Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Chemistry
versão On-line ISSN 1996-840X
versão impressa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.70 Durban 2017
http://dx.doi.org/10.17159/0379-4350/2017/v70a8
RESEARCH ARTICLE
Synthesis, spectroscopic and DFT Characterization of 4β-(4-tert-Butylphenoxy)phthalocyanine positional isomers for non-linear optical absorption
Denisha GoundenI, II; Grace N. NgubeniII; Marcel S. LouzadaII; Samson KheneII; Jonathan BrittonII; Nolwazi NombonaI, *
ISchool of Chemistry and Physics, University of KwaZulu-Natal, Durban, 4000, South Africa
IIDepartment of Chemistry, Rhodes University, Grahamstown, 6140, South Africa
ABSTRACT
In this work the synthesis, spectral characterization and non-linear optical properties of metal-free 4y/-(4-ferf-butyl-phenoxy)phthalocyanine isomers are described and compared to the previously reported alpha derivative. The second-order nonlinear optical properties of the phthalocyanine isomers were investigated using the Z-scan technique and compared to the theoretical data obtained from density functional theory (DFT) and time dependent density functional theory (TD-DFT) calculations. Z-scan results indicated strong non-linear behaviour, revealing reverse saturable absorption (RSA) profiles for all four isomers. The experimental Bexp values showed the following trend: C4h (9.31 X 1010 mMW1)>D2h (7.89 X 1010 mMW-1)>Cs (7.32 X 10-10 mMW-1)>C2v (1.77 X 10-10 mMW-1). These results were similar to that obtained with the 4a-(4-to-f-butylphenoxy)phthalo-cyanines as the C2v and Cs isomers were found to have the lowest Bexp values compared to other symmetries. The 4ß-(4-tert-butylphenoxy)phthalocyanine C4h isomer was found to show better non-linear optical properties compared to all other isomers.
Keywords: Phthalocyanine, non-linear optical absorption, density functional theory, magnetic circular dichroism.
1. Introduction
Since their discovery, phthalocyanines (Pcs) have been utilized in diverse applications which include photovoltaic energy con-version,1 photodynamic therapy,2 electrocatalysis3 and semi-conductors.4 The use of Pcs for non-linear optical (NLO) absorption has found much interest in recent times5 due to its network of p-conjugated delocalized electrons. When interacting with strong electromagnetic fields such as laser radiation, the highly conjugated Pc structure promotes preferable polarizability and as well as fast distribution charge.6 Phthalocyanines are optical limiters that display reverse saturable absorption (RSA).7,8 This mechanism involves a sequential two photon absorption from the ground state (S0) to a higher excited state (S1). During excited state absorption, molecules are promoted from S1 to a higher excited state (Sn). Phthalocyanines are able to absorb intense light and upon their relaxation back to the ground state, they are able to intersystem cross from S1 to the excited triplet (T1) state. At this point the intense light would result in triplet-triplet absorption (T1 ® Tn) making Pcs excellent optical limiters.9,10
It is recognized that the asymmetrical electronic structure of Pcs induced by the introduction and variation of peripheral substituents enhances NLO properties. When peripheral sub-stituents are considered, a condensation reaction with a single phthalonitrile precursor can result in four constitutional isomers with Cs,C2v,C4h and D2h symmetries.6 Symmetry has been found to influence the NLO properties of Pcs.5 It has been reported that Pcs of lower symmetry show greater optical properties compared to Pcs of higher symmetry.11 For the purpose of this study, the isomers are labelled Cs,C2v,C4h and D2h to aid with the comparison to metallated phthalocyanine (MPc) complexes in literature.12,13 In this work, the Z-scan technique combined with DFT calculations will be employed to investigate the NLO properties of the four constitutional isomers of 43-(4-tert-butyl-phenoxy)phthalocyanine. Pure isomers may highlight interesting NLO properties which cannot be evaluated in a mixture of isomers.
2. Experimental
2.1. Materials
Acetic anhydride (Ac2O), acetone, 25 % ammonia solution (NH4OH), formamide (HCONH2), methanol (MeOH) and potassium carbonate (K2CO3) were obtained from Sigma-Aldrich or Merck. Dimethylformamide (DMF) was obtained from Sigma-Aldrich and dried using molecular sieves before use. Chloroform (CHCl3), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), 1-pentanol, high purity silica gel, silica gel TLC 60 F254 sheets, 4-tert-butylphenol and thionyl chloride (SOCl2) were purchased from Sigma-Aldrich. 4-Nitrophthalonitrile was synthesized and purified according to literature.14
2.2. Equipment
Thin layer chromatography was done on silica gel 60 F254 sheets. Column chromatography was achieved on silica gel 60 A (63-200 μιη). UV-vis absorption spectra were recorded on a Shimadzu UV-2250 spectrophotometer. Fourier transform infrared (FT-IR) spectra were obtained using a Perkin-Elmer Spectrum 100 FT-IR Spectrometer, equipped with a diamond crystal ATR accessory. Magnetic circular dichroism (MCD) spectra were recorded on a Chirascan Plus spectropolarimeter fitted with a permanent magnet, producing a magnetic field of
1T (tesla). Mass spectroscopy data were obtained using a Bruker AutoFLEX III smart-beam MALDI-TOF mass spectrometer, with an a-cyano-4-hydroxycinnamic acid matrix in the positive ion mode. 1H-NMR spectra were recorded using a Bruker EMX 400 MHz NMR spectrometer. Fluorescence lifetimes were recorded using a time correlated single photon counting (TCSPC) technique perfomed on a FluoTime 300 Easy Tau spectrometer (PicoQuant GmbH). A diode laser (LDH-P-670 with 20 MHz repetition rate, 44 ps pulse width, Pico-Quant GmbH) was used to excite the samples at 670 nm. Fluorescence was detected with a Peltier cooled photomultiplier tube (PMT) (PMA-C 192-M, PicoQuant GmBh). Decay curves measured at maximum emission. Lifetimes were obtained through the deconvolution of these curves using the FLUOFIT software program (PicoQuant GmbH, Germany). The standard Z-scan technique was employed to evaluate the nonlinear optical properties of the H2Pc isomers. Phthalocyanines T1-Tn absorption was achieved using a frequency-doubled Nd:YAG laser (Quanta-Ray, 1.5 J/10 ns FWHM pulse duration). The laser studies were performed using a near Gaussian transverse mode at 532 nm, utilizing a repetition rate of 10 Hz and an energy range of 0.1 μJ-0.1 mJ, monitored with an energy detector (Coherant J5-09). Mounting thermal nonlinearities were prevented by the low repetition rate of the laser. The laser beam was specifically filtered to limit the higher order modes and was focused using a 15 cm focal length lens. The stability of the system was achieved when there was no observable damage observed either between multiple runs or when the Pc samples were changed.
2.3. Synthesis of 4-ferf-Butylphenoxy Phthalocyanine (tBuH2Pc) Isomers
The isomers were synthesized using a procedure previously described.15 Briefly, 4-(4-íerí-butylphenoxy)phthalonitrile (0.75 g, 2.70 mmol) was transferred to a round-bottom flask to which pentanol (6 mL) was added. The mixture was refluxed with DBU (6 drops) under nitrogen for 5 h. Thereafter, the green slurry was allowed to cool to room temperature and MeOH (20 mL) was used to precipitate the crude dark green product. Excess MeOH was added and the mixture was left to stir for 1 h. The green solid was filtered under vacuum and left in air to dry overnight. The green product was loaded onto a silica gel column and the isomers were separated. The first three fractions (A, B and C) were eluted using DCM and each fraction was a different shade of green (Fig. 1). The fourth fraction (D) eluted as the darkest shade of green using MeOH and DCM (v/v; 1:5) as the solvent system. Figure 2 shows the four positional isomers (C4h,D2hh Cs and C2v)of4b-(4-feri-butylphenoxy)phthalocya- nines.
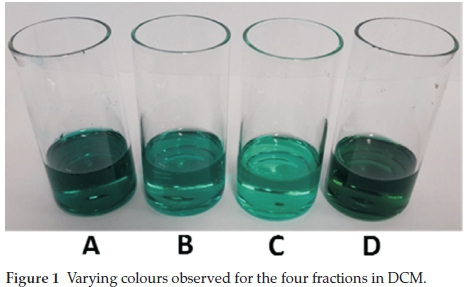
Fraction A: Yield: 100 mg (10 %). UV-vis (DCM): λmax nm (loge): 702 (5.02); 667 (4.97); 641 (4.58); 608 (4.39); 403 (4.35); 339 (4.39). IR (vmax cm-1): 1236 (C-O-C); 1367 (CH3); 1465, 1509, 1605 (C = C); 2853, 2922, 2955 (-C-H); 3042, 3294 (=C-H). MS (MALDI-TOF): Calculated: 1107 m/z. Found: 1109 m/z. Ή NMR (CDCl3): δ ppm 7.9-7.5 (28 - H, m, Pc; Ar-H); 1.5 (36-H, s, But)
Fraction B: Yield: 200 mg(20 %). UV-vis (DCM):λmaxnm (loge): 702 (4.69); 667 (4.63); 641(4.20); 607(4.01); 401 (4.01); 340(4.38). IR (vmax cm-1): 1234 (C-O-c); 1363 (CH3); 1465, 1506, 1602 (C=C); 2853, 2922, 2955 (-C-H); 3043, 3287 (=C-H). MS (MALDI-TOf): Calculated: 1107 m/z. Found: 1109 m/z. Ή NMR (CDCl3): δ ppm 7.9-7.5 (28 - H, m, Pc; Ar-H); 1-1.5 (36-H, s, But)
Fraction C: Yield: 150 mg(15 %). UV-vis (DCM):λmaxnm (loge): 702 (4.98); 667 (4.92); 640 (4.53); 608 (4.34); 400 (4.35); 340 (4.70).IR (vmax cm-1): 1238 (C-O-C); 1368(CH3); 1465, 1507, 1604 (C=C); 2853, 2923, 2955 (-C-H), 3047, 3295 (=C-H). MS (MALDI-TOf): Calculated: 1107 m/z. Found: 1109 m/z. Ή NMR (CDCl3): δ ppm 7.9-7.5 (28 - H, m, Pc; Ar-H); 1.5 (36-H, s, But)
Fraction D: Yield: 60 mg (6 %). UV-vis (DCM): λmax nm (log e): 705 (4.86); 673 (4.82); 649 (4.61); 621 (4.47); 391 (4.54); 338(4.84). IR (vmax cm-1): 1231 (C-O-C); 1366 (CH3); 1474, 1505, 1601 (C=C); 2851, 2921, 2955 (-C-H); 3041, 3286 (=C-H). MS (MALDI-TOf): Calculated: 1107 m/z. Found: 1109 m/z. Ή NMR (CDCl3): δ ppm 7.9-7.5 (28 - H, m, Pc; Ar-H); 1.5 (36-H, s, But)
2.4. Computational Details
The geometry optimizations of the H2Pc isomers were carried out by means of the B3LYP density functional with the 6-31G(d) basis set as applied in the Gaussian 03 software. The B3LYP exchange-correlation density functional uses Becke's method via his B88 exchange functional and the Lee-Yang Parr correlation functional, which incorporates a combination of semi-empirical Hartree-Fock and DFT exchange.16-19 The Gaussview 4.1 interface was employed for the visualizations of molecular orbitals (MOs) as well as other relevant properties.20 An identical method was used to perform single point energy calculations in order to determine the NLO response (ß) in accordance to literature techniques21 using H2(OH)4Pc as the standard compound. With H2(OH)4Pc used as the model compound, TD-DFT calculations were carried out using the B3LYP functional of the Gaussian 09 software package with 6-31G(d) basis set.22
2.4.1. Second-order NLO Optics Calculations
The equation used to calculate the magnitude of the first hyperpolarizability ofa3x3x3 matrix with 10 components (Equation (S1) in Supplementary Material) was determined by performing DFT calculations according to literature.21
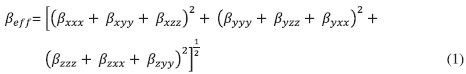
Since the ßjjkvalues provided by the Gaussian 03 software are reported in Debye, A2, the calculated ßeff values were converted to electrostatic units (esu), (1Å2 = 1 x 10-30 esu).
2.4.2. Octupolar and Dipolar Contribution Calculations
DFT calculation of hyper-Rayleigh Scattering (HRS) response coefficient (ßHRS) was carried out in order to calculate the first static hyperpolarizability (second-order hyperpolarizability), following literature methods23-26 The advantage of this method is that octupolar and dipolar second-order NLO contributions are theoretically separated. The values of both dipolar (ßJ=1) and octupolar (ßJ=3) are known to be significantly influenced by the number of electrons in the system.23 Due to symmetry constraints there is no permanent dipole moment for octupolar molecules,25 hence octupolar molecules present an isotropic ß tensor.
It is to be noted that the equations presented below are only valid in the off-resonance region. The following equations can be used to calculate the (ßHRS) response; however, reference [21] method as indicated above was used to calculate the second-order nonlinear coefficient. In Equation (2), ( ß2ZZZj and ( ß 2ZXX) are the orientation average of the molecular ß tensor components.
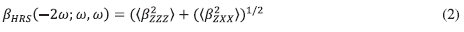
The molecular B tensor were calculated using Equations (3) and (4)23:
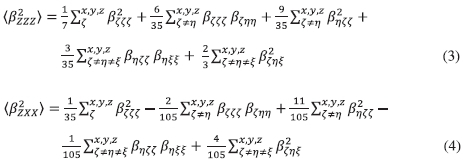
Furthermore, the molecular geometric information is given by the depolarization ratio (DR), which is expressed by DR = (ß2zzz)/(ß2zxk). The nature of the symmetric Rank-3 ß tensor is further clarified by decomposing (ß2HRS) as the sum of the dipolar (ßJ=1) and octupolar (ßJ=3) tensorial components,25 which are expressed as Equations (6) and (7):
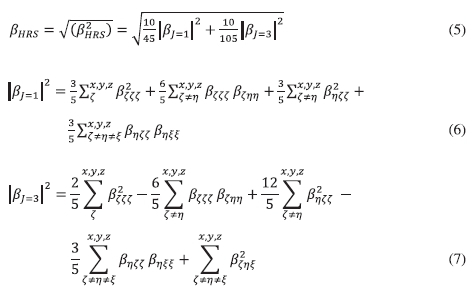
The ratio of octupolar [ΦJ=3= p/(1 + p)] and dipolar [Φ J=1 = 1/(1 + p)] contribution to the hyperpolarizability tensor is determined by substituting the nonlinear anisotropy parameter P =1 ßJ=3|/| ßJ=1|. The nonlinear anisotropy parameter values run from 0 (pure dipole) to ¥ (pure octupole).
The theoretical normalized HRS intensity  is determined by using Bersohn's expression,25 Equation (8), which assumes a general elliptically polarized incident light propagating along the X direction. Equation (8) further assumes that the intensity of the harmonic light is scattered at 90 ° along the Y direction and the vertically (V) polarized light along the Z axis.
is determined by using Bersohn's expression,25 Equation (8), which assumes a general elliptically polarized incident light propagating along the X direction. Equation (8) further assumes that the intensity of the harmonic light is scattered at 90 ° along the Y direction and the vertically (V) polarized light along the Z axis.
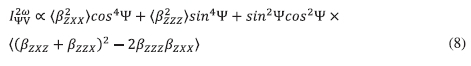
The orientational averages <(ßzxz + ßzzx)2 -2ßzzzßzxx) in Equation (8) is expressed as Equation (9):
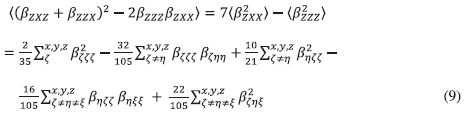
3. Results and Discussion
3.1. Synthesis and Characterization 4ß-(4-ferf-Butyl- phenoxy)phthalocyanines (tBuH2Pc) Isomers
The 4-nitrophthalonitrile was converted to the 4-tert-butyl phenoxy phthalonitrile through a base catalyzed displacement of the nitro functional group by 4-tert-butyl phenol. Cyclo-tetramerization of the 4-tert-butyl phenoxy phthalonitrile yielded the corresponding tBuH2Pc isomers. The tBuH2Pc was soluble in organic solvents such as chloroform (CHCl3), DCM and THF. The dark green complex underwent purification and isomer separation by column chromatography.
The C-O-C vibration at 1248 cm-1 verified the formation of the 4-(4-tert-butylphenoxy)phthalonitrile. Conversion into the corresponding tBuH2Pc was indicated by the loss of the intense -CN stretch at 2232 cm-1. MALDI-TOF spectral data revealed molecular ion peaks at 1109 m/z for all isomers. This mass differs from the expected mass by 2 protons due to possible protonation of the nitrogen atoms of the Pc. This mass confirms the proposed
Pc structure. Ring protonation is common during the ionization process of Pcs.27 The 1H NMR of the isomers in CDCl3 showed similar and broad aromatic peaks between 7 and 8 ppm and the integration of the peaks gave a total of 28 protons. The extended delocalized aromatic p electron cloud causes extensive stacking of the planar Pc macrocycles.28 This aggregation significantly affects proton chemical shifts and results in weak and broad peaks.29 Differentiation between the protons was difficult as the 4-tert butyl aromatic protons were deshielded such that the signals overlapped with those of the Pc ring. The internal protons which are usually observed between 2 and 6 ppm, were not observed for all isomers as the localization of these chemical shifts are also highly influenced by aggregation.
3.2. Electronic Absorption Spectroscopy, Magnetic Circular Dichroism (MCD) Spectroscopy and TD-DFT Calculations
UV-vis spectra obtained were typical for unmetallated Pcs and all fractions showed similar absorption (Fig. 3). The spectra showed a split Q band in the red region and the B band in the blue region. The B and Q bands are characteristic to Gouterman's 4-orbital model30 for D4h symmetry of a metal porphyrin. The Q band observed in the 667-705 nm region for the isomers are associated with x/y-polarization. This is linked primarily to excitation from the highest occupied molecular orbital (HOMO, a1u) to the lowest unoccupied molecular orbital (LUMO, eg). The B bands (assigned B1 and B2) correspond to transitions from the a2u and b2u to the eg level.31 The Q bands were blue shifted compared to the a derivative.13 This shift is as a result of an increase in the HOMO-LUMO energy gap which is highly influenced by electron density. The electron-withdrawing nature of the substituents at the alpha position results in the lowering of the energy gap.
Magnetic circular dichroism (MCD) was used to obtain information regarding the degeneracy of ground and excited electronic states of the isomers. The absorption and MCD spectra are shown in Fig. 3 for Fractions A and D. Fraction A displayed similar spectral properties as Fractions B and C. The Q band of Fraction D displays a slight deviation showing less splitting, attributed to lower symmetry than the other isomers. The MCD spectra show the B0 term, demonstrating that the B1 and B2 bands appear in the 300-400 nm region.32 The absorption between 400 and 470 nm was attributed to the n → π transitions. This transition occurs either as a result of the lone pairs on the outer oxygen atoms13,33 or the destabilization of the sp3 hybridized oxygen atoms on the p-MOs.34 Figure 4 supports the n → π transitions being due to the delocalization of molecular orbitals on the oxygen atom for the four regio isomers. When comparing the optimized energies of the isomers, no significant difference was observed. This indicates that changing the position of the substituents does not change the minimum energy of this compound.
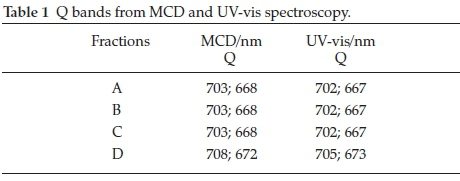
The curves observed in the 650-700 nm region of the MCD spectra each have a centre essentially at the same wavelength as that of the maximum absorption. A comparison of the UV-vis and MCD spectra lead to an unambiguous assignment of the Q bands for the fractions displayed in Table 1.
The TD-DFT results (Fig. 5) were identical for the isomers and were consistent with the experimental absorption spectra. The assignment of the Q and B bands are based on the allowed transitions between the molecular orbitals, which does not account for the intensity of the peaks. The Q band arises from the a1u (HOMO) to the eg (LUMO) and the B band from a2u and b2u orbitals to the eg orbital. The point groups of unmetallated Pc isomers lack a four-fold symmetry. This property results in the Q band being split into x- and y-polarized bands as observed in the UV-vis spectra. The TD-DFT calculations were performed under vacuum conditions and the discrepancy in peak intensity between the experimental and theoretical data could be due to solvent effects.35
3.3. Fluorescence Spectra
The excitation spectra (Fig. 6) for Fractions A-C were identical to their respective absorption spectra. The excitation spectra of Fraction D differed from its absorption spectra with changes to the position and shape of the Q band. This suggests a slight change of symmetry upon excitation of the Fraction D isomer.
The differences that are observed in the absorbance and excitation spectra in Fraction D are also attributed to aggregation in the latter. Since aggregates do not fluoresce, a similar behaviour is not seen in the excitation spectrum. There were no symmetry changes observed on the emission spectra of the tBuH2Pcs.
Table 2 shows the excitation and emission spectra, fluorescence lifetime (t) and anisotropy rotational correlation time (f) of the isomers. The fluorescence lifetimes of the isomers were measured and ranged between 5.5 and 5.8 ns, within the typical range observed for monomeric Pc compounds.13,36,37 The fluorescence lifetimes observed for the b derivative are found to be greater than those observed for the a derivative. The chi-square values were between 0.99 and 1.01, describing a good fit of the mono-exponential decay curves to the experimental data. The rotational correlation time measures the time taken for each of the four isomers to rotate one radian. Fractions A-D measured 0.42, 0.43, 0.40 and 0.49 correlation times (ns) respectively. The Fraction D appears to have a greater correlation time than the other isomers. This trend has been reported in a similar study of isomers by Ngubeni et al.13Rotational correlation times are influ Nenced by solvent viscosity, the shape and size of the molecule.38 The isomers showed differences in rotational correlation times. Since the experiments were carried out in DCM and the isomers have the same molecular weight, this difference can then be associated with the orientation of the isomer structures. Fraction D showed the largest correlation time indicating that this isomer may experience larger solvent resistance during its rotations.13 This also suggests that the molecular structure of the isomer affects t, as it exhibits a shorter t than the other isomers. Typical correlation times for Pcs are within the 0.3-4 ns range.39 The molecular volume of each isomer was determined using the following Equation (10)40:

were η the solvent viscosity, V the molecular volume, k is the Boltzman constant and T the absolute temperature. The calculated molecular volume values are shown in Table 2. The experimental molecular volumes for the isomers were 4.26 X 10-27 m3, 4.31 X 10-27 m3, 4.07 X 10-27 m3 and 4.93 X 10-27 m3, respectively. These experimental volumes were within range of the theoretically calculated molecular volume of 1.29 X 10-27 m3 for an unsubstituted Pc compound.13 In comparison with the a derivative, these values are significantly larger and this suggests greater solvent interaction for the ß derivative.
3.4. Characterization of the Positional Isomers using Non-linear Optical (NLO) Parameters and DFT Calculations
The Z-scan technique was the experimental method used to measure nonlinear absorption. The open aperture Z-scan experiment was carried out in DCM. The curves were similar for all the isomers, displaying strong non-linear absorption behaviour with reverse saturable absorption (RSA) profiles (Fig. 7). The second-order hyperpolarizability, (ßeff) values were calculated using Equation (11) and can be seen in Table 3. The ßeff value characterizes the strength of nonlinearity of the non-linear plots. Non-linear fits of q0(zs) (Fig. 7) were constructed to obtain the experimental ßexp values of the respective isomers. Theßexp values are compared with those reported previously for the a deriva-tives41,42 and display the following trend: Fraction A (9.31 X 10-10 mMW-1)> Fraction B (7.89 X 10-10 mMW-1)> Fraction C (7.32 X 10-10 mMW-1) > Fraction D (1.77 X 10-10 mMW-1). Substitution at the a positions causes complex charge distribution which significantly affects the molecular orbitals of the Pc ring. It is expected that substitution at the β position will have no effect on the Pc's molecular orbitals. As a result the influence of substitution position can be used to relate its effect on NLO properties.
The ßexp observed for the β derivative are smaller than those observed for the a derivative, this correlates well with the fluorescence lifetimes. Since long fluorescence lifetimes suggests low triplet state population, a low populated triplet state results in a low ßexp value.43.
The symmetry of the four fractions was confirmed by means of DFT analysis of the calculated ßeff values, as ßeff values are sensitive to symmetry.44 Table 3 shows the measured and DFT calculated ßeff values used to certify the symmetry of each isomer.
The symmetry assignment was found to follow a similar trend to symmetry assignment for 4a-(4-tert-butylphenoxy)phtha-locyanines,13 apart from the C2v and Cs symmetries. The C2v ßexp value for 4a-(4-tert-butylphenoxy)phthalocyanines was found to be higher with respect to Cs symmetry. However, similar to the 4a-(4-tert-butylphenoxy)phthalocyanines the C2v and Cs ßexp values were found to be the lowest compared to other symmetries. Similar to 4a-(4-tert-butylphenoxy)phthalocyanines, the theoretical beff values indicate that the C4h has the highest NLO response. The ßeff values for C4h and D2h isomers of 4ß-(4-tert-butylphenoxy)phthalocyanines where found to have a very small difference, making it ambiguous to assign based on theoretical calculations only. Hence the assignment was also based on the assignment observed for 4a-(4-tert-butylphenoxy)phtha-locyanines.13
The optimized geometries of the isomers were simulated by DFT calculations (Fig. 8). The theoretical molecular anisotropy ratio (p), depolarization ratio (DR), dipolar (f J=1) and octupolar (Φ j=3) contributions values were calculated using Equations (2-9) respectively and can be viewed in Table 4.
The dipolar (Φ J=1) and octupolar (Φ j=3) contribution trend of the four positional isomers (Table 4) suggest the following trend of decreasing dipolar contribution C4h >D2h >Cs >C2v. This study has not been done for the a derivative hence no comparison can be made.
The molecular anisotropy ratio (o) was employed to evaluate the ratio of dipolar and octupolar contributions to the hyperpolarizability tensor. The o ratio ranges between 0 (pure dipolar) and∞ (pure octupolar).23 The ρ for the four isomers suggest that C2v has the most octupolar contribution. The theoretical ßeff values follow a similar trend to dipolar contribution, the greater the dipolar contribution the greater the ßeff values.
To compare the relative magnitude of the dipolar and octupolar components of the second-order NLO response, fJ=1 and f J=3 values were plotted with respect to p. Figure 9 provides a quantitative classification of the NLO system with regard to dipolar or octupolar character of the isomers. The C4h and D2h isomers fall to the left of the ρ = 1 axis indicating a pronounced dipolar character. The Cs and C2v isomers fall to the right of the p = 1 axis indicating a pronounced octupolar character.
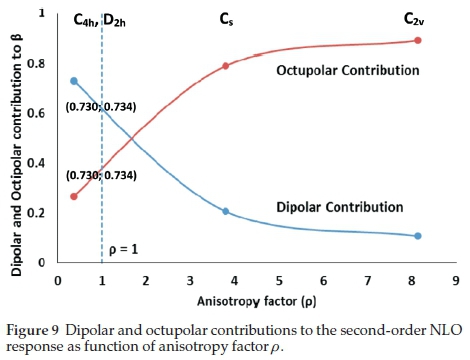
The depolarization ratio (DR) was assessed to provide supporting information on the molecular geometries of the isomers. The minimal depolarization for dipoles is (p=0)Dmin = 4/27 = 0.14845 and the maximum depolarization for pure octupoles is (p = ∞)Dmax = 2/3 = 0.667.45,46 The Cs and C2v isomers had DR values of 0.400 and 0.499 reflecting their octupolar character. The C4h and D2h isomers had DR values of 0.047 and 0.045 reflecting their strong dipolar character towards NLO activity.
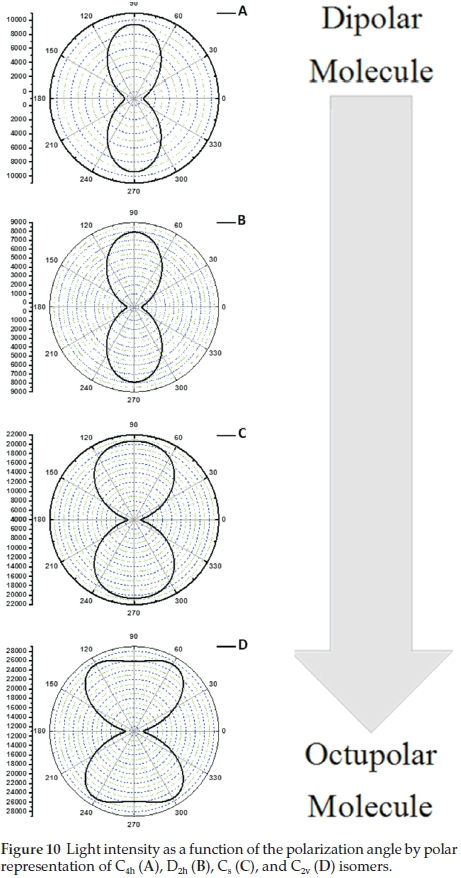
Figure 10 displays the polar plot of the harmonic light intensity as a function of the incoming fundamental beam polarization angle. The graphs provide a graphical interpretation of the dipolar and octupolar parameters of the isomers. The results coincide with Fig. 9, indicating that the C4h and D2h isomers can be considered dipolar molecules with dipolar contributions of 73 % each. The Cs and C2v isomers display strong octupolar character with 79 % and 89 % octupolar contributions respectively. Octupolar molecules have been used as NLO chromo-phores as they lack a permanent dipole moment. They can form noncentrosymmetric bulk materials to obtain a large macroscopic second-order NLO response.23,46,47 Due to their larger octupolar contributions the Cs (79 %) and C2v (89 %) H2Pc isomers are more suitable in films (or bulk material) for NLO applications, which makes use of second-order hyperpolarizability than the C4h (73 %) and D2h (73 %) tBuH2Pc isomers.
4. Conclusion
According to the characterization techniques used, it can be concluded that the proposed isomers of metal-free 4ß-(4-tert-butylphenoxy)phthalocyanine were successfully synthesized and separated. The experimental volume values of the isomers were determined to be within range of the theoretically calculated molecular volume of 1.29 x 10-27 m3 for an unsubstituted Pc compound. The fluorescence lifetimes of the isomers were measured and found to be in the 5.5-5.8 ns range, which is typical for monomeric Pc compounds. The non-linear optical behaviour of the isomers showed reverse saturable absorption with the higher symmetry C4h isomer displaying enhanced NLO properties. A similar ßexp value trend was observed for the 4a-(4-tert-butylphenoxy)phthalocyanine.13 The isomers with high dipolar
contribution (C4h (73 %) and D2h (73 %)) showed better NLO absorption coefficient with respect to other isomers in solution. However, theoretically calculated octupolar ratio suggest that Cs (79 %) and C2v (89 %) H2Pc isomers are better suited for application as films compared to C4h (73 %) and D2h (73 %) H2Pc isomers. This applies for applications which make use of second-order hyperpolarizability (ßeff).
Acknowledgements
This work was supported by the Department of Science, Republic of South Africa, and the National Research Foundation through the Thuthuka funding instrument (Grant number 94046). The authors are thankful to the University of KwaZulu-Natal and Nanotechnology Innovation Center (NIC) based at Rhodes University.
References
1 W. Shi, B. Peng, Y. Guo, L. Lin, T. Peng and R. Li, Synthesis of asymmetric zinc phthalocyanine with bulky diphenylthiophenol substituents and its photovoltaic performance for dye-sensitized solar cells, J. Photochem. Photobiol., A, 2016, 321, 248-256. [ Links ]
2 N. Nombona, K. Maduray, E. Antunes, A. Karsten and T. Nyokong Synthesis of phthalocyanine conjugates with gold nanoparticles and liposomes for photodynamic therapy, J. Photochem. Photobiol., B, 2012, 107, 35-4. [ Links ]
3 E.T. Saka, G. Sarkia, H. Kantekin and A. Koca, Electrochemical, spectroelectrochemical and catalytical properties of new Cu(II) and Co(II) phthalocyanines, Synth. Met., 2016, 214, 82-91. [ Links ]
4 Z. Wang, M. Lee, E.S. Choi, J. Poston and M.S. Seehra, Low temperature, high magnetic field investigations of the nature of magnetism in the molecular semiconductorß-cobalt phthalocyanine (C32H16CoN8), J. Magn. Magn. Mater., 2016,407, 83-86. [ Links ]
5 G. de la Torre, P. Vazquez, F. Agulló-López and T. Torres, Role of structural factors in the nonlinear optical properties of phthalocyanines and related compounds, Chem. Rev., 2004, 104, 3723-3750. [ Links ]
6 A. Bilgin, B. Ertem and Y. Gök, Highly organosoluble metal-free phthalocyanines and metallophthalocyanines: synthesis and characterization, Eur. J. Inorg., 2007,12, 1703-1712. [ Links ]
7 N. Venkatram, D. Narayana Rao, L. Giribabu and S. Venugopal Rao, Femtosecond nonlinear optical properties of alkoxy phthalocyanines at 800 nm studied using Z-Scan technique, Chem. Phys. Lett., 2008, 464, 211-215. [ Links ]
8 R.S.S. Kumar, S. Venugopal Rao, L. Giribabu and D. Narayana Rao, Femtosecond and nanosecond nonlinear optical properties of alkyl phthalocyanines studied using Z-scan technique, Chem. Phys. Lett., 2007, 447, 274-278. [ Links ]
9 J.P. Fitzgerald, P.D. Huffman, I.A. Brenner, J.J. Wathen, G. Beadie, R.G.S. Pong, J.S. Shirk and S.R. Flom, Synthesis, chemical characterization and nonlinear optical properties of thallium(III) phthalo- cyanine halide complexes, Opt. Mater. Express, 2015, 5, 1560-1578. [ Links ]
10 Y. Zhang, Z. Zhao, C. Yao, L. Yang, J. Li and P. Yuan, The nonlinear absorption and optical limiting in phenoxy-phthalocyanines liquid in nano- and femto-second regime: experimental studies, Opt. Laser Technol., 2014, 58, 207-214. [ Links ]
11 O.M. Bankole and T. Nyokong, Nonlinear optical response of a low symmetry phthalocyanine in the presence of gold nanoparticles when in solution or embedded in poly acrylic acid polymer thin films, J. Photochem. Photobiol., A, 2016, 320, 8-17. [ Links ]
12 J. Mack and N. Kobayashi, Low symmetry phthalocyanines and their analogues, Chem. Rev, 2011,111, 281-321. [ Links ]
13 G. Ngubeni, J. Britton, J. Mack, E. New, I, Hancox, M. Walker, T. Nyokong, S.T. Jones and S. Khene, Spectroscopic and nonlinear optical properties of the four positional isomers of 4a-(4-tert-butyl- phenoxy)phthalocyanines, J. Mater. Chem. C, 2015, 3, 10705-10714. [ Links ]
14 J.G. Young and W. Onyebuagu, Synthesis and characterization of di-disubstituted phthalocyanines, J. Org. Chem.,1990,55,2155-2159. [ Links ]
15 P. Tau and T. Nyokong, Synthesis and electrochemical characterisation of a- andb-tetra-substituted oxo(phthalocyaninato) titanium(IV) complexes, Polyhedron, 2006, 25, 1802-1810. [ Links ]
16 A.D. Becke, Density-functional thermochemistry. III. The role of exact exchange J. Chem. Phys. 1993, 98, 5648-5652. [ Links ]
17 C. Lee, W. Yang and R.G. Parr, Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density, Phys. Rev. B, 1988, 37, 785-789. [ Links ]
18 S.H. Vosko, L. Wilk and M. Nusair, Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis, Can. J. Phys., 1980, 58, 1200-1211. [ Links ]
19 P.J. Stephens, F.J. Devlin, C.F. Chabalowski and M.J. Frisch, Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields, J. Phys. Chem. 1994, 98, 11623-11627. [ Links ]
20 M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, J.A. Montgomery Jr., T. Vreven, K.N. Kudin, J.C. Burant, J.M. Millam, S.S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G.A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J.E. Knox, H.P. Hratchian, J.B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, P.Y. Ayala, K. Morokuma, G.A. Voth, P. Salvador, J.J. Dannenberg, V.G. Zakrzewski, S. Dapprich, A.D. Daniels, M.C. Strain, O. Farkas, D.K. Malick, A.D. Rabuck, K. Raghavachari, J.B. Foresman, J.V.D.J. Fox, T. Keith, M.A. Al-Laham, C.Y. Peng, A. Nanayakkara, M. Challacombe, P.M.W. Gill, B. Johnson, W. Chen, M.W. Wong, C. Gonzalez and J.A. Pople, Gaussian 03, Revision E.01, Gaussian, Inc., Wallingford, CT, 2004. [ Links ]
21 P.S. Liyanage, R.M. de Silva and K.M. Nalin de Silva, Nonlinear optical (NLO) properties of novel organometallic complexes: high accuracy density functional theory (DFT) calculations, J. Mol. Struct.: THEOCHEM, 2003, 639, 195-201. [ Links ]
22 M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G.A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H.P. Hratchian, A.F. Izmaylov, J. Bloino, G. Zheng, J.L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J.A. Montgomery, Jr., J.E. Peralta, F. Ogliaro, M. Bearpark, J.J. Heyd, E. Brothers, K.N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J.C. Burant, S.S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J.M. Millam, M. Klene, J.E. Knox, J.B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, R.L. Martin, K. Morokuma, V.G. Zakrzewski, G.A. Voth, P. Salvador, J.J. Dannenberg, S. Dapprich, A.D. Daniels, Ö. Farkas, J.B. Foresman, J. V. Ortiz, J. Cioslowski and D.J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT, 2009. [ Links ]
23 L. Zhang, D. Qi, L. Zhao, C. Chen, Y. Bian and W. Li, Density functional theory study on subtriazaporphyrin derivatives: dipolar/octupolar contribution to the second-order nonlinear optical activity, J. Phys. Chem, 2012, 116, 10249-10256. [ Links ]
24 A. Plaquet, M. Guillaume, B. Champagne, F. Castet, L. Ducasse, J. Pozzo and V. Rodriguez, In silico optimization of merocyanine- spiropyran compounds as second-order nonlinear optical molecular switches, Phys. Chem. Chem. Phys, 2008, 10, 6223-6232. [ Links ]
25 F. Castet, E. Bogdan, A. Plaquet, L. Ducasse, B. Champagne and V. Rodriguez, Reference molecules for nonlinear optics: a joint experimental and theoretical investigation, J. Chem. Phys., 2012, 136,024506-0245015. [ Links ]
26 P. Chandra Ray, Size and shape dependent second order nonlinear optical properties of nanomaterials and its application in biological and chemical sensing, Chem Rev., 2010,110, 5332-5365. [ Links ]
27 A.Y Tolbin, VE. Pushkarev, G.F. Nikitin and L.G. Tomilova, Hetero- ligand and heteronuclear clamshell-type phthalocyanines: selective preparation, spectral properties, and synthetic application, Tetrahedron Lett, 2009, 50, 4848-850. [ Links ]
28 K. Palewska, J. Sworakowski and J. Lipinski, Molecular aggregation in soluble phthalocyanines - Chemical interactions vs. p-stacking, Opt. Mater., 2012, 34, 1717-1724. [ Links ]
29 S. Sergeyev, E. Pouzet, O. Debever, J. Levin, J. Gierschner, J. Cornil, R.G. Aspe and Y.H. Geerts, Liquid crystalline octaalkoxycarbonyl phthalocyanines: design, synthesis, electronic structure, self- aggregation and mesomorphism,J. Mater. Chem.,2007,17,1777-1784. [ Links ]
30 M. Gouterman, The Porphyrins, Vol. III, Part A, (D. Dolphin, ed.), Academic Press, New York, USA, 1978. [ Links ]
31 M. Ozer, A. Altmdal, A.R. Ozkaya, M. Bulut and O. Bekaroglu, Synthesis, characterization and some properties of novel bis(penta- fluorophenyl)methoxyl substituted metal free and metallophthalo- cyanines, Polyhedron, 2006, 25, 3593-3602. [ Links ]
32 H. Isago, Optical Spectra of Phthalocyanines and Related Compounds -A Guide for Beginners, Springer, Tsukuba, Japan, 2015. [ Links ]
33 Z. Gasyna, N. Kobayashi and M.J. Stillman, Optical absorption and magnetic circular dichroism studies of hydrogen, copper(II), zinc(II), nickel(II), and cobalt(II) crown ether-substituted monomeric and dimeric phthalocyanines, J. Chem. Soc, Dalton Trans., 1989, 12, 2397-2405. [ Links ]
34 J. Mack, N. Kobayashi and M.J. Stillman, Re-examination of the emission properties of alkoxy-and thioalkyl-substituted phthalocyanines, J. Inorg. Biochem, 2010, 104, 310-317. [ Links ]
35 M. Dulski, M. Kempa, P. Kozub, J. Wójcik, M. Rojkiewicz, P. Kus ,A. Szurko, A. Ratuszna and R. Wrzalik, DFT/TD-DFT study of solvent effect as well the substituents influence on the different features of TPP derivatives for PDT application, Spectrochim. Acta, Part A, 2013, 104, 315-327. [ Links ]
36 Y. Yilmaz, J. Mack, M.K. Sener,M. Sönmez and T. Nyokong Synthesis, photophysicochemical properties and TD-DFT calculations of tetrakis(2-benzoyl-4-chlorophenoxy) phthalocyanines, J. Porphyrins Phthalocyanines, 2014, 18, 326-335. [ Links ]
37 J.W. Owens and M. Robins, Phthalocyanine photophysics and photosensitizer efficiency on human embryonic lung fibroblasts, J. Porphyrins Phthalocyanines, 2001, 5, 460-64. [ Links ]
38 P. Tau and T. Nyokong, Electrochemical characterisation of tetra- and octa-substituted oxo(phthalocyaninato)titanium(IV) complexes, Electrochim. Acta, 2007, 52, 3641-3650. [ Links ]
39 Z. Petrásiek and D. Phillips, A time-resolved study of concentration quenching of disulfonated aluminium phthalocyanine fluorescence, Photochem. Photobiol. Sci., 2003, 2, 236-244. [ Links ]
40 J.W Borst, M.A. Hink, A. van Hoek and A.J.WG. Visser, Effects of refractive index and viscosity on fluorescence and anisotropy decays of enhanced cyan and yellow fluorescent protein, J. Fluoresc., 2005,15, 153-160. [ Links ]
41 E.M. García-Frutos, S.M. O'Flaherty, E.M. Maya, G. de la Torre, W Blau, P. Vázqueza and T. Torres, Alkynyl substituted phthalocyanine derivatives as targets for optical limiting, J. Mater. Chem., 2003, 13, 749-753. [ Links ]
42 C. Nitschke, S.M. O'Flaherty, M. Kroll, J.J. Doyle and W.J. Blau, Optical properties of zinc phthalocyanine nanoparticle dispersions, Chem. Phys. Lett., 2003, 383, 555-560. [ Links ]
43 M.Y. Berezin and S. Achilefu, Fluorescence lifetime measurements and biological imaging, Chem. Rev., 2010,110, 2641-2684. [ Links ]
44 T. Verbiest, S.V. Elshocht, A. Persoons, C. Nuckolls, K.E. Phillips and T.J. Katz, Second-order nonlinear optical properties of highly symmetric chiral thin films, Langmuir, 2001,17, 4685-687. [ Links ]
45 N.B. McKeown, Phthalocyanine Materials: Synthesis, Structure and Function, Cambridge University Press, Cambridge, 1998. [ Links ]
46 M.M. Ayhan, A. Singh, E. Jeanneau, V. Ahsen, J. Zyss, I. Ledoux-Rak, A. Gül Gürek, C. Hirel, Y. Bretonnière and C. Andraud, ABAB homoleptic bis(phthalocyaninato) lanthanide(iii) complexes: original octupolar design leading to giant quadratic hyperpolarizability, Inorg. Chem, 2014, 53, 4359-4370. [ Links ]
47 Y. Lee, S. Jeon and M. Cho, Molecular polarizability and first hyperpolarizability of octupolar molecules: donor-substituted triphenylmethane dyes, J. Am. Chem. Soc., 1998, 120, 10921-10927. [ Links ]
Received 19 September 2016
Revised 6 January 2017
Accepted 8 January 2017
* To whom correspondence should be addressed. E-mail: nombonan@ukzn.ac.za
Supplementary Data
The supplementary data is available in pdf: [Supplementary data]














