodegradation.14 According to Fenoll et al.,TS dissipated entirely even before 120 minutes of photodegradation experiment.15 Ohkawa and Inui have devised yeast gene expression systems in plants that enhance phytoremediation as well as tolerance to chemical residues in the environment.16 Biotically, TS was found to be degraded by bacteria, Ochro-bactrum sp.17
The fate of Triasulfuron in the environment was revealed by its adsorption and desorption behaviour in geographically distinct soils samples.18 Despite higher application rates, Triasulfuron has not been studied comprehensively in an agricultural country like Pakistan. Environmental state of Pakistan is already in chaos due to the use of various agrochemicals and lack of sufficient knowledge for proper use and disposal. Thus the present study investigates Triasulfuron sorption behaviour in selected soils and draws a clear environmental trend depicting the dependence of sorption on soils' physicochemical characteristics.
2. Experimental
2.1. Reagents
Analytical grade acetone and methanol with 99.9 % purity were used supplied by Fluka Company. Anhydrous powder of sodium chloride and calcium chloride with analytical grade Triasulfuron herbicide of Fluka Company was used.
Adsorption and desorption of Triasulfuron with selected agricultural soils were studied employing standard batch equilibrium method. All experiments were done in replicates. Pesticides solutions of known volume at known concentrations in 0.1 M NaCl for adsorption and 0.1 M CaCl2 for desorption were added to prepared tested soils of known weight. Soil pesticide mixture was then agitated, centrifuged and filtered. Finally, filtered aqueous phase was analyzed by analytical techniques.19,20,21,22
2.2. Soil Sampling
Four soil samples of 4 kg were collected from four geographically discrete areas of Pakistan; Sialkot (Punjab) (32.4925°N, 74.5310°E), Ormara (Punjab) (25.2666°N, 64.6096°E), Gilgit (Gilgit Baltistan) (35.9202°N, 74.3080°E) and Karachi (Sindh) (24.8615°N, 67.0099°E). Samples were collected from agricultural field in each district with no or zero use of any pesticide through random sampling at a depth of 0-6 cm, and were stored in clean polythene bags and transferred to lab for further experimentation and analysis. These were air-dried and mixed thoroughly to attain uniformity. Samples were disintegrated by using mortar and pestle and then preserved in sealed labelled Petri dishes for experiment. All the physicochemical parameters including soil texture, pH, soil organic matter (OM), total organic carbon (TOC), cation exchange capacity (CEC), total soil nitrogen (N) and potassium (K) were checked.
2.3. Sorption Experiment
Adsorption and desorption of experiments was carried out at laboratory isothermal and ambient conditions (temperature (25 ± 1 °C) employing batch equilibrium method following OECD Guideline for the Testing of Chemicals.19,20,2
2.3.1. Adsorption Experiment
All experiments were done under isothermal conditions at ambient room temperature. All samples were run in duplicates for the experiment. Pesticide solutions were prepared in distilled water and stored at 4 °C, whereas Triasulfuron herbicide of different concentrations of 0.25,0.5,0.75,1.0,2.5,5.0 and 7.5 ppm were prepared. Pesticide and soil ratio was kept at 1:20. Depending on the selected herbicide concentration, 10 mL of 0.1 M NaCl was added as background electrolyte in each concentration. Each sample consisted of 0.5 g of soil mixed with 10 mL of pesticide solution in 1:10 soil and solution ratio, placed in a 15 mL capped centrifuge tubes. The tubes were continuously shaken on an orbital shaker at 90 rpm overnight at room temperature (25 °C).23 In order to achieve equilibrium, the adsorption process was done in replicates for each concentration. A blank sample containing only dissolved herbicide and 0.1 M sodium chloride background electrolyte without soil was prepared and treated in parallel with each set of batch experiment in order to calculate the losses and possible deterioration during the experiment. The equilibrated solution was centri-fuged at 3000 rpm for 25 min at 25 °C. The supernatants were filtered through 0.2 am nucleopore syringe filter and filtered aliquots were taken for investigation of the pesticides using a spectrophotometer. The wavelength was taken at lmax395 nm .
2.3.2. Desorption Experiment
Once the adsorption was done, the remainder of the supernatant was discarded and immediately replaced by 9 mL freshly prepared 0.01M calcium chloride solution. The samples were left overnight on a shaker at 90 rpm. The tubes containing the samples were centrifuged, the sample was filtered and analyzed for desorbed herbicide by UV spectrophotometer. Desorption studies were performed in duplicate.
The analytical method used for analysis of the pesticide sorption in the present research was UV-Visible spectrophotometry using BMS 1602 UV-Visible spectrophotometer (Biotechnology Medical Services K. Group, U.S.A). Adsorption and desorption behaviour of Triasulfuron in 16 concentrations including duplicates was studied by UV-Visible spectrophotometer. UV spectra of Triasulfuron was recorded at 230 nm . To obtain the peak for Triasulfuron, the pesticide solution was run at a wavelength range of 200 to 300 nm .
2.4. Data Analysis
The amount of the Triasulfuron herbicide adsorbed (μg g-1 of soil) was calculated by using Equation 1.

where Cs is the amount adsorbed, V is the volume of solution, m is grams of soil taken, Cb is equilibrium concentration of blank and Ca is equilibrium concentration of treatment supernatant. The adsorption values obtained from Equation (1) were used to construct linear type of isotherm Equation 2.

where Kd(ads) is linear or sorption equilibrium distribution coefficient in (mL μg-1). Ceis the concentration (μg mL-1) at the equilibrium concentration. Desorption is expressed as micrograms adsorbed per gram of soil (μg g-1 soil) and was calculated from the difference of solution remaining in the soil after the supernatant was poured off. The sorption equilibrium distribution coefficient Kd(des) in (mL μg-1) was calculated by Equation 3.

The adsorption isotherms of pesticides in all the soils fitted to the Freundlich adsorption relationship, Equation 4.

where Cs is the amount of adsorbed (μg g-1), Ceis the equilibrium concentration (μg mL-1)and Kf and n are constants. The Freundlich constant normalized to organic carbon (Kfoc) was calculated by using Equation 5.

where Kfoc and Kf are related by Equation 6.

The equilibrium organic matter (KOM) by normalizing Kd or by normalizing Kf with the content of organic matter was evaluated according to Equation 7 and Equation 8, respectively.

3. Results and Discussion
In the present research estimation of adsorption and desorption behaviour of Triasulfuron on selected soils was done to gain sorption values or sorption coefficients. The adsorption and desorption coefficients (distribution coefficient (Kd), Freundlich constant (Kf), standard deviation (S), Regression coefficients ( R2), Gibbs free energy (AG) and hysteresis (H) of Triasulfuron on different soils were determined as a function of characteristics of soils like SOM, CEC, pH, soil texture etc. Therefore various soil types of different characteristics were investigated in the present study in order to understand and predict extensive interactions of Triasulfuron with naturally occurring soils.
3.1. Characteristics of Soil Samples
To study adsorption and desorption behaviour of Triasulfuron four agricultural regions of Pakistan were selected for soil collection including Sialkot, Ormara, Gilgit and Karachi. The selection of sampling areas was based on the use of selected pesticides. Soil sampling was done by using the composites and random sampling while soil preparation was done by standard method. The soil physiochemical properties that are considered to be most important for sorption studies of pesticide are texture, organic matter (SOM), organic carbon (TOC), and pH. Other soil characteristics which may have influence on the sorption studies of a pesticide are the cation exchange capacity (CEC) and total nitrogen content (TN).19,24,25 These physical and chemical properties of four soils were determined and illustrated in Table 1.
The highest values of EC were obtained for sample 4 while lowest was observed for sample 1 (Table 1). This implies that though sample 4 showed more salinity as compare to rest of soil samples yet overall salinity is in medium ranges for all studied samples.
Sample 3 was characterized as acidic soil as compared to other three studied soils which were identified as alkaline (sample 2 and sample 4) and neutral soils (sample 1) by their pH analysis. However, all four soil samples are neither highly acidic nor highly alkaline.
OM is the most influential soil property especially in the present study. As depicted in Table 1, comparatively highest value of OM was found for forest soil 3 and least values of organic matter was found for coastal sample 4. Sample 2 has shown more potential to hold the positively charged ions as depicted by the CEC values. Sample 2 have comparatively more negatively charged situates on the surface which consequently adsorb and hold more cations by electrostatic forces as compare to other soil samples.26
Texture analysis of the soil samples depicts highest values of clay, silt and sand in Table 1. Highest value of sand content were found for sample 1 and highest value of clay content were found for sample 3 and vice versa.
In present study values of clay content varied among 13 % to 49.1 % whereas values of sand were ranging from 15 % to 77 % and values of silt follow the range from6%to25% thus demonstrating diverse range of characteristics. Sorption behaviour is highly evaluated on the bases of clay and sand content.
3.2. Adsorption Isotherms
In order to check the sorption behaviour of Triasulfuron in selected soils, the adsorbed and desorbed Triasulfuron concentration (ug mL-1) was plotted against equilibrium concentration of the Triasulfuron in each soil sample (Figs. 1 & 2). The experiments were run in duplicates in order to achieve the values for linear and Freundlich adsorption graphs. Linear and Freundlich adsorption parameters have been shown in Table 2 for selected soils. All soils exhibited C-type isotherm for adsorption of Triasulfuron with values of R2 approximating 1. C-type isotherm depicted that Triasulfuron was highly hydrophobic and thus it was remarkably distributed in soils posing challenges of environmental mitigation. Isotherms were further used for derivation of parameters like Kd, Kf, R2, AG and hysteresis (H).
3.2.1. Linear Adsorption Coefficient
Kd is the most important parameter of linear isotherm also known as partition/distribution coeffcient. Linear adsorption coefficient Kd was different in all soils and ranged in 4.2-11.9 μg mL-1 with soil 3 showing maximum adsorption of Triasulfuron and soil 4 was marked with lowest adsorption potential. Kd is considered as a fundamental parameter to enumerate risk and transport potential of pesticide. Besides Kd(ads) and movement of pesticide in soil are inversely correlated. Higher Kd(ads) value indicates strong affnity (less movement) of a pesticide for a soil while lower values of Kd specifies less affinity and increased possibility of pesticide movement through soils.22 Highest Kd(ads) was found for sample 3 while lowest adsorption Kd was found for sample 4 (Table 2). This indicates strong affinity of Triasulfuron for sample 3 which has resulted in highest adsorption. Whereas sample 4 has more risks of movement of Triasulfuron that has been evidenced by its Kd(ads) values. Decreasing order of Kd(ads) of Triasulfuron for all soils is as follows:
Soil 3 > soil 1 > soil 2 > soil 4
Strong adsorption of Triasulfuron can be attributed to its highest organic matter (matter (3.5 %), total organic carbon (2.03 %) and lowest pH (6.2) in addition to highest clay content (61 %).27 While lowest value of Kd(ads)was shown by soil 4 which is 4.2 μg mL-1 owing to low SOM and clay which could act as medium for enhanced adsorption. Pusino and coworkers studied the sorption behaviour of Triasulfuron in Italian soils. Their results were in accordance with the results of the current study indicating pH to be the most important factor governing the rate of adsorption.28 More studies are focused towards adsorption of Triasulfuron in neutral to acidic soils but Sarmah et al. have studied adsorption in alkaline soils of Australian origin. Their results clearly depicted the inverse relation of high pH soils with adsorption.29
3.2.2. Freundlich Adsorption Coefficients
Freundlich adsorption model was applied to study adsorption kinetics on the heterogeneous soil surface. Kf for Triasulfuron on selected soil samples are presented in Table 2 and all the four soil samples for Freundlich coeffient Kf were ranging from 2.1 μg mL-1 to 8.8 μg mL-1. Soil sample 3 expressed highest adsorption of 8.8 μg mL-1. The Freundlich constant Kf is an estimated indicator of capacity of pesticides adsorption. As Kf increases adsorption of pesticide also increases while desorption decreases. Desorption of pesticide increases with decreasing Kf(ads) This is because high value of Kf depicts least movement of pesticides through soil while lower Kf is indicator of least affinity of pesticides with soils.30 This elevation in Kf is because of higher soil organic matter (SOM) of soil sample which was 3.5 % as there is positive correlation between SOM and Kd. Another factor justifying the greater adsorption of Triasulfuron in soil 3 is the na value. Value of nais inversely proportion to the adsorption capability of soil. When n is equal to 1 the partition between the phases is not dependent on the concentration.31 It can be seen for soil 3 where na value is approximately equal to 1 (0.9). Lower values of n are indicative of higher adsorption and lower desorption while higher values of n are indicative of the low adsorption and enhanced desorption thus n is demonstrating inverse correlation with adsorption of pesticides.31 In present study nadsrange from 0.9 to 3.3. Similarly the nd values are used to check the intensity of desorption occurring in the soil samples.
3.2.3. Gibbs Free Energy Change (ΔG)
The free energy change ΔG for adsorption of the Triasulfuron on the soil samples is shown in Table 2. The values of ΔG were in the range -11 to -16 kJ mol-1. The negative value of AG indicated that the interaction of Triasulfuron on the selected soils was spontaneous and exothermic behaviour of the interaction.20 Moreover it is reported ΔG showed efficiency of reaction i.e. if pesticide sorption studies specify the absolute value of ΔG £ 40 kj mol-1, then it would depict physical adsorption of that pesticide with the particular soil otherwise it would indicate negative adsorption.32 In present research the ΔG values of Triasulfuron were proposing the physical adsorption of Triasulfuron with the soils involve weak Van der Waal's forces. Hence it is the weakest category of adsorption.31
3.2.4. Leachibility Index
The predicted capacity for Triasulfuron retention normalized to the OC content (i.e. Koc) distinguished expressively in studied soils as shown in Table 2. Koc and Kom are called Leachibility Index. Weaker adsorption as already depicted by lower negative values for AG was governing factor for higher mobility of Triasulfuron in tested soils. Higher Koc values were marked by strong affinity of pesticides towards soils and hence their revulsion towards movement.33 The strength of adsorption was directly dependent upon Koc values, thus soils having higher Koc values were not prone to fast leaching of Triasulfuron particularly soil 3 (1232 μg mL-1) restricting the faster mobity of Triasulfuron. While for the poor adsorbent soil 4, Koc value is oc lowest (352 μg mL-1) hence providing suitable conditions for Triasulfuron leaching to lower pedospheric compartments and thus posing ecological challenges with higher costs for mitigation. Koc and Kfoc determined the transportation and motile nature of Triasulfuron in different soil layers. As the attachment of Triasulfuron was on soil organic matter of soil textural class thus soil organic matter governed the Triasulfuron mobility in selected soils.34 Thus by augmenting the organic matter, total organic content, clay and by plummeting pH, adsorptive interactions can be enhanced resulting in decreased mobilities. Similarly the Fruendlich model organic coefficient also played decicive role in prediction of pesticide movement in soils. If Kfoc values are greater than 1000 μg mL-1, pesticides show higher movement in soil and Kfoc values lying between 150 μg mL-1 and 500 μg mL-1 are moderately mobile in soil and when more than 500 μg mL-1 so mobility is slow. Mobility in soil sample 3 was very slow as Kfoc is 909 μg mL-1 and sample 1, 2 and 4 show moderate movement as their values are less than 500 μg mL-1.Kfoc values ranged from 177 to 909 μg mL-1 with highest value allocated to soil 3.
3.3. Desorption Isotherms
All soils yielded C-type desorption isotherms for Triasulfuron for desorption studies as well. In order to achieve desorption graphs the samples were run in duplicates to ensure correctness. Desorption is reverse of adsorption so in alkaline soils more desorption occurs and in low pH soils desorption process takes place slowly. Kd(des) values varied between 5.4 to 14.0 μg mL-1. Soil 4 exhibited higher desorption Kd(des) = 14.0μgmL-1 for possessing all physicochemical parameters that enhances the escape of poorly adsorbed Triasulfuron molecules. Soils 1 and 3 had higher OM, higher clay content, higher CEC, good texture and low pH which developed durable adsorptive interaction with Triasulfuron due to which they were not desorbed readily. Soil 10 showed higest desorption values because of higher sand content which enhanced desorption. Moreover desorption values increased with increasing pH in the following order:
Soil 3 < soil 1 < soil 2 < soil 4
3.3.1. Hysteresis Phenomena
The ratio between n(des) and n(ads) measures the extent of irre-versibility of adsorption process, the ratio called the apparent hysteresis indicated greater or lesser irreversibility of adsorption in all soil samples. Largely binding by physical sorption is reversible whereas binding by chemisorption is irreversible. Furthermore chemisorbed chemicals can be incompletely desorbed depending upon desorption methodology or the nature of chemical bond.21 In present study desorption hysteresis coefficient, H was calculated (Table 3). The values of H were around unity indicating quick sorption in soil. The highest hysteresis was observed in Sialkot with 2.2 % organic matter. Although the occurrence of hysteresis indicates that the adsorption isotherms are slightly different from the desorption isotherms, however value of H close to 1 means that desorption process took place almost as quickly as adsorption did. The high value of Kd(ads) as compared Kd(des) shows that adsorption is irreversible. Desorption hysteresis can be related to the immobilization of pesticicdes in soil resulting from the irreversible chemical binding.35 Desorption hysteresis coefficient (H) in tested four soils ranged from 0.4 to 1.1.
3.3.2. Fitness of Sorption Models
The lower standard errors (S.E.) and higher values of coefficients, r2 are used to measure the goodness of fit or best fitting isotherm to the experimental data.36 R2 was obtained by fitting experimental data into the linear model in Tables 2 and 3. Linear model is found to be best fitted model for adsorption and desorption studies of Triasulfuron because of its higher values of R square (0.8 to 0.9 for adsorption and 0.8 to 0.9 for desorption) with lower S.E. (0.5 to 1.7 of adsorption and 0.07 to 0.2 for desorption).
Freundlich adsorption model was applied to study adsorption kinetics on the heterogeneous soil surface. The data presented in Table 3 fitted the empirical Equation 3 proposed by Freundlich model. The S.E and r2 of the each soil for Triasulfuron were presenting best-fitting Freundlich isotherm to the experimental data as illustrated in Tables 2 and 3. R2 of Freundlich adsorption isotherm were in the range from 0.9 (90 %) to 0.8 (80 %) and related S.E was varied from 0.03 to 0.08. The values of desorption r2 follow the range of 0.8 to 0.9 with S.E. 0.06 to 0.27. However, lower values of r2 and higher values of S.E. illustrate the worst fitted model while present study verified linear and Freundlich good fitted models for pesticides adsorption and desorption studies.36
3.4. Statistical Analysis
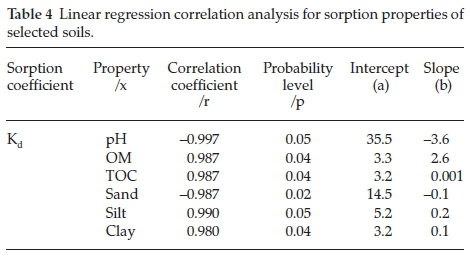
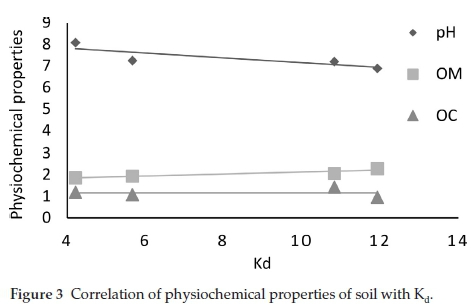
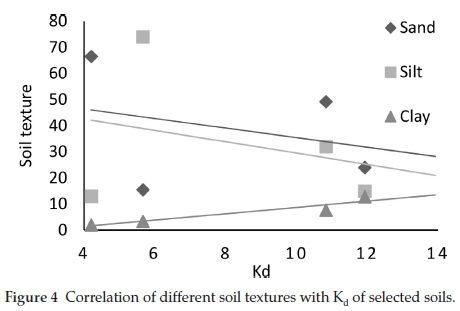
Further statistical investigations were done regarding the relationship between Kd(ads) and the physiochemical properties of soil including OM, TOC and pH and between Kd(ads) and soil textures (Table 4) (Figs. 3 & 4). Linear regression analysis was performed to analyze the effect on Kd of the physiochemical properties (Table 4). The analysis provided with the knowledge that pH is negatively correlated (r = -0.997) to Kd(ads) while OM and TOC were seen to be positively correlated (r = 0.987 and r = 0.987, respectively). Textural composition of soils also affected Triasulfuron. Kd and clay were found positively correlated (r=0.980) while presence of sand negatively impacted the Triasulfuron adsorption (r=-0.987). According to Fig. 3 the lowering pH augments the adsorption of Triasulfuron in soils. In a nutshell, soil pH values are accountable for the dissociation or protonation processes of both the Triasulfuron and the adsorbent surfaces (soils), whereas an increase in adsorption was observed with the increasing percentage of OM and TOC. The results also showed that the soil 3, having highest Kd(ads) value (11.9 g-1), contains relatively high percentage of soil OM (3.5 %), proving the fact that soil OM is directly proportional with the rate of adsorption occurring in that particular soil. Also the CEC values indicate that comparatively higher CEC values usually enhance adsorption either by ion exchange or surface precipitation.
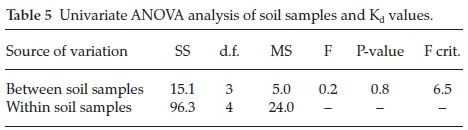
In order to compare means of soil samples and adsorption distribution coefficient Kd a univariate ANOVA test was performed on all four soil samples along with their Kd values. In the one way ANOVA sum of squares (SS), mean square (MS), F statistics (F), P-value and F critical values (F crit) were compared within the same groups and between groups (Table 5). For above test we assume that all observations are originating from normal distributions and all the four groups of soils have equal variances. In this case, the P-value (0.8) is greater than the alpha value (a) (0.05) so we do not reject our null hypothesis (H0) i.e, the assumption we made earlier. Also it can be observed from the table the F critical value (F crit) (6.5). This is the number given to us such that any number greater than the F crit value would cause us to reject our null hypothesis (H0). In this experiment the F statistics value (0.2) falls much lower than the F crit value hence it does not lie in the rejection region.
The effect of pH, OM and TOC on Kd was further studied in Minitab 17. Several residual graphs were plotted which determine the goodness of fit in ANOVA. From the residual plots we determine that the ordinary least square assumptions are being met. Satisfying these assumptions indicates, the ordinary least squares regression will produce unbiased coefficient estimates with the minimum variance. Normal probability plots of residuals, residuals versus fits and residuals versus order of data were plotted in Minitab (Fig. 5). Normal probability plot of residuals shows that our data are distributed normally. The residuals versus fits plot determine that our data has a constant variance.
4. Conclusion
Sorption of sulfonylurea herbicide, Triasulfuron (1-[2-(2-chloroethoxy)phenyl]sulfonyl-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl)urea) was performed with four different soil samples using Batch equilibrium method. The data obtained fitted well with linear and Freundlich models generating C-type isotherms in all cases. It was observed that adsorption tendency of Triasulfuron was highly dependent upon physiochemical make-up of soil samples, including pH, organic matter and texture. Adsorption was measured to be increasing with high values of soil organic matter and clay content and decreasing with increased pH. Statistical tools including ANOVA and regression were utilized to statistically evaluate the experimental results. Results of Gibbs free energy (AG) confirmed physical adsorption of Triasulfuron in soils. Triasulfuron exhibit lower to medium mobility with in selected soil samples. Desorption experiments showed inverse relation of herbicide with soil physiochemical properties than adsorption.
References
1 Z. Hussain, F. Munsif, K.B. Marwat, K. Ali, R.A. Afridi and S. Bibi, Studies on efficacy of different herbicides against weeds in potato crop in Peshawar, Pak. J. Bot., 2013, 45, 487-491. [ Links ]
2 H. Asiabi, Y. Yamini and M. Moradi, Determination of sulfonylurea herbicides in soil samples via supercritical fluid extraction followed by nanostructured supramolecular solvent microextraction, J. Super-crit. Fluid, 2013, 84, 20-28. [ Links ]
3 T. Bahrampor and P.S. Ziveh, Effects of residue sulfonylurea herbicides on wheat, Intl. J. Agron. Plant. Prod., 2013, 4, 2707-2713. [ Links ]
4 Y. Menchari, M. Annabi, H. Bahri and K. Latiri, Herbicides use in wheat crop in Tunisia: trends, variability and relation with weed resistance development, 2016, DOI: 10.5171/2016.990377. [ Links ]
5 D.R. Bajya, T. Parween, M.C. Lakharan and S.K. Raza, Efficacy of new formulations of triasulfuron on weeds in wheat (Triticum aestivum) and their residual effects on succeeding maize (Zea mays), Indian J. Agron., 2015, 60, 57-60. [ Links ]
6 M.S. Hayyat, M.E. Safdar, M. Akram and Z. Iqbal, Screening of herbicides for efficient control of broadleaf weeds in wheat (Triticum aestivum L.), Pak. J. Weed Sci. Res., 2016, 22, 365-379. [ Links ]
7 K.G. Chhipa and V. Nepalia, Effect of weed control and phosphorus sources on productivity of wheat (Triticum aestivum), Indian J. Agri. Res., 2015, 49. [ Links ]
8 R.S. Chhokar, R.K. Sharma, S.C. Gill and R.P Meena, Herbicides for broad-leaved weeds management in wheat, Indian J. Weed Sci., 2015, 47, 353-361. [ Links ]
9 A. Mehmeti, F. Musa, J. Demaj, M. Kamberi, I. Rusinovci and R. Kastrati, The effect of herbicides on the chemical content of wheat grain, Agr. Forest., 2016, 62, 117-123. [ Links ]
10 K.M. Pickett and V.D. Zheljazkov, Screening of preemergence and postemergence herbicides for weed control in dill (Anethum graveo-lens), fennel (Foeniculum vulgare), coriander (Coriandrum sativum), and basil (Ocimum basilicum), in medicinal and aromatic crops: production, phytochemistry, and utilization, Am. Chem. Soc., 2016, 103-119. [ Links ]
11 L. Bandzuchová, R. Selesovská, T. Navrátil and J. Chýlková, Sensitive voltammetric method for determination of herbicide Triasulfuron using silver solid amalgam electrode. Electrochim. Acta., 2013,113,1-8. [ Links ]
12 M. Knezevic, R. Balicevic, M. Ravlic and I. Ravlic, Effects of soil tillage and post-emergence herbicides on weed control and yield of winter wheat. In 48th Croatian and 8th International Symposium on Agriculture, (2014). [ Links ]
13 S.B. Singh, T.K. Das and G. Kulshrestha, Persistence of herbicide fenoxaprop ethyl and its acid metabolite in soil and wheat crop under Indian tropical conditions, J. Environ. Sci. Health., Part B., 2013, 48, 324-330. [ Links ]
14 S. Sondhia, Herbicides residues in soil, water, plants and non-targeted organisms and human health implications: an Indian perspective, Indian J. Weed Sci, 2014, 46, 66-85. [ Links ]
15 J. Fenoll, P. Hellín, P. Flores, C. M. Martinez and S. Navarro, Photocatalytic degradation of five sulfonylurea herbicides in aqueous semiconductor suspensions under natural sunlight, Chemosphere, 2012, 87, 954-961. [ Links ]
16 H. Ohkawa and H. Inui, Metabolism of agrochemicals and related environmental chemicals based on cytochrome P450s in mammals and plants, Pest Manag. Sci., 2015, 71, 824-828. [ Links ]
17 W Zhao, L. Xu, D. Li, X. Li, C. Wang, M. Zheng, C. Pan and L. Qiu, Biodegradation of thifensulfuron-methyl by Ochrobactrum sp. in liquid medium and soil, Biotechnol. Lett., 2015, 37, 1385-1392. [ Links ]
18 K. Fenner, S. Canonica, L.P. Wackett and M. Elsner, Evaluating pesticide degradation in the environment: blind spots and emerging opportunities. Science., 2013, 341, 752-758. [ Links ]
19 OECD Guideline for the Testing of Chemicals. Adsorption-Desorption Using a Batch Equilibrium Method, 2005. [ Links ]
20 M.R. Shariff, Effect of co-pesticide on adsorption-desorption process on agricultural soils, Int. J. Eng. Res. Develop., 2012,1, 55-69. [ Links ]
21 K.M. Doretto, L.M. Peruchi and S. Rath, Sorption and desorption of sulfadimethoxine, sulfaquinoxaline and sulfamethazine antimicrobials in Brazilian soils, Sci. Total Environ., 2014,476, 406-414 [ Links ]
22 S.Y. Gebremariam, M.W. Beutel, D.R. Yonge, M. Flury and J.B. Harsh, Adsorption and desorption of chlorpyrifos to soils and sediments. In Reviews of Environmental Contamination and Toxicolog., Springer, New York, 2012, 123-175. [ Links ]
23 P. Liang, J. Wang, G. Liu and J. Guan, Determination of sulfonylurea herbicides in food crops by matrix solid-phase dispersion extraction coupled with high-performance liquid chromatography, Food Anal. Methods, 2014, 7, 1530-1535. [ Links ]
24 A.M. Khairatul, C.K. Ngan and B.S. Ismail, Adsorption and leaching studies of molinate, carbofuran and propiconazole in Muda agricultural soils, J. Trop. Agric. Food Sci., 2013,41, 127-136. [ Links ]
25 U.S. EPA. 2008. EPA's Report on the Environment (ROE). U.S. Environmental Protection Agency, Washington, D.C., EPA/600/R-07/045F (NTIS PB2008-112484). [ Links ]
26 E. Kondrlova, D. Igaz, J. Horak and K. Halaszova, Soil texture analysis by optical method: laboratory experiment on sample preparation prior to analysis. International Multidisciplinary Scientific Geo-Conference: SGEM: Surveying Geology & Mining Ecology Management, 2013, 677. [ Links ]
27 K.R. Krishna and L. Philip, Adsorption and desorption characteristics of lindane, carbofuran and methyl parathion on various Indian soils, J. Hazard. Mater., 2008,160, 559-567. [ Links ]
28 A. Pusino, M.G. Fiori, I. Braschi and C. Gessa, Adsorption and desorption of triasulfuron by soil, J. Agri. Food Chem., 2003, 51, 5350-5354. [ Links ]
29 A.K. Sarmah, R.S. Kookana and A.M. Alston, Fate and behaviour of triasulfuron, metsulfuron-methyl, and chlorsulfuron in the Australian soil environment: a review, Aus. J. Agri. Res, 1998, 49, 775-790. [ Links ]
30 A.O. Dada, A.P. Olalekan, A.M. Olatunya and O. Dada, Langmuir, Freundlich, Temkin and Dubinin-Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk, IOSR J. Appl. Chem., 2012, 3, 38-45. [ Links ]
31 J. Ananpattarachai and P. Kajitvichyanukul, Adsorption and degradation of 2-chlorophenol by TiO2/AC and TiO2/CB in photocatalytic process, Chem. Eng., 2014,42, 157-162. [ Links ]
32 K.S. Ahmad, N. Rashid and M. Zakaria, Adsorption and desorption characteristic of metsulfuron methyl in Pakistani soils, J. Chem. Soc. Pak., 2015, 37, 380-388. [ Links ]
33 A. Pereira, E. Silva and M.J. Cerejeira, Applicability of the new 60 am polyethylene glycol solid-phase microextraction fiber assembly for the simultaneous analysis of six pesticides in water, J. Chromatogr. Sci., 2014, 52(5), 423-428. [ Links ]
34 M. Gavrilescu, Fate of pesticides in the environment and its bio- remediation, Eng. Life Sci., 2005, 5(6), 497. [ Links ]
35 Q. Wu, Z. Li, H. Hong, R. Li and W.T. Jiang, Desorption of cipro-floxacin from clay mineral surfaces, Water Res., 2013, 47, 259-68. [ Links ]
36 C. Chen, W. Zhou and D. Lin, Sorption characteristics of N-nitrosodi- methylamine onto biochar from aqueous solution. Bioresource Technol, 2015,179, 359-366. [ Links ]
Received 14 March 2017
Revised 5 October 2017
Accepted 14 November 2017
* E-mail: chemist.phd33@yahoo.com / chemist.phd33@fjwu.edu.pk














