Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.70 Durban 2017
http://dx.doi.org/10.17159/0379-4350/2017/v70a6
RESEARCH ARTICLE
Effect of benzamide on the corrosion inhibition of mild steel in sulphuric acid
Cleophas A. LotoI, II, *; Roland T. LotoI; Olufunmilayo O. JosephI
IDepartment of Mechanical Engineering, Covenant University, Canaan Land, Ota, Nigeria
IIDepartment of Chemical, Metallurgical & Materials Engineering, Tshwane University of Technology, Pretoria, South Africa
ABSTRACT
The effect of benzamide as a chemical inhibitor on mild steel corrosion in 0.5M H2SO4 was studied at ambient temperature. The experimental work was performed with gravimetric and potentiostatic polarization measurement methods. Potentiostatic polarization measurement was performed with a potentiostat (Autolab PGSTAT 30 ECO CHIMIE) interfaced with a computer for data acquisition and analysis. The benzamide inhibitor achieved very effective corrosion inhibition of the steel specimens in the H2SO4 test medium. The inhibition performance increased with increasing concentration of the inhibitor. Benzamide's best performance was achieved with the 4 g 200 mL-1 H2SO4 concentration and closely followed by the 3 g 200 mL-1 of the H2SO4.In 0.5 M H2SO4, the 4 g and 3 g 200 mL-1 H2SO4 gave the optimal performance with weight loss of 2.99 g at 480 h of the experiment, respectively. The corrosion rate for 4 g's was 6.4 mm yr-1. The experiment also achieved polarization resistance values of 3.98 and 2.37E + 01Ω; corrosion rate, CR, of 7.48E + 00and1.26E + 01 mm yr1 and current density (Icorr) values of 6.45E-04 and 1.08E - 03 A cm-2, respectively. The corrosion inhibition efficiency values are, respectively, 60 and 70 % for both 3 g and 4 g 200 mL-1 H2SO4 concentrations at 48 h. Results of ba and bc indicated a mixed type inhibitor. Benzamide adsorption on the steel's surface obeys the Freundlich adsorption isotherm.
Keywords: Electrochemical corrosion, benzamide, inhibitor, sulphuric acid, mild steel, polarization.
1. Introduction
The uniqueness of mild steel among other metallic materials can undoubtedly be associated with its very wide application and its usefulness in domestic, services, construction, marine, industrial and engineering purposes. The challenge, however, is that the metal is subject to environmental/corrosive degradation in service. Chemical inhibitors - the chemical compounds that are adsorbed on the metal surfaces to control, prevent and/or minimize destructive corrosion reactions processes are used, among other means to mitigate this destructive phenomenon. The significance of this metal protection process in diverse areas of applications has necessitated the keen interest in research in corrosion chemical inhibition by various corrosion scientists among others.1-5
In this work, benzamide (Fig. 1) was used as the corrosion chemical inhibitor for mild steel in sulphuric acid. This chemical's other names include benzoic acid amide; benzoylamide, phenylcarboxyamide, and phenylcarboxamide. Benzamide is a derivative of benzoic acid and has the chemical formula C6H5CONH2 or C7H7NO. It has a molecular weight (M.W.) of 121.13658 g mol-1. It can be described as a crystal obtained by the action of ammonia on benzoyl chloride. Among its many uses is as a base for antipsychotic treatments.6 Also, chemical derivatives of benzamide are widely used in therapeutics. These have been described to include analgesics (e.g. salicylamide), antiemetics (e.g. metoclopramide), antipsychotics (e.g. sultopride) and other agents.7
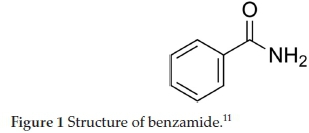
Benzamide was used to study the mechanism of photocatalytic decomposition of aqueous solution of acetic acid, acetamide and acetonitrile in the presence of semiconductors.8 It was, in addition, used to develop a versatile screening method to study biotransformations using (+)-y-lactamase enzyme.9 On radioiodination by different labelling procedures, benzamide has been reported to result in large-scale production of radioiodinated benzamides that have potential therapeutic application for patients with metastatic malignant melanoma.10
As previously expressed,12 sulphuric acid, used as the test medium in this work, is a highly corrosive, strong mineral acid with many industrial uses. Molecule of sulphuric acid, H2SO4 has more than one ionizable hydrogen atoms and is thus called polyprotic acid. The ionization of this acid occurs in two steps, with the molecule losing one proton at a time:13
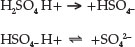
Sulphuric acid, H2SO4, which is a strong acid, is fully dissociated, and all the displaceable hydrogen in the acid is present in solution as hydrogen ion, H+.
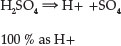
Its complete dissociation enhances more reactive corrosion reactions. Sulphuric acid is a powerful protonating agent. It is also a moderately strong oxidizing agent. The acid is also a powerful dehydrating agent. In dilute solution, sulphuric acid is a strong dibasic acid forming two series of salts.14 Its multifarious use in diverse areas of industry accounts for its being selected for use in this work.
The structure and chemistry of benzamide is expected to give an appreciable amount of electrochemical activities of corrosion inhibition of the mild steel in the corrosive sulphuric acid environment used in this work. It is anticipated that the results emanating from this research work will be of technological and economic benefits.
2. Materials and Methods
2.1. Materials Preparation
The cylindrical mild steel sample of 13 mm diameter was each cut into average size of 13 mm x 10 mm coupons for weight loss measurements and also for potentiostatic polarization measurements. Test samples used for the weight loss experiment were de-scaled with a wire brush, ground with various grades of emery paper and then polished to 6 μπι. They were further rinsed in distilled water to remove any corrosion products and then cleaned with acetone to degrease. The samples were fully immersed thereafter preventing further exposure to moisture in the atmosphere. Another set of samples for the corrosion polarization experiments were cleaned in the same manner as those for the weight loss experiment. They were subsequently mounted in resin to ensure that only the tested surface of the sample was exposed to the corrosive medium. Before mounting, copper wire was spot-welded to each of the samples. The surface of the samples were thoroughly cleaned and prepared for experimental use with silicon carbide papers of up to 1000 grade before being cleansed in distilled water and dried with acetone. Different weights of benzamide -1,2,3, and 4 g were made and then prepared into the various per cent concentrations used.
2.2. Experimental Setup
The experiments were set up in six different environments, one control experiment and five other experiments with different concentrations of the inhibitor (benzamide) in 0.5 M H2SO4. The benzamide inhibitor concentrations used were: 1, 2, 3, and 4 g 200 mL-1 H2SO4, respectively.
2.3. Weight Loss Experiments
Weighed test species were fully and separately immersed in 200 mL of the test environment at specific concentrations of the inhibitors for 480 h at room temperature. The method involves exposing a specimen of material to a process environment over a period of time, taking the weight of the sample at a time interval. Each of the test samples was taken out every 48 h, washed with distilled water, rinsed with acetone, dried and re-weighed. Plots of weight loss (g), corrosion rate (mm yr-1) and percentage inhibition efficiency (% IE) versus exposure time in hours were made from the readings recorded in the tables.
From the weight loss data, corrosion rate (Cr) values were calculated from the equation:
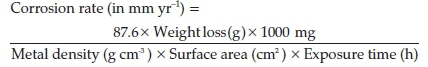
2.4. Potentiostatic Polarization Experiments
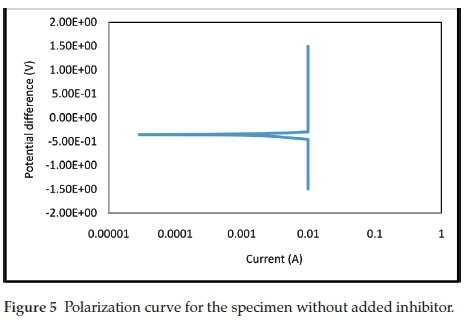
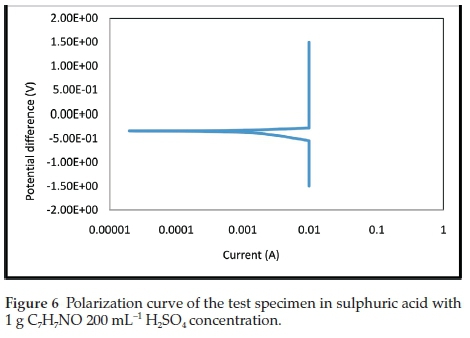
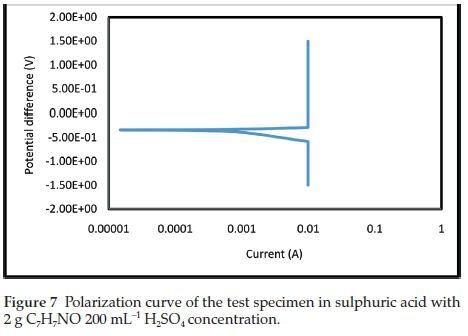
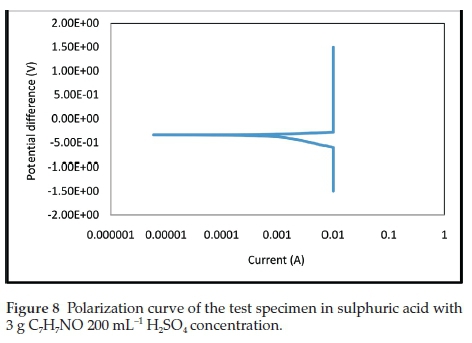
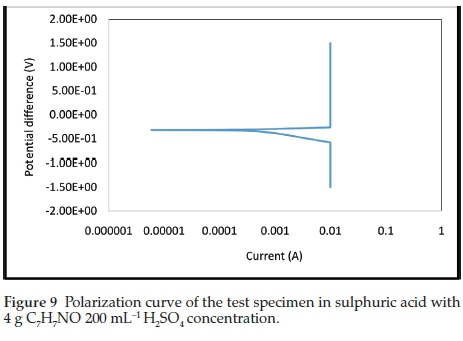
The electrochemical polarization techniques were performed on the prepared test specimens immersed in the sulphuric acid test environment with and without the addition of various inhibitor concentrations. Tafel plots were generated in this experiment by polarizing the specimen about 300 mV in the anodic direction (positive-going potential) and cathodically (negative-going potential) from the corrosion potential, Ecorr. The resulting current is plotted on a logarithmic scale. The data generated include current density, corrosion rate, and polarization resistance. The result summary is presented in Table 1. The curves generated from the results are given in Figs. 5- 9.
2.5. Optical Microscopy/Micrographs
Photomicrographs were made of the unused and of the relevant and selected surfaces of the test samples after use for weight loss experiments.
3. Results and Discussion
The results of the experiments carried out revealed the different weight loss and corrosion rate values of mild steel. Based on the data obtained from the experiment, table was drawn and graphs were plotted.
3.1. Weight Loss Method
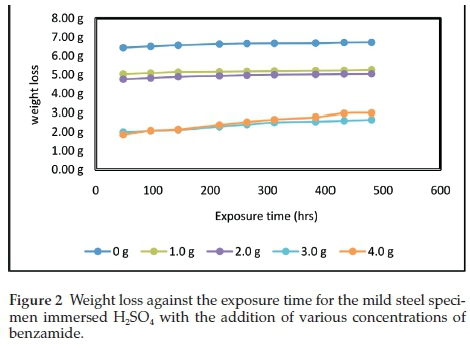
The results obtained for the weight loss of the mild steel immersed in 0.5 M H2SO4 with and without benzamide (C7H7NO) addition in different concentrations are presented in Fig. 2 and the corresponding corrosion rate in Fig. 3. The test without benzamide recorded the highest amount of weight loss that ranged between 6.44 and 6.72 mg during the duration of the experiment. The weight loss decreased with the increase in the inhibitor concentration. The lowest weight loss values were achieved by the 3 and 4 g of benzamide in 200 mL-1 H2SO4. These inhibitor concentrations recorded about the same values of weight loss from the beginning up to the 264 h (11 days) of the experiment; after which the 3 g 200 mL-1 H2SO4 concentration showed lower weight loss to the end of the experiment. In general, the benzamide inhibitor could be said to be effective in corrosion inhibition of mild steel in the sulphuric acid strength (0.5 M) used.
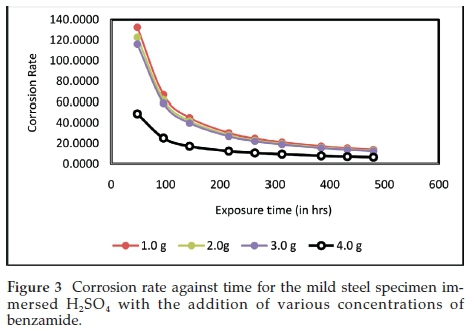
The curves in Fig. 3 show that corrosion rate decreased with exposure time for all the tested specimens at all the concentrations used. The observed phenomenon could be associated with the slower corrosion reactions emanating from the weak test environment due to contamination by the corroding test metal in solution. The graph further reveals that the sample with the highest inhibitor concentration had the lowest corrosion rate. Furthermore corrosion deposit from the corroding electrode could cause passivation on the electrode surface. Passivation refers to a material becoming less affected by environmental factors. It involves a shielding outer layer of base material which may be applied as micro coating, or which occurs spontaneously in nature.
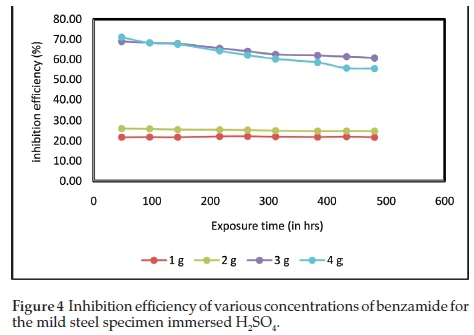
Figure 4 shows the curves of inhibition efficiency with the exposure time. The results of inhibitor efficiency reveal that after 48 h (2 days), 4 g of benzamide in 200 mL H2SO4 showed the highest inhibition efficiency which was slightly above 70 %. After 480 h (20 days) of exposure time in sulphuric acid, 3 g and4gofbenzamide in 200 mL H2SO4 gave a good performance achieving inhibition efficiency of 61 % and 55.5 %,respec-tively.
3.2. Potentiostatic Polarization Method
The electrochemical variables such as corrosion potential (Ecorr), corrosion current (icorr), cathodic Tafel constant (bc), anodic Tafel constant (ba), corrosion rate (mm yr-1) and polarization resistance (Ω) with their values are presented in Table 1. The curves generated for different concentrations of benzamide used in the test medium as inhibitor of mild steel are presented in Figs. 5-9.
In Table 1, the concentration 4 g 200 mL-1 H2SO4, with the lowest current density (Icorr) exhibits the best inhibitive characteristics. It also has the lowest corrosion rate and the highest polarization resistance which show it be the most effective concentration. There is an anodic and cathodic Tafel constant for all the inhibitor concentrations. This indicates that benzamide is a mixed type inhibitor. The control experiment that has no inhibitor addition had the highest corrosion magnitude as indicated by the overall polarization results.
This polarization test shows that benzamide significantly alters the electrochemical process responsible for corrosion. In addition, changes in the cathodic and anodic Tafel constants in the presence of benzamide in contrast with the control concentration signify the suppression of redox reactions associated with the corrosion process by the surface blocking effect of the inhibitor generated film. The inhibitive action of the inhibitor is related to its adsorption and formation of a compact barrier film on the metal electrode surface. This is further indicated by the values of the corrosion current of the tests with inhibitor in comparison with the corrosion current test values of the tests without inhibitor addition - the control experimental results.
3.3. Thermodynamics and Adsorption Studies
Molecular adsorption can further be used to explain corrosion inhibition. The chemical structures of organic compounds, the distribution of charge in molecule, the nature and surface charge of metal and the type of aggressive media are known to influence the adsorption process.15
Investigation of the likely mode of adsorption is necessary. This is achieved by putting to test the experimental data obtained with several adsorption isotherms. The value of k which is the adsorption equilibrium constant and the standard free energy of adsorption have been evaluated based on the relation written below16:
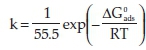
where 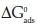 sis the standard free energy of adsorption;
sis the standard free energy of adsorption;
R is the molar gas constant and T is the absolute temperature.
The negative values of  obtained indicates the spontaneous adsorption process and the stability of the adsorbed inhibitor layer on the metal surface. The values of the standard free energy of adsorption for1gto4g200 mL-1 benzamine inhibitor concentration used are within the range of -19 to -20 kJ mol-1; thus characterizing the physiosorption mode of adsorption. Studies have shown that
obtained indicates the spontaneous adsorption process and the stability of the adsorbed inhibitor layer on the metal surface. The values of the standard free energy of adsorption for1gto4g200 mL-1 benzamine inhibitor concentration used are within the range of -19 to -20 kJ mol-1; thus characterizing the physiosorption mode of adsorption. Studies have shown that 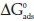 values that are -20 kJ mol-1 and above, i.e. less negative are consistent with physical adsorption (physiosorption) which involves electrostatic interaction between charged atoms and the charged metal, while those around -40 kJ mol-1 and more negative are generally associated with chemical adsorption.
values that are -20 kJ mol-1 and above, i.e. less negative are consistent with physical adsorption (physiosorption) which involves electrostatic interaction between charged atoms and the charged metal, while those around -40 kJ mol-1 and more negative are generally associated with chemical adsorption.
Freundlich adsorption isothermgives the best fit and it is given by the relations17:
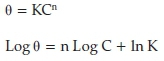
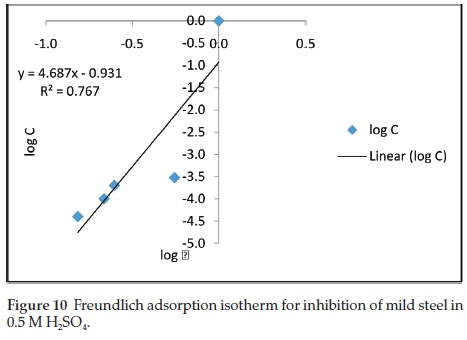
where θ is the degree of surface coverage, K and n are coefficients and C is the inhibitor concentration. The linear regression obtained by plotting the graph of Log C against Log θ is 0.770 as presented in Fig. 10.
3.4. Optical Microscopy (Photomicrographs)
Two representative micrographs made among others are presented in Figs. 11 and 12.
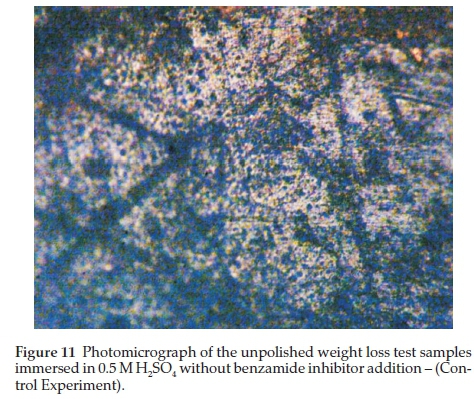
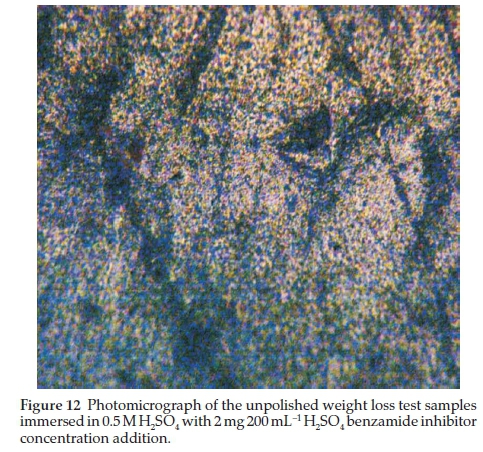
Figure 11 shows the micrograph of the unpolished weight loss test samples immersed in 0.5 M H2SO4 without benzamide inhibitor addition - this is the control experiment. Though the quality of the photomicrograph is low, severe corrosion of the surface could be observed.
In Fig. 12, the low concentration of the benzamide, 2 mg 200 mL-1 H2SO4, still show improved surface corrosion resistance of the mild steel in sulphuric acid. It thus confirms the effectiveness of benzamide as an inhibitor of the metal in the strong acid. As earlier explained, benzamide provided the surface film on the metal that stood as a barrier between the metal and the acid test environment. This reduced the active corrosion reactions at the metal-acid interface.
Benzamide consists among others, nitrogen (N), and oxygen (O). These are heteroatoms, present in the ring structure of benzamide's chemical constituents. These constituents are known18 to have remarkable inhibitory effect which facilitates their adsorption on the metal surface in the sequence O < N. The inhibition efficiency of an inhibitor such as benzamide, may therefore, depend also on the structure of the inhibitor itself. This includes the number of adsorption active centres in the molecule, the charge density, the molecular size, the mode of adsorption and the formation of metallic complexes.19
In general, compounds of organic origin are known to have good corrosion inhibition characteristics for mild steel in acidic environments.20-25 From this research work, benzamine as an organic compound can be considered as one of such compounds with very appreciable corrosion inhibition in strong acid like H2SO4 even at low concentrations as obtained in this investigation. Benzamide can also be considered as an environmentally friendly inhibitor among many others that are currently receiving significant research attention in corrosion protection studies.26-33
The results of the electrochemical tests obtained here are very much in agreement with the gravimetric tests. An appreciable effectiveness of the inhibitory properties of benzamide in the H2SO4 test environments is achieved. The results of the extract concentrations affected both the anodic and cathodic reactions according to the Tafel slope (ba and bc) values in Table 1 and thus confirms the benzamide inhibitor to be a mixed type inhibitor in sulphuric acid test environment. Thermodynamic evaluation confirms the adsorption mechanism to be physiosorption on the steel surface. As earlier mentioned, the adsorption obeyed the Freundlich adsorption isotherm.
4. Conclusion
From the experiments carried out and from careful analysis and comparisons of the various test samples against the controls, these following conclusions are made:
• Benzamide acted as a mixed inhibitor for mild steel in acidic medium (sulphuric acid). It exhibited up to 70 % inhibition efficiency in a concentration of 4 g 200 mL-1 H2SO4 over 48 h of exposure time.
• Benzamide exhibited more than 70 % inhibition efficiency after 48 h with 4 g 200 mL-1 H2SO4 of benzamide while a concentration of 3 g 200 mL-1 H2SO4 exhibited just below an efficiency of 70 % at the same time interval. According to the weight-loss experiments, a concentration of 3 g200 mL-1 H2SO4 of benzamide performed better than 4 g 200 mL-1 H2SO4 of benzamide after 480 h.
• The effectiveness of corrosion inhibition performance of benzamide on mild steel as a chemical inhibitor in the strong H2SO4 is demonstrated in the overall results profile of potentiodynamic polarization
• Adsorption of benzamine on the steel surface was observed to obey the Freundlich adsorption isotherms.
Acknowledgements
The laboratory contribution of Tolulope Abiodun is acknowledged. The authors thank the Department of Mechanical Engineering, Covenant University, Ota, Nigeria, for the use of laboratory research facilities.
References
1 R. Döner, M. Solmaz, M. Özcan and G. Kardaş, Experimental and theoretical studies of thiazoles as corrosion inhibitors for mild steel in sulphuric acid solution, Corros. Sci. 2011, 53, 2902. [ Links ]
2 R. Solmaz, Investigation of the inhibition effect of 5-((E)-4-phenyl- buta-1,3 dienylidene amino)-1, 3,4-thiadiazole-2-thiol Schiff base on mild steel corrosion in hydrochloric acid, Corros. Sci., 2010, 52, 3321 [ Links ]
3 K. Parameswari, S. Chitra, A. Selvaraj, S. Brindha and M. Menaga, Investigation of benzothiazole derivatives as corrosion inhibitors for mild steel, Port. Elect. Acta, 2012, 30,126. [ Links ]
4 R.T. Loto, C.A. Loto, A. P. Popoola and W. Kupolati, Corrosion inhibition Effect of N, N'-diphenylthiourea on the electrochemical characteristics of mild steel in dilute acidic environments, J. Chem. Soc. Pak., 2016, 38(2), 222-233. [ Links ]
5 C.A. Loto, O.S.I. Fayomi and R.T. Loto, Electrochemical corrosion resistance and inhibition behaviour of martensitic stainless steel in hydrochloric acid, Der Pharm. Chem, 2015, 7 (10) 102-111. [ Links ]
6 http://www.cocoa.uk.com/seratis/content/treatments/antipsychot-ics/atypicalantipsychotics/benzamides.html, Medical Dictionary, 2012 Farlex and Printers (Retrieved 28 May, 2016). [ Links ]
7 http://medical-dictionary.thefreedictionary.com/benzamide (Retrieved 28 May, 2016). [ Links ]
8 K.H. Park and J.H. Kim, Photocatalytic decompositions of carboxylic acid derivatives by semiconductors, Bull. Korean Chem. Soc., 1991, 12(4), 439. [ Links ]
9 A.M. Hickey, B. Ngamsom, C. Wiles, G.M. Greenway, P. Watts and J.A. Littlechild, A micro reactor for the study of biotransformations by a cross-linked gamma-lactamase enzyme, Biotech J., 2009, 4(4), 510-516. [ Links ]
10 W. Mier, C. Kratochwil, J.C. Hassel, F.L. Giesel, B. Beijer, J.W. Babich, M. Friebe, M. Eisenhut, A. Enk and U. Haberkorn, Radiopharma-ceutical therapy of patients with metastasized melanoma with the melanin-binding benzamide 131I-BA52. J. Nucl. Med., 2014, 55(1), 9-14. [ Links ]
11 http://www.sigmaaldrich.com/catalog/product/aldrich/1358287la-ng=en®ion=NG#cited_4 (Retrieved 27 May, 2016). [ Links ]
12 C.A. Loto, O.S.I. Fayomi, R.T. Loto and A.P.I. Popoola, Electrochemical corrosion resistance and susceptibility of 12Cr martensitic stainless steel in H2SO4, J. Chem. Pharm. Res., 2015, 7(10): 39-8. [ Links ]
13 Ionization of Acids - http://chem.wisc.edu/deptfiles/gencherri/sstutorial/Text12/ Tx124/tx124.html (Retrieved 16 May 2015). [ Links ]
14 Sulphuric Acid - http://www.ucc.ie/academic/chem/dolchem/html/comp/h2so4.html (Retrieved 16 May 2015). [ Links ]
15 C.O. Akalezi, C.K. Enenebaku and E.E. Oguzie, Inhibition of acid corrosion of mild steel by biomass extracts from the Petersianthus macrocarpus plant. J. Mater. Environ. Sci., 2013,4(2), 217-226. [ Links ]
16 A.L. Nnanna, C.O. Nwadiuko, D.N. Ekekwe, F.C. Ukpabi, C.S. Udensi, B.K. Okeoma, N.B. Onwuagba and M.I. Mejeha, Adsorption and inhibitive properties of leaf extract of Newbouldia leavis as a green inhibitor for aluminium alloy in H2SO4Amer. J. Mater. Sci., 2011,1(2), 143-148, DOI: 10.5923/j. materials. 20110102.24 [ Links ]
17 E.B. Ituen and U.E. Udo, Phytochemical profile, adsorptive and inhibitive behaviour of costus after extracts on aluminium corrosion in hydrochloric acid, Der. Chem. Sin, 2012, 3(6), 1394-1405. [ Links ]
18 B. Donnelly, T.C. Downier, R. Grzeskowiak, H.R. Hamburg and D. Short, The effect of electronic delocalization in organic groups R in substituted thiocarbamoyl R_CS_NH2 and related compounds on inhibition efficiency, Corros. Sci., 1978, 18, 109 -116. [ Links ]
19 A. Chetouani, B. Hammouti,, T. Benhadda and M. Daoudi, Inhibitive action of bipyrazolic type organic compounds towards corrosion of pure iron in acidic media, App. Surf. Sci., 2005, 249, 375-385. [ Links ]
20 T.G. Trabanelli, Inhibitors - an old remedy for a new challenge, Corrosion, 1991,47(6), 410-419. [ Links ]
21 S.S. Abd El Rehim, S.A.M. Refay, F. Taha, M.B. Saleh and R.A. Ahmed, Corrosion inhibition of mild steel in acidic medium using 2-amino thiophenoland 2-cyanomethyl benzothiazole, J. Appl. Electrochem, 2001, 31, 429-35. [ Links ]
22 B.A. Abd El-Nabey, E. Khamis, M.Sh Ramadan and A. El-Gindy, Application of the kinetic-thermodynamic model for inhibition of acid corrosion of steel by inhibitors containing sulfur and nitrogen, Corrosion, 1996, 52(9), 671-679. [ Links ]
23 F. Bentiss, M. Lagrenee and M. Traisnel, 2,5-Bis(n-pyridyl)-1,3,4- oxadiazoles as corrosion inhibitors for mild steel in acidic media, Corrosion, 2000, 56,(7), 733-742. [ Links ]
24 J.M. Bastidas, J.L. Polo, E. Cano and C.L. Torres, Trybutylamine as corrosion inhibitor for mild steel in hydrochloric acid, J. Mater. Sci., 2000, 35, 2637-2642. [ Links ]
25 S.S. Abd El-Rehim, M.A.M. Ibrahim and K.F. Khaled, 4-Amino- antipyrine as an inhibitor of mild steel corrosion in HCl solution. J. Appl. Electrochem, 29, 593-599. [ Links ]
26 N.A. Negm, N.G. Kandile, E.A. Badr and M.A. Mohammed, Gravimetric and electrochemical evaluation of environmentally friendly nonionic corrosion inhibitors for carbon steel in 1M HCl, Corros. Sci., 2012, 65, 94-103. [ Links ]
27 A.M. Abdel-Gaber, B.A., Abd-El-Nabey, E. Khamis and D.E. Abd-El-Khalek, A natural extract as scale and corrosion inhibitor for steel surface in brine solution, Desalination, 2011, 278(1-2), 337-342. [ Links ]
28 M. Salasi, T. Sharabi, E. Roayaei, E. and M. Aliofkhazraei, The electro chemical behaviour of environment-friendly inhibitors of silicate and phosphonate in corrosion control of carbon steel in soft water media, Mater. Chem. Phys., 2007,104(1), 183-190. [ Links ]
29 G. Blustein and R. Romagnoli, Zinc basic benzoate as eco-friendly steel corrosion inhibitor pigment for anticorrosive epoxy-coatings, Coll. Surf. A: Physicochem. Eng. Aspects, 2006, 290(1-3), 7-18. [ Links ]
30 P. Bommersbach, C. Alemany-Dumont, J.P. Millet and B. Normand, Formation and behaviour study of an environment-friendly corrosion inhibitor by electrochemical methods, Electrochim. Acta, 2005, 51(6) 1076-1084. [ Links ]
31 A. Lecante, F. Robert, P.A. Blandinières and C. Roos, Anti-corrosive properties of S. tinctoria and G. ouregou alkaloid extracts on low carbon steel, Curr. Appl. Phys., 2011,11(3), 714-724. [ Links ]
32 K. Radojcic, S. Berkovic, J. Kovac, J. and F. Vorkapic, Natural honey and black radish juice as tin corrosion inhibitors, Corros. Sci., 2008, 50(5), 1498-1504. [ Links ]
33 P.C. Okafor, M.E. Ikpi, I.E. Uwah, E.E. Ebenso, U.J. Ekpe, U.J. and S.A. Umoren, Inhibitory action of Phyllanthus amarus extracts on the corrosion of mild steel in acidic media, Corros. Sci., 2008, 50(8), 2310-2317 [ Links ]
Received 22 July 2016
Revised 14 October 2016
Accepted 27 January 2017
* To whom correspondence should be addressed. E-mail: akinloto@gmail.com / cleophas.loto@covenantuniversity.edu.ng




![Synthesis and characterization of new bis-symmetrical adipoyl, terepthaloyl, chiral diimido-di-L-alanine diesters and chiral phthaloyl-L-alanine ester of tripropoxy p-tert-butyl calix[4]arene and study of their hosting ability for alanine and Na+](/img/en/prev.gif)









