Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Chemistry
versão On-line ISSN 1996-840X
versão impressa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.70 Durban 2017
http://dx.doi.org/10.17159/0379-4350/2017/v70a18
RESEARCH ARTICLE
Synthesis and photocatalytic activity of monolithic Fe2O3/TiO2
Wei ChangI', *; Maojin ZhangI; Xiaosai RenI; Andrew MillerII
ISchool of Environmental and Chemical Engineering, Xian Polytechnic University, Xian 710048, China
IIEmporia State University, Emporia, KS, 66801-5087, USA
ABSTRACT
The monolithic Fe2O3/TiO2 composites were synthesized via a one-pot sol-gel process with titanium tetrachloride as a Ti source and ferric chloride as a Fe source. The monolithic Fe2O3/TiO2 composites were characterized by scanning electron microscopy, powder XRD, N2/adsorption-desorption, fluorescence and UV-vis diffuse reflectance spectroscopy. The photocatalytic activity of the Fe2O3/TiO2 composites were evaluated by degradation of bisphenol A. The results indicated that, with the doping of Fe, the surface area of the monolithic Fe2O/TiO2 increased, the forbidden band width decreased and the electron-hole recombination rate decreased. The monolithic Fe2O3/TiO2 composite with Fe/Ti molar ratio of 0.03 had the best photocatalytic activity for degradation of bisphenol A.
Keywords: Sol-gel process, Fe2O3/TiO2, photocatalysis.
1. Introduction
In recent years, with the development of industry, the pollution of water has become a serious problem to the normal ecological environment. Typically, pharmaceuticals and personal care products (PPCPs), as a series of new reported contaminants, have had a persistent and unrecoverable impact on the environment even though their concentration in the environment is very low. Bisphenol A (BPA), one of the PPCPs, may potentially create adverse health effects. It is reported that BPA has been frequently detected in rivers and drinking water and is difficult to remove effectively with traditional methods.1,2
Nano TiO2 is an efficient photocatalyst and has attracted much attention with the increasing environmental problems because of its stable chemical properties, non-toxic character and no secondary pollution.3 However, TiO2 has a wide band gap energy of about 3.2 eV for the anatase structure and 3.0 eV for rutile structures and thus only a small fraction of the solar spectrum (5 %) is absorbed.4 On the other hand, the recombination probability of photo-generated electrons and holes is higher, leading to a lower photocatalytic efficiency. To extend the photo-response region of TiO2 from the UV to visible region and to overcome this electron-hole recombination, some different methods have been applied, such as doping with Nd, Cu, Fe and Ag,5-9 noble metal deposition,10-13 sensitization,14 composite formation,15 and the coupling of TiO2 with other metal oxides.16-20 Among them, ferric oxide is known to have a narrow band gap of 2.2 eV and can be used as a visible light photocatalyst in industrial applications due to its low cost, non-toxicity and high chemical stability.21 Fe2O3 modified TiO2was investigated extensively for its potential application in degradation of organic pollutants. It has been shown that combination of Fe2O3 and different TiO2 nanostructure can not only extend the spectra absorption range, but can also develop a defect structure for the separation of photo-generated electrons and holes. In addition, most of the reported materials have a nanostructure, which is difficult to separate after the photocatalytic reaction process. The film type photocatalysts increased the recovery efficiency but reduced the specific surface area, resulting in low photocatalytic activity.22
Therefore, how to remove all kinds of contaminants effectively and in an environmentally-friendly manner is becoming a focused area of current research. In this study, the monolithic Fe2O3/TiO2 was synthesized by the sol-gel process. The monolithic material has a macro and mesopore structure. Meanwhile, the Fe doping extends the range of the light response of the material. The photocatalytic activity of the monolithic Fe2O3/TiO2 was evaluated by degradation of BPA in aqueous solution using a 500 W xenon lamp.
2. Experimental
2.1. Materials
Titanium tetrachloride (TiCl4) was obtained from Tianjin Fuchen Chemical Reagents Co., Ltd. Poloxamer (F127) was purchased from BASF chemical company in Germany. Absolute ethyl alcohol (CH3CH2OH), furfuryl alcohol (FA,C5H6O2), ferric trichloride (FeCl3.6H2O), and bisphenol A(BPA) were obtained from Sinopharm Chemical Reagents Co., Ltd. Millipore water (R>18MQ*cm-1) was used in all experiments. All reagents were used as received.
2.2. Characterization
The morphology of all the samples was observed by scanning electron microscopy (SEM) performed on a QUATA FEG 450 field emission instrument operated at an accelerating voltage of 5.0 kV. The crystal structure of the samples were analyzed by XRD (Rigaku, Dmax-Rapid II) with Cu-Ka radiation (1 = 0.15418 nm, 40 kV, 40 mA). The scanning range was from 20 ° to 80 °. The surface areas of samples were determined from nitrogen adsorption-desorption isotherms at liquid nitrogen temperature using a 2390 Micromertics instrument. The photo-luminescence (PL) spectra of the samples were recorded by RF-5301PC (Japan) spectrofluorometer at the excitation wavelength of 350 nm. The scanning speed was 1200 nm min-1, the PMT voltage 700 V and the width of the excitation slit and emission slit was 10.0 nm. UV-vis diffuse reflectance spectra were recorded at room temperature on a Lambda 950 instrument.
2.3. Preparation of Monolithic Te2OjTiO2and TiO2
Firstly, 2.6 mL TiCl4 was slowly added into 56 mL ethyl alcohol in ice water bath, and then, 1.8 g F127 was dissolved completely in the above solution. Secondly, 225 μL FeC3 solution, and 2.27 mL FA were added to the mixture, stirring for 15 min. The molar ratio of Fe/Ti was 0.005, 0.01, 0.02, 0.03, respectively. The mixed solution was kept in an oven at 60 °C for 5 days, and then at 130 °C for 3 days. Finally, the sample was calcined at 450 °C for 2 h. The obtained samples with Fe/Ti molar ratio of 0.005, 0.01, 0.02,0.03 were marked as FT0.5, FT1, FT2, FT3, respectively. The prepared sample without iron was marked as FT0.23
2.4. Photocatalytic Activity Test
Photocatalytic performance of the prepared samples were investigated by degrading BPA. The photocatalytic experiments were conducted in a photocatalytic reactor (BL-GHX-V). In the experiment, a 500 W xenon lamp (190-1100 nm) was employed as the light source. Prior to irradiation, 50 mL of BPA (2 X 10-5 mol L-1) aqueous solution containing 30 mg sample was magnetically stirred in the dark for 60 min to achieve adsorption/desorption equilibrium and a good dispersion. During the photoreaction, the samples were collected at regular intervals (1 h) and centrifuged to remove the sample. The supernatant solution before and after degradation was analyzed by a UV-visible spectrophotometer (UV-2450, Shimadzu) at 225 nm. The remaining BPA concentration (%) after various time intervals could be estimated with the following equation:
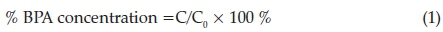
where C0 is initial concentration of BPA insolution,while Cisthe concentration at t h, respectively. The test process was repeated three times.
3. Results and Discussion
3.1. SEM of the As-prepared Material
Figure 1a, b, c, d shows the images of the prepared samples. It can be observed that the samples presented a honeycomb cell structure but with irregular continuous macropores. The macropore structure permits the liquid to enter and the light to penetrate, which is beneficial for organic molecular adsorption and photocatalytic degradation. Furthermore the monolith is easy to recover when it is used for wastewater treatment. The chemical composition of the samples was further characterized by EDS. Figs. 1e, 1f, 1g and 1h show the EDS spectra of the FT0, FT1, FT2 and FT3 samples, respectively. It indicates that the FT0 sample was, apart from O, mainly composed of Ti, while Fe and Ti were found in the other prepared samples.
3.2. X-Ray Diffraction (XRD) Patterns
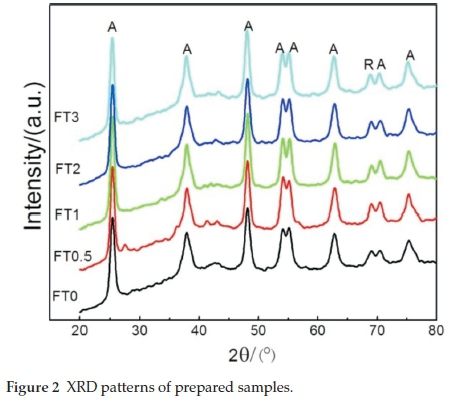
The XRD patterns of Fe2O3/TiO2 photocatalysts are shown in Fig. 2. The diffraction peak of Fe2O3/TiO2 photocatalysts at about 2Θ = 25.2 °, 37.8 °, 48.1 °, 54.1 °, 55.0 °, 62.7 °, 70.3 °, 75.1 ° could be perfectly indexed to the (101, (004, (200, (105), (211, (204), (215) crystal faces of anatase TiO2, respectively. And diffraction peaks atabout2q = 68.8°could be perfectly indexed to the (301) crystal faces of rutile TiO2. Meanwhile, with the increase of the Fe content, the position of the diffraction peak and intensity have changed little, and no new diffraction peaks appear. It indicates that the doping of Fe2O3 hardly affected the TiO2 crystallinity. It can be inferred that, because the ionic radius of Fe3+ (0.64 A) is only slightly smaller than that of Ti4+ (0.68 A), the Fe3+ can enter the lattice of TiO2 replace Ti4+.4,24,25
3.3. Nitrogen Adsorption-Desorption
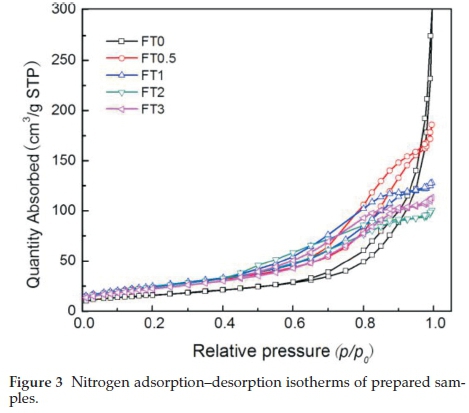
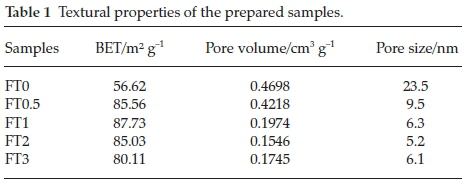
The nitrogen adsorption-desorption isotherms of Fe2O3/TiO2 photocatalysts are shown in Fig. 3. As can be seen from Fig. 3, all the samples have a type IV hysteresis loop according to the IUPAC classification. With the doping of iron, the appearance of the hysteresis loop moved to lower pressure, indicating that the pore size of the samples decreased. The specific surface areas of the samples calculated by the BET model and the pore size distribution calculated by the BJH model are shown in Table 1, which suggests that the specific surface area of the samples increased after doping iron. The average pore diameter of the prepared samples was between 5~25 nm, showing that the samples have mesoporous structure. The results suggested that Fe doping would affect the porous structure of the monolithic TiO2.
3.4. Photoluminescence Spectroscopy
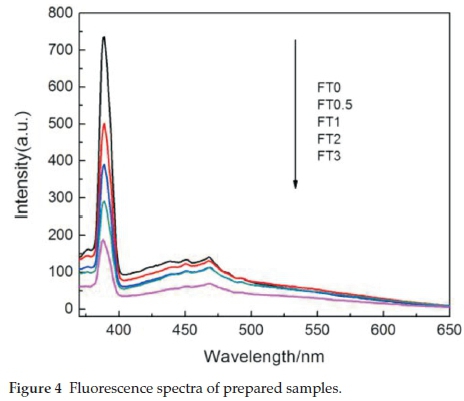
The photoluminescence spectra of all samples excited at 350 nm are shown in Fig. 4. The photoluminescence spectroscopy of semiconductor caused by the electronic-hole recombination, and the smaller the carrier recombination rate was, the lower photoluminescence intensity.26,27 As can be seen in Fig. 4, the intensity of PL decreased with the increasing Fe content, which implies the electron-hole recombination rate tends to be lower with the doping of Fe in the monoliths and hence have higher separation efficiency for electronic-hole pairs. When the recombination rate decreases, more photo-generated charge carriers can participate in the photochemical transformation, resulting in the enhancement of photocatalytic activity.28 It can be inferred that the Fe incorporated into the TiO2 can act as the electron-trapping agent to promote the electron-hole separation.
3.5. UV-vis Diffuse Reflectance Spectra
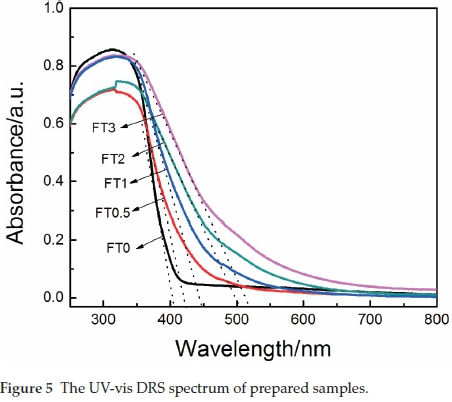
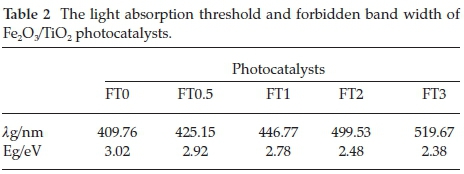
Figure 5 displays the DRS of monolithic TiO2 and Fe2O/TiO2 samples. Compared with the absorption spectrum of monolithic TiO2, there is an obvious absorption extending to more than 400 nm for the Fe2O/TiO2 sample, which is ascribed to the contribution from Fe2O3. According to the extension method,29 the light absorption threshold and forbidden band gap of samples were evaluated and the results are shown in Table 2. The band gap of monolithic TiO2 was calculated to be 3.02 eV The forbidden band gap decreased after doping with Fe, and when Fe2O/TiO2 composite with Fe/Ti molar ratio of 0.03, the forbidden band gap decreased to 2.38 eV. This is because the electronic structure of TiO2 were interfered after doping of Fe3+. Meanwhile, the d-d orbital transition (2T2g → 2A2g,2T1g) of Fe3+ or the charge transition (Fe3+ +Fe3+ → Fe2+ +Fe4+) between Fe3+ and Fe3+ can also cause the absorption band red shift.6
3.6. Photocatalytic Activity
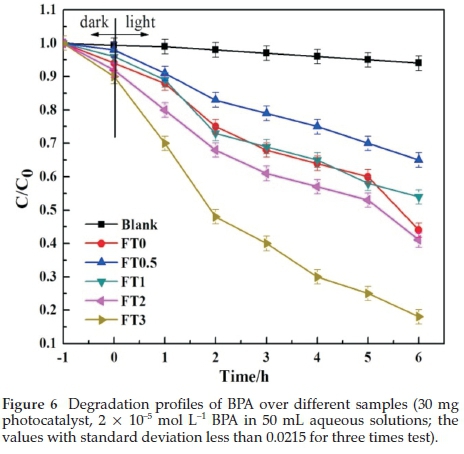
The photocatalytic activities of monolithic TiO2,Fe2O/TiO2 were evaluated by degradation of BPA in water with illumination. Control experiment indicates that BPA can be slightly degraded under 500 W xenon lamp irradiation without catalysts, which indicated that the photocatalytic process played the major role in the BPA degradation. Fig. 6 shows the degradation profiles of BPA wastewater under illumination with various photocatalysts. As one can see, the degradation of BPA by the FT3 is the most significant among all the prepared samples, about 85 % of BPA was degraded after 6 h. The improved activity can be ascribed to the narrow band gap and lower electronic-hole recombination rate of FT3. At the same time, doping of appropriate amounts of Fe can form more Fe (OH)2+.It is favourable for the photocatalytic reaction to have phenol hydroxyl groups and weak acidity. Therefore, a moderate amount of doping of Fe can improve the degradation rate of BPA effectively.
4. Conclusions
The monolithic Fe2O3/TiO2 photocatalysts were successfully synthesized via a one-pot sol-gel process. With the doping of Fe, the surface area of the monolithic Fe2O3/TiO2 increased, the absorption edge appeared red shifted and the electronic-hole recombination rate decreased and its quantum efficiency increased. Appropriate iron doping can improve the degradation rate of BPA effectively. In addition, the monolithic Fe2O/TiO2 photocatalysts are easier to recover in a practical application.
Acknowledgements
This work was supported by the Funds of Xi'an Polytechnic University Subject Construction (107090817), and the Cooperative Innovational Center for Technical Textiles, Shaanxi Province (2015ZX-25).
References
1 M. Auriol, Y.F. Meknassi and R.D. Tyagi, Endocrine disrupting compounds removal from wastewater, a new challenge, Process Biochem., 2006, 41, 525-539. [ Links ]
2 C. Kuo, C. Wu and H. Lin, Photocatalytic degradation of bisphenol A in a visible light/TiO2 system, Desalination, 2010, 256, 37-42. [ Links ]
3 N.S. Begum and H.M.F. Ahmed, Synthesis of nanocrystalline TiO2 thin films by liquid phase deposition technique and its application for photocatalytic degradation studies, Bull. Mater. Sci., 2008, 31, 43-48. [ Links ]
4 B. Kiliç, N. Gedik, S.P. Mucur, A.S. Hergul and E. Gür, Band gap engineering and modifying surface of TiO2, nanostructures by Fe2O3, for enhanced-performance of dye sensitized solar cell. Mater. Sci. Semicond. Process., 2015, 31, 363-371. [ Links ]
5 H. Sun, G. Zhou, S. Liu, H. Ang, M. Tadé and S. Wang, Visible light responsive titania photocatalysts codoped by nitrogen and metal (Fe, Ni, Ag, or Pt) for remediation of aqueous pollutants, Chem. Eng. J., 2013, 231, 18-25. [ Links ]
6 S. Wang, J.S. Lian and WT Zheng, Photocatalytic property of Fe doped anatase and rutile TiO2 nanocrystal particles prepared by sol-gel technique, Appl. Surf. Sci., 2012,263, 260-265. [ Links ]
7 W Lv, S.Y. Chen and D.L. Chen, Preparation of Zn doped TiO2 composite nanofibers by electrospinning, J. Mater. Sci., 2010,124,217-221. [ Links ]
8 S. Ding, G.W Zhu and R.W. Wang, Synthesis of carbon-doped mesoporous TiO2 visible-light photocatalytic materials via one pot solvo thermal method, Chem. J. Chinese Univ., 2014, 35,1016-1022. [ Links ]
9 M. Nasirian, C.F. Bustillolecompte and M. Mehrvar, Photocatalytic efficiency of Fe2O/TiO2 for the degradation of typical dyes in textile industries: effects of calcination temperature and UV-assisted thermal synthesis, J. Environ. Manage, 2017,196, 487-498. [ Links ]
10 P. Xu, Y.S. Li and C. Liu, Preparation and visible-light driven photocatalytic performance of mesoporous titania doped with silver, J. Chinese Ceramic Soc., 2014,14, 1195-1202. [ Links ]
11 M.V. Dozzi, S. Brocato, G. Marra, G. Tozzola, L. Meda and E. Selli, Aqueous ammonia abatement on Pt-and Ru-modified TiO2: selectivity effects of the metal nanoparticles deposition method, Catal. Today, 2017, 287,148-154. [ Links ]
12 C. Noberi, F. Kaya and C. Kaya, Synthesis, structure and characterization of hydrothermally synthesised Ag-TiO2, nano-structures onto Ni filters using electrophoretic deposition, Ceram. Int., 2016, 42(15), 17202-17209. [ Links ]
13 K. Tahir, A. Ahmad, B. Li, A.U. Khan, S. Nazir, S. Khan, Z.U.H. Khan and S.U. Khan, Preparation, characterization and an efficient photocatalytic activity of Au/TiO2 nanocomposite preparedby green deposition method, Mater. Lett, 2016,178, 56-59. [ Links ]
14 M. Gharagozlou and R. Bayati, Photocatalytic characteristics of single phase Fe-doped anatase TiO2 nanoparticles sensitized with vitamin B12, Mater. Res. Bull., 2014, 61, 340-347. [ Links ]
15 S.S. Lee, H. Bai, Z. Liu and D. Sun, Optimization and an insightful properties - Activity study of electrospun TiO2/CuO composite nanofibers for efficient photocatalytic H2 generation, Appl. Catal. B: Environ, 2013,140-141, 68-81. [ Links ]
16 A. Luengnaruemitchai, K. Srihamat, C. Pojanavaraphan and R. Wanchanthuek, Activity of Au/Fe2O3-TiO2, catalyst for preferential CO oxidation, Int. J. Hydrogen Energy, 2015, 40(39), 13443-13455. [ Links ]
17 J.Y. Li, Z.X. Song, P. Ning, Q.L. Zhang, X. Liu, H. Li and Z.Z. Huang, Influence of calcination temperature on selective catalytic reduction of NOx with NH3 over CeO2-WO3/TiO2 catalyst, J. Rare Earths, 2015, 33(7), 726-735. [ Links ]
18 M. Naimi-Joubani, M. Shirzad-Siboni, J.K. Yang, M. Gholami and M. Farzadkia, Photocatalytic reduction of hexavalent chromium with illuminated ZnO/TiO2composite,J. Ind. Eng. Chem.,2015,22,317-323. [ Links ]
19 K.R. Wee, B.D. Sherman, M.K. Brennaman, M.V. Sheridan, A. Nayak, L. Alibabaei and T.J. Meyer, An aqueous, organic dye derivatized SnO/TiO2 core/shell photoanode, J. Mater. Chem. A, 2016, 4(8), 2969-2975. [ Links ]
20 W. Deng, Q.G. Dai,Y.J. Lao, B.B. Shi andX.Y. Wang, Low temperature catalytic combustion of 1,2-dichlorobenzene over CeO2-TiO2 mixed oxide catalysts, Appl. Catal. B: Environ, 2016,181, 848-861. [ Links ]
21 J.Q. Li, D.F. Wang, Z.Y. Guo and Z.F. Zhu, Preparation, characterization and visible-light-driven photocatalytic activity of Fe-incorporated TiO2 microspheres photocatalysts, Appl. Surf. Sci., 2012, 263, 382-388. [ Links ]
22 S. Leong, A. Razmjou, K. Wang, K. Hapgood and X. Zhang, TiO2 based photocatalytic membranes: a review, J. Membr. Sci., 2014, 472, 167-184. [ Links ]
23 G.L. Drisko, A. Zelcer and X.D.Wang, Synthesis and photocatalytic activity of titania monoliths prepared with controlled macro- and mesopore structure, Appl. Mater. Inter., 2012,4, 4123-130. [ Links ]
24 J.F. Lei,X.P. Li, W.S. Li, Photocatalytic degradation of methyl orange on arrayed porous iron-doped anatase TiO2. J. Solid State Electrochem, 2012,16, 625-632. [ Links ]
25 Q.Q. Wang, S.H. Xu and F.L.Shen, Preparation and characterization of TiO2 photo-catalysts co-doped with iron (III) and lanthanum for the degradation of organic pollutants. Appl. Surf. Sci., 2011, 257, 7671-7677. [ Links ]
26 N. Wu, Q.F. Wei and L. Chen, Preparation, characterization and photocatalytic properties of Fe3+doped TiO2nanofibers, J. Textile Res., 2011, 32, 5-9. [ Links ]
27 G.H. Xu and J.G. Yu, Visible-light-induced photo-electrochemical behaviors of Fe modified TiO2 nanotube arrays, Nanoscale, 2011, 3, 3138-3144. [ Links ]
28 S.D. Deldkar, H.M. Yadav, S.N. Achary, S.S. Meena and S.H. Pawar, Structural refinement and photocatalytic activity of Fe-doped anatase TiO2 nanoparticles, Appl. Surf. Sci., 2012, 263, 536-545. [ Links ]
29 G.R. Yang, Q. Zhang, W. Chang and W. Yan, Fabrication of Cd1-xZnxS/ TiO2 heterostructures with enhanced photocatalytic activity, J. Alloy. Compd., 2013, 580, 29-36. [ Links ]
Received 14 March 2017
Revised 11 September 2017
Accepted 13 September 2017
* To whom correspondence should be addressed. E-mail: changwei72@163.com














