Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Chemistry
versão On-line ISSN 1996-840X
versão impressa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.69 Durban 2016
http://dx.doi.org/10.17159/0379-4350/2016/v69a6
RESEARCH ARTICLE
In vitro Investigation of the Antimicrobial Activity of a Series of Lipophilic Phenols and Naphthols
Thavendran GovenderI; Usha GovindenI; Chunderika MocktarI; Hendrik G. KrugerI; Jelena VeljkovicII; Nikola CindroII; Damir BobinacII; Ivana ZabcicII; Kata MlinariC-MajerskiII; Nikola BasaricII,*
ICatalysis and Peptide Research Unit, School of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
IIDepartment of Organic Chemistry and Biochemistry, Ruder Boskovic Institute, Bijenicka cesta 54,10 000 Zagreb, Croatia
ABSTRACT
Five groups of phenols/naphthols (42 compounds in total) were synthesized and screened against Gram-positive Staphylococcus aureus and Bacillus subtilis, Gram-negative Escherichia coli and Klebsiella pneumoniae, and the fungus Candida albicans. Whereas compounds were found inactive against Gram-negative bacteria, potent activities against Gram-positive bacteria were observed. The activities correlate with the ability of molecules to form quinone methides, suggesting potential new modes of action.
Keywords: Antimicrobial activity, phenols, naphthols, quinone methides
1. Introduction
Bacterial infections are responsible for a vast number of human diseases. Moreover, development of bacterial resistence to common antibiotics is stimulating intensive research devoted to discovery of new targets in combating the bacteria. In search for new and improved antibiotics with new mechanism of action, it is important to screen many new libraries of compounds.
Antimicrobial activity of simple phenols,1 and their carboxylic acid derivatives has been investigated.2 It is generally accepted that phenols and benzyl alcohols exert antimicrobial action3-6 on the non-specific ability to alter membranes in Gram-negative bacteria.7-10 On the other hand, a more specific mode of action of phenolic compounds has been suggested, especially against Gram-positive bacteria, since the bactericidal concentrations are not dependent on the type of cell wall.11 It was suggested that phenolic compounds inhibit DNA synthesis, due to an effect associated with the inhibition of RNA and protein synthesis.11 Furthermore, antibacterial properties are ascribed to a large number of food products due to the presence of phenolic natural compounds.12 Examples include berries,13 vine,14 tropical vegetables,15 and mushrooms.16 Recently, an investigation of antimicrobial activities have been conducted on a series of naphthol derivatives of Mannich bases.17 The latter series of compounds can in principle be transformed to quinone methides (QM) intermediates during metabolic processes. QMs are interesting substrates in the development of antimicrobial agents since it was demonstrated that they act as alkylating agents and inhibitors of serine proteases and β-lactamases.18 Depending on their structure, they are mechanism-based enzyme inhibitors, or suicide substrates. When QMs are formed from cyclic compounds such as coumarins, they usually induce cross-linking of the enzyme in the reaction with a histidine. On the contrary, free QM formed in the active site of the enzyme usually reacts with neighbouring tryptophan residues (Scheme 1).18
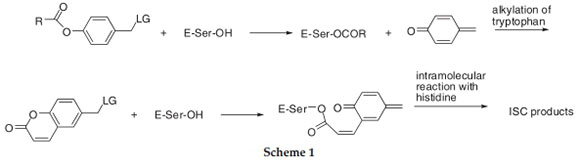
We became interested in the photochemical generation of QMs with sterically congested lipophylic substituents19,20 and their antiproliferative activities.21-24 A series of simple phenols,19 biphenyls,20-22 naphthols,23 and anthrols24 were synthesized and their antiproliferative activities were investigated. Since compounds that generate QMs are known β-lactamase inhibitors,18 we were also prompted to investigate their antimicrobic activities. Potentially, they can be used in combination with β-lactam derivatives as antibiotics. Herein we report an investigation of the antimicrobial activity of five series of lipophilic derivatives of phenols and naphthols and/or benzyl alcohols (Figs. 1-5) with the aim of developing a SAR relationship. Moreover, many of our derivatives bear an adamantyl substituent, and the antibacterial activity of a number of adamantane derivatives has been reported.25-29 The in vitro antimicrobial testing was performed on five strains of microorganisms, Gram-positive Staphylococcus aureus and Bacillus subtilis, Gram-negative Escherichia coli and Klebsiella pneumoniae, and the fungus Candida albicans.

2. Matherials and Methods
2.1. Compounds
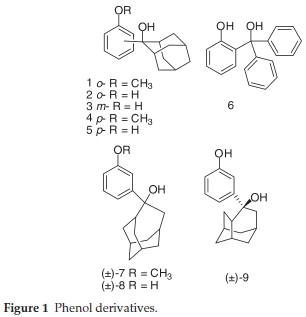
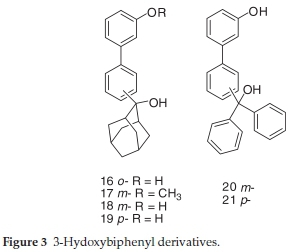
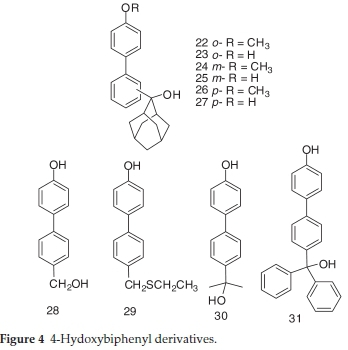
The structures of the investigated compounds comprise phenols (Fig. 1), 2-hydroxybiphenyls (Fig. 2), 3-hydroxy-biphenyls (Fig. 3), 4-hydroxybiphenyls (Fig. 4), naphthols (Fig. 5) or their corresponding methoxy or QM derivatives. All compounds were prepared according to previously published procedures.19-23 Chiral compounds 7-9 were synthesized as racemic mixtures.
2.1.1. Synthesis of Phenol Derivatives 1-9
2-Hydroxy-2-(2-methoxyphenyl)adamantane (1) was prepared in a Grignard reaction from 2-adamantanone and 2-bromanisol in 50 % yield according to the published procedure.19 Characterization of product 1 compares well with literature (mp = 87-88 °C).19 1H NMR (CDCl3, 300 MHz) δ/ppm: 7.44 (dd, 1H, J = 1.5 Hz, J = 7.7 Hz), 7.21 (dt, 1H, J = 1.5 Hz, J = 8.1 Hz), 6.93 (dt, 1H, J = 1.5 Hz, J = 7.7 Hz), 6.90 (d, 1H, J = 8.1 Hz), 3.85 (s, 3H), 3.42 (s, 1H, OH), 2.66 (br s, 2H), 2.54 (br s, 1H), 2.50 (br s, 1H), 1.65-1.90 (m, 8H), 1.61 (d, J = 12.5 Hz, 2H).
2-Hydroxy-2-(2-hydroxyphenyl)adamantane (2) was prepared from 1 by treatment with BBr3 in 45 % yield according to the published procedure.19 Characterization of product 2 compares well with literature (mp = 170-171 °C).191H NMR (DMSO-d6,300 MHz) d/ppm: 9.28 (s, 1H, OH), 7.32 (dd, 1H, J = 1.2 Hz, J = 7.7 Hz), 7.05 (dt, 1H, J = 1.5 Hz, J = 8.5Hz), 6.73-6.82 (m, 2H) 4.93 (s, 1H, OH), 2.61 (br s, 2H), 2.44 (br s, 1H), 2.40 (br s, 1H), 1.60-1.50 (m, 8H), 1.51 (d, 2H, J = 12 Hz).
2-Hydroxy-2-(3-hydroxyphenyl)adamantane (3) was prepared from 2-hydroxy-2-(3-methoxyphenyl)adamantane by treatment with BBr3 in 85 % yield according to the published procedure.19 Characterization of product 3 compares well with literature (mp = 160-161 °C).191H NMR (DMSO-d6,600 MHz) d/ppm: 9.17 (s, 1H, OH), 7.11 (t, 1H, J = 7.7 Hz), 6.87-6.90 (m, 2H), 6.60 (ddd, 1H, J = 0.9 Hz, J = 2.3 Hz, J = 7.7 Hz), 4.48 (s, 1H, OH), 2.37 (br s, 3H), 2.35 (br s, 1H), 1.79 (br s, 1H), 1.52-1.68 (m, 9H).
2-Hydroxy-2-(4-methoxyphenyl)adamantane (4) was prepared in a Grignard reaction from 2-adamantanone and 4-bromanisol in 65 % yield according to the published procedure.19 Characterization of product 4 compares well with literature (mp = 104-105 °C).191H NMR (CDCl3, 300 MHz) d/ppm: 7.46 (d, 2H, J = 8.8 Hz), 6.89 (d, 2H, J = 8.8 Hz), 3.80 (s, 3H, OCH3), 2.52 (br s, 2H), 2.42 (br s, 1H), 2.38 (br s, 1H), 1.89 (br s, 1H), 1.65-1.75 (m, 9H), 1.49 (br s, 1H, OH).
2-Hydroxy-2-(4-hydroxyphenyl)adamantane (5) was prepared from 4 by treatment with BBr3 in 90 % yield according to the published procedure.19 Characterization of product 5 compares well with literature (mp = 301-302 °C).191H NMR (DMSO-d6,300 MHz) d/ppm: 9.20 (s, 1H, OH), 7.24 (d, 2H, J = 8.6 Hz), 6.71 (d, 2H, J = 8.6 Hz), 4.30 (s, 1H, OH), 2.37 (br s, 3H), 2.31 (br s, 1H), 1.78 (br s, 1H), 1.50-1.68 (m, 9H).
Diphenyl-(2-hydoxyphenyl)methanol (6) was prepared from diphenyl-(2-methoxyphenyl)methanol by treatment with BBr3 in 90 % yield according to the published procedure30. Characterization of product 6 compares well with literature (mp = 148-150 °C).301H NMR (CDCl3, 300 MHz) d/ppm: 8.10 (s, 1H, OH), 6.40-7.40 (m, 14H), 3.70 (s, 1H, OH).
4-(3-Methoxyphenyl)homoadamantan-4-ol (7) was prepared in a Grignard reaction from homoadamantan-4-one and 3-bromanisol in 67 % yield according to the published proce-dure.31 Characterization of product 7 compares well with literature (mp = 130-132 °C).31 1H NMR (CDCl3,300 MHz) δ/ppm: 7.25 (t, 1H, J = 8.0 Hz), 7.19 7.12 (m, 2H), 6.78 (d, 1H, J = 8.0 Hz), 3.81 (s, 3H), 2.81 (dd, 1H, J = 3.8,15.0 Hz), 2.48 (d, 1H, J = 14.0 Hz), 2.22-2.03 (m, 3H), 2.01-1.89 (m, 4H), 1.89-1.68 (m, 4H), 1.60-1.47 (m, 4H).
4-(3-Hydroxyphenyl)homoadamantan-4-ol (8) was prepared from 7 by treatment with sodium thiolate in 90 % yield according to the published procedure.19,31 Characterization of product 8 compares well with literature(mp = 162-164 °C).311H NMR (DMSO-d6,300 MHz) d/ppm: 9.14 (s, 1H), 7.07 (dd (t), 1H, J = 7.8 Hz), 7.00 (dd (t), 1H, J = 2.0 Hz), 6.96 (d, 1H, J = 7.8 Hz), 6.56 (dd, 1H, J = 2.0, 7.8 Hz), 4.81 (s, 1H), 2.61-2.50 (m, 2H), 2.13-1.95 (m, 2H), 1.91-1.65 (m, 7H), 1.59-1.33 (m, 5H).
exo-4-(3-Hydroxyphenyl)protoadamantan-4-ol (9) was prepared from exo-4-(3-methoxyphenyl)protoadamantan-4-ol by treatment with sodium thiolate in 46 % yield according to the published procedure (mp = 176-178 °C).19,31 Characterization of product 9 compares well with literature.311H NMR (DMSO-d6, 300 MHz) δ/ppm 9.13 (s, 1H), 7.07 (dd (t), 1H, J = 7.8 Hz), 6.91-6.86 (m, 2H), 6.57 (dd, 1H, J = 1.6, 7.6 Hz), 4.56 (s, 1H), 2.58 (dd (t), 1H, J = 8.2 Hz), 2.46-2.36 (m, 1H), 2.33-2.24 (m, 1H), 2.07 (bs, 4H), 1.74 (d, 1H, J = 13.0 Hz), 1.67-1.40 (m, 3H), 1.29 (dd (t), 2H, J = 13.0 Hz), 1.19 (d, 1H, J = 12.0 Hz).
2.1.2. Synthesis of 2-Hydroxybiphenyl Derivatives 10-15
2-(2-Hydroxyadamantan-2-yl)-2'-methoxybiphenyl (10) was prepared in a Grignard reaction from adamantan-2-one and 2-bromo-2'-methoxybiphenyl in 76 % yield according to the published procedure.20 Characterization of product 10 compares well with literature (mp = 114-115 °C).20 1H NMR (CDCl3, 600 MHz) d/ppm: 7.68 (d, 1H, J = 8.1 Hz), 7.38 (dt, 1H, J = 1.6 Hz, J = 8.1 Hz), 7.34 (dt, 1H, J = 1.7Hz, J = 8.1 Hz), 7.27 (dt, 1H, J = 1.1 Hz, J = 7.4 Hz), 7.18 (dd, 1H, J =1.6Hz, J = 7.4 Hz), 7.00-7.04 (m, 2H), 6.93 (d, 1H, J = 8.3 Hz), 3.86 (br s, 1H, OH), 3.74 (s, 3H, OCH3) 2.59 (br s, 1H), 2.34 (dt, 1H, J = 2.2 Hz, J = 12.4 Hz), 2.11 (dt, 1H, J = 2.2 Hz, J = 12.4 Hz), 1.80-1.90 (m, 2H), 1.60-1.72 (m, 5H), 1.54 (dt, 1H, J = 2.2 Hz, J = 12.4 Hz), 1.40 (dt, 1H, J = 2.2 Hz, J = 12.4 Hz), 1.32 (dq, 1H, J = 3.2 Hz, J = 12.4 Hz), 1.23 (dq, 1H, J = 3.2 Hz, J = 12.4 Hz).
2-(2-Hydroxyadamantan-2-yl)-2'-hydroxybiphenyl (11) was prepared from 10 by treatment with sodium thiolate in 60 % yield according to the published procedure.20 Characterization of product 11 compares well with literature (mp = 194-196 °C).20 1H NMR (DMSO-d6, 300 MHz), d/ppm: 9.83 (s, 1H, OH), 7.60 (d, 1H, J = 7.4 Hz), 7.36 (dt, 1H J =1.5Hz, J = 7.3 Hz), 7.26 (dt, 1H J = 0.9 Hz, J= 7.3 Hz), 7.17 (dt, 1H J= 1.5 Hz, J= 7.5 Hz), 7.09 (dd, 1H, J = 1.5 Hz, J = 7.5 Hz), 6.95 (dd, 1H, J = 1.5 Hz, J = 7.5 Hz), 6.83-6.90 (m, 2H), 5.30 (s, 1H, OH), 2.50 (br s, 1H), 2.24 (d, 1H, J = 12.1 Hz), 2.08 (br s, 2H), 1.75 (d, 1H, J = 11.6 Hz), 1.44-1.69 (m, 6H), 1.36 (d, 1H, J = 12.1 Hz), 1.29 (d, 1H, J = 12.1 Hz), 1.21 (d, 1H, J = 11.6 Hz).
3-(2-Hydroxyadamantan-2-yl)-2'-hydroxybiphenyl (12) was prepared from 3-(2-hydroxyadamantan-2-yl)-2'-methoxy-biphenyl by treatment with BBr3 in 69 % yield according to the published procedure.20 Characterization of product 12 compares well with literature (mp = 179-181 °C).20 1H NMR (DMSO-d6,300 MHz) δ/ppm: 9.45 (s, 1H, OH), 7.62 (br s, 1H), 7.32-7.44 (m, 3H), 7.23 (dd, 1H, J = 1.6 Hz, J = 7.5 Hz), 7.14 (dt, 1H, J =1.6Hz, J = 8.0 Hz), 6.92 (dd, 1H, J = 0.8 Hz, J = 8.0 Hz), 6.87 (dt, 1H, J = 1.0 Hz, J = 7.4 Hz), 4.60 (br s, 1H, OH), 2.50 (br s, 2H), 2.41 (br s, 1H), 2.38 (br s, 1H), 1.55-1.85 (m, 10H).
4-(2-Hydroxyadamantan-2-yl)-2'-hydroxybiphenyl (13) was prepared from 4-(2-hydroxyadamantan-2-yl)-2'-methoxy-biphenyl by treatment with BBr3 in 40 % yield according to the published procedure.20 Characterization of product 13 compares well with literature (mp = 215-217 °C).201H NMR (DMSO-d6,300 MHz), d/ppm: 9.48 (s, 1H, OH), 7.52 (d, 2H, J = 8.6 Hz), 7.48 (d, 2H, J= 8.6 Hz), 7.25 (dd, 1H J= 1.5 Hz, J= 7.4 Hz), 7.13 (dt, 1H J=1.5 Hz, J = 8.1 Hz), 6.93 (dd, 1H, J = 1.0Hz, J = 8.1 Hz), 6.86 (dt, 1H J = 1.0 Hz, J = 7.4 Hz), 4.57 (s, 1H, OH), 2.50 (br s, 1H), 2.42 (br s, 1H), 2.38 (br s, 1H), 1.82 (br s, 1H), 1.55-1.75 (m, 10 H).
4-[2-(N-ethylaminoadamantan-2-yl)]-2'-hydroxybiphenyl hydrochloride (14) was prepared from 4-(2-azidoadamantan-2-yl)-2'-hydroxybiphenyl by reduction with Raney Ni in 27 % yield according to the published procedure (mp = 242-245 °C).20 Characterization of product 14 compares well with literature.20 1H NMR (DMSO-d6,300 MHz) d/ppm: 9.69 (s, 1H, OH), 8.43 (br s, 2H, NH2), 7.69 (d, 2H, J = 7.6 Hz), 7.65 (d, 2H, J = 7.6 Hz), 7.31 (dd, 1H, J = 7.3 Hz, J = 1.3 Hz), 7.18 (dt, 1H, J = 8.0 Hz, J = 1.5 Hz), 6.99 (d, 1H, J = 8.0 Hz), 6.89 (dd, 1H, J = 7.3 Hz, J = 8.0 Hz), 3.35 (q, 2H, J = 7.4 Hz), 2.90 (br s, 2H), 2.61 (br s, 1H), 2.47 (br s, 1H), 2.42 (br s, 1H), 1.94 (br s, 1H), 1.62-1.86 (m, 8H), 1.05 (t, 3H, J = 7.4 Hz).
Spiro[adamantane-2,9'-(4'-hydroxy)fluorene] (15) was prepared from 10 by treatment with BBr3 in 99 % yield according to the published procedure.20 Characterization of product 15 compares well with literature (mp = 158-160 °C).201H NMR (CDCl3, 600 MHz) d/ppm: 8.31 (dd, 1H, J = 1.0 Hz, J = 7.7 Hz), 8.11 (d, 1H, J = 8.1 Hz), 7.71 (d, 1H, J = 7.9 Hz), 7.37 (dt, 1H, J = 0.8 Hz, J = 7.6 Hz), 7.26 (dt, 1H, J = 1.5 Hz, J = 7.5 Hz), 7.12 (t, 1H, J = 7.9 Hz), 6.72 (d, 1H, J = 7.9 Hz), 5.08 (s, 1H, OH), 2.85-3.00 (m, 4H), 2.18 (br s, 2H), 1.98 (br s, 2H), 1.70-1.80 (m, 4H), 1.55-1.60 (m, 2H).
2.1.3. Synthesis of 3-Hydroxybiphenyl Derivatives 16-21
2-(2-Hydroxyadamantan-2-yl)-3'-hydroxybiphenyl (16) was prepared from 2-(2-hydroxyadamantan-2-yl)-3'-methoxy-biphenylby treatment with sodium thiolate in 20 % yield according to the published procedure.22 Characterization of product 16 compares well with literature (mp = 119-120 °C).221H NMR (DMSO-d6,600 MHz) d/ppm: 9.26 (s, 1H, OH), 7.56 (d, 1H, J = 7.9 Hz), 7.32 (dt, 1H, J = 1.4 Hz, J = 8.1 Hz), 7.22 (t, 1H, J = 7.3 Hz), 7.08 (t, 1H, J = 7.8 Hz), 6.98 (dd, 1H, J = 1.4 Hz, J = 7.5 Hz), 6.80-6.86 (m, 2H), 6.67 (dd, 1H, J = 2.2 Hz, J = 8.1 Hz), 4.68 (s, 1H, OH), 2.24 (br s, 2H), 2.19 (br s, 1H), 2.17 (br s, 1H), 1.63 (br s, 1H), 1.59 (br s, 1H), 1.40-1.55 (m, 6H), 1.28-1.36 (m, 2H).
3-(2-Hydroxyadamantan-2-yl)-3'-methoxybiphenyl (17) was prepared in a Grignard reaction from 3-bromo-3'-methoxy-biphenyl and 2-adamantanone in 86 % yield according to the published procedure.22 Characterization of product 17 compares well with literature.22 1H NMR (CDCl3, 300 MHz) δ/ppm: 7.74 (s, 1H), 7.46-7.56 (m, 2H), 7.43 (t, 1H, J = 7.5 Hz), 7.35 (t, 1H, J = 7.9 Hz), 7.17 (d, 1H, J = 7.7 Hz), 7.11 (t, 1H, J = 2.0 Hz), 6.89 (dd, 1H, J = 2.3 Hz, J = 8.1 Hz), 3.86 (s, 3H, OCH3), 2.62 (br s, 1H), 2.45 (br s, 1H), 2.41 (br s, 1H), 1.92 (br s, 1H), 1.68-1.82 (m, 10H).
3-(2-Hydroxyadamantan-2-yl)-3'-hydroxybiphenyl (18) was prepared from 17 by treatment with BBr3 in 83 % yield according to the published procedure.22 Characterization of product 18 compares well with literature (mp = 181-183 °C).221H NMR (DMSO-d6, 300 MHz) d/ppm: 9.49 (br s, 1H, OH), 7.65 (s, 1H), 7.36-7.50 (m, 3H), 7.25 (t, 1H, J = 7.8 Hz), 7.00-7.08 (m, 2H), 6.76 (dd, 1H, J = 1.2 Hz, J = 7.8 Hz), 2.50 (br s, 2H), 2.42 (br s, 1H), 2.38 (br s, 1H), 1.82 (br s, 1H), 1.52-1.76 (m, 10H).
4-(2-Hydroxyadamantan-2-yl)-3'-hydroxybiphenyl (19) was prepared from 4-(2-hydroxyadamantan-2-yl)-3'-methoxy-biphenyl by treatment with BBr3 in 34 % yield according to the published procedure.22 Characterization of product 19 compares well with literature (mp = 201-203 °C).22 1H NMR (DMSO-d6,300 MHz) δ/ppm: 9.50 (br s, 1H, OH), 7.56 (d, 2H, J = 8.6 Hz), 7.53 (d, 2H, J = 8.6 Hz), 7.24 (t, 1H, J = 7.6 Hz), 7.06 (d, 1H, J = 7.6 Hz), 7.02 (s, 1H), 6.75 (dd, 1H, J = 1.2 Hz, J = 7.9 Hz), 2.50 (br s, 2H), 2.41 (br s, 1H), 2.38 (br s 1H), 1.82 (br s 1H), 1.54-1.72 (m, 10H).
(3 -Hydroxybiphenyl-3-yl)diphenylmethanol (20) was prepared from (3'-methoxybiphenyl-3-yl)diphenylmethanol by treatment with BBr3 in 96 % yield according to the published procedure.22 Characterization of product 20 compares well with literature (mp = 141-142 °C).221H NMR (DMSO-d6, 600 MHz) δ/ppm: 9.47 (s, 1H, OH), 7.50 (t, 1H, J = 1.4 Hz), 7.48 (dd, 1H, J =1.7Hz, J = 7.7 Hz), 7.36 (t, 1H, J = 7.7 Hz), 7.32 (dd,4H, J = 7.2 Hz, J = 7.6 Hz), 7.23-7.28 (m, 6H), 7.21 (t, 1H, J = 7.8 Hz), 7.13 (dd, 1H, J = 1.5 Hz, J = 7.7 Hz), 6.95 (dd, 1H, J = 1.3 Hz, J = 7.7 Hz), 6.92 (t, 1H, J = 2.0 Hz), 6.73 (dd, 1H, J = 8.0 Hz, J = 2.3 Hz), 6.51 (s, 1H, OH).
(3'-Hydroxybiphenyl-4-yl)diphenylmethanol (21) was prepared from (3'-methoxybiphenyl-4-yl)diphenylmethanol by treatment with BBr3 in 98 % yield according to the published procedure.22 Characterization of product 21 compares well with literature (mp = 168-170 °C).221H NMR (DMSO-d6, 600 MHz) δ/ppm: 9.49 (s, 1H, OH), 7.54 (d, 2H, J = 8.4 Hz), 7.32 (dd, 4H, J = 7.2 Hz, J = 7.7 Hz), 7.22-7.30 (m, 9H), 7.06 (d, 1H, J = 7.6 Hz), 7.02 (t, 1H, J = 2.0 Hz), 6.75 (dd, 1H, J = 1.6Hz, J = 8.0 Hz), 6.46 (s, 1H, OH).
2.1.4. Synthesis of 4-Hydroxybiphenyl Derivatives 22-31
2-(2-Hydroxyadamantan-2-yl)-4'-methoxybiphenyl (22) was prepared in a Grignard reaction from 2-bromo-4'-methoxy-biphenyl and 2-adamantanone in 80 % yield according to the published procedure.21 Characterization of product 22 compares well with literature (mp = 125-130 °C).211H NMR (CDCl3, 300 MHz) δ/ppm: 7.61 (d, 1H, J = 8.0 Hz), 7.32 (t, 1H, J = 8.0 Hz), 7.28 (d, 2H, J = 8.5 Hz), 7.25 (t, 2H, J = 8.0 Hz), 7.10 (d, 1H, J = 8.0 Hz), 3.84 (s, 3H), 2.27 (s, 2H), 2.17 (br s, 1H), 2.15 (br s, 1H), 1.81 (s, 1H), 1.73 (br s, 1H), 1.62-1.69 (m, 3H), 1,60 (br. s, 2H), 1.52-1.57 (m, 2H), 1.49 (d, 2H, J = 12.4 Hz).
2-(2-Hydroxyadamantan-2-yl)-4'-hydroxybiphenyl (23) was prepared from 22 by treatment with sodium thiolate in 69 % yield according to the published procedure.21 Characterization of product 23 compares well with literature (mp = 160-163 °C).21 1H NMR (DMSO-d6, 300 MHz) d/ppm: 9.29 (s, 1H, OH), 7.55 (d, 1H, J =7.7Hz), 7.29(t,1H, J =7.7Hz), 7.16-7.26(m,3H),6.98 (dd, 1H, J = 1.0 Hz, J = 7.2 Hz), 6.70 (d, 2H, J = 8.4 Hz), 4.73 (s, 1H, OH), 2.10-2.30 (m, 4H), 1.20-1.70 (m, 10 H).
3-(2-Hydroxyadamantan-2-yl)-4'-methoxybiphenyl (24)was prepared in a Grignard reaction from 3-bromo-4'-methoxy-biphenyl and 2-adamantanone in 78 % yield according to the published procedure.21 Characterization of product 24 compares well with literature (mp = 103-105 °C).211H NMR (CDCl3, 300 MHz), d/ppm: 7.71 (br s, 1H), 7.51 (d, 2H, J = 8.8 Hz), 7.27-7.49 (m, 3H), 6.97 (d, 2H, J = 8.8 Hz), 3.84 (s, 3H), 2.62 (br s, 2H), 2.45 (br s, 1H), 2.41 (br s, 1H), 1.91 (br s, 1H), 1.68-1.80 (m, 10H).
3-(2-Hydroxyadamantan-2-yl)-4'-hydroxybiphenyl (25) was prepared from 24 by treatment with BBr3 in 79 % yield according to the published procedure.21 Characterization of product 25 compares well with literature (mp = 155-157 °C).211H NMR (DMSO-d6,300 MHz) d/ppm: 9.50 (br s, OH), 7.63 (br s, 1H), 7.46 (d, 2H, J = 8.6 Hz), 7.35-7.44 (m, 3H), 6.85 (d, 2H, J = 8.0 Hz), 2.52 (br s, 2H), 2.42 (br s, 1H), 2.38 (br s, 1H), 1,81 (br s, 1H), 1.55-1.70 (m, 10H).
4-(2-Hydroxyadamantan-2-yl)-4'-methoxybiphenyl (26)was prepared in a Grignard reaction from 4-bromo-4'-methoxy-biphenyl and 2-adamantanone in 65 % yield according to the published procedure.21 Characterization of product 26 compares well with literature (mp = 186-187 °C).211H NMR (CDCl3, 300 MHz) d/ppm: 7.58 (d, 2H, J = 8.8 Hz), 7.54 (d, 2H, J = 8.8 Hz), 7.53 (d, 2H, J = 8.8 Hz), 6.97 (d, 2H, J = 8.8 Hz), 3.84 (s, 3H), 2.59 (br s, 2H), 2.45 (br s, 1H), 2.40 (br s, 1H), 1.91 (br s, 1H), 1.70-1.80 (m, 10H).
4-(2-Hydroxyadamantan-2-yl)-4'-hydroxybiphenyl (27) was prepared from 26 by treatment with BBr3 in 67 % yield according to the published procedure.21 Characterization of product 27 compares well with literature (mp = 225-228 °C).211H NMR (DMSO-d6, 300 MHz) d/ppm: 9.48 (br s, OH), 7.42-7.61 (m, 6H), 6.84 (d, 2H, J = 8.5Hz), 2.47 (br s, 2H), 2.41 (br s, 1H), 2.38 (br s, 1H), 1.81 (br s, 1H), 1.53-1.73 (m, 10H).
4-Hydroxymethyl-4'-hydroxybiphenyl (28) was obtained from 4-methoxy-4'-hydroxybiphenyl with sodium thiolate in 23 % yield according to the published procedure.21 Characterization of product 28 compares well with literature (mp = 195-196 °C).21 1H NMR (DMSO-d6, 300 MHz), d/ppm: 9.50 (s, 1H, OH), 7.51 (d, 2H, J = 8.5 Hz), 7.46 (d, 2H, J = 8.5 Hz), 7.33 (d, 2H, J = 8.5 Hz), 6.83 (d, 2H, J = 8.6 Hz), 5.15 (br s, 1H, OH), 4.50 (s, 2H).
4-Thioethylmethyl-4'-hydroxybiphenyl (29) was obtained from 4-methoxy-4'-hydroxybiphenyl with sodium thiolate in 75 % yield according to the published procedure.21 Characterization of product 29 compares well with literature.211H NMR (CDCl3, 300 MHz), d/ppm: 7.48 (d, 2H, J = 8.3 Hz), 7.47 (d, 2H, J = 8.3 Hz), 7.36 (d, 2H, J = 8.3 Hz), 6.89 (d, 2H, J = 8.6 Hz), 4.84 (s, 1H, OH), 3.75 (s, 2H), 2.47 (q, 2H, J = 7.4 Hz), 1.25 (t, 3H, J = 7.4 Hz). 13C NMR (CDCl3, 75 MHz), d/ppm: 155.0 (s), 139.2 (s), 136.9 (s), 133.5 (s), 129.0 (d), 128.1 (d), 126.6 (d), 115.6 (d), 35.5 (t), 25.2 (t), 14.2 (q).
4-Hydroxy-4'-(2-hydroxypropan-2-yl)biphenyl (30) was obtained from 4-methoxy-4'-(2-hydroxypropan-2-yl)biphenyl with sodium thiolate in 80 % yield according to the published procedure.21 Characterization of product 30 compares well with literature (mp = 178-180 °C).211H NMR (DMSO-d6, 300 MHz), d/ppm: 9.48 (s, 1H, OH), 7.48 (s, 4H), 7.45 (d, 2H, J = 8.6 Hz), 6.83 (d, 2H, J = 8.6 Hz), 4.98 (br s, 1H, OH), 1.44 (s, 6H).
4'-(Hydroxydiphenylmethyl)biphenyl-4-ol (31) was obtained from (4'-methoxybiphenyl-4-yl)diphenylmethanol with BBr3 in 83 % yield according to the published procedure.21 Characterization of product 31 compares well with literature (mp = 274-276 °C).21 1H NMR (CDCl3, 300 MHz) δ/ppm: 9.50 (br s 1H, OH),7.49(t,4H, J = 8.7Hz),7.18-7.34(m,12H),6.83(d,2H, J = 8.6 Hz), 6.41 (br s, 1H, OH).
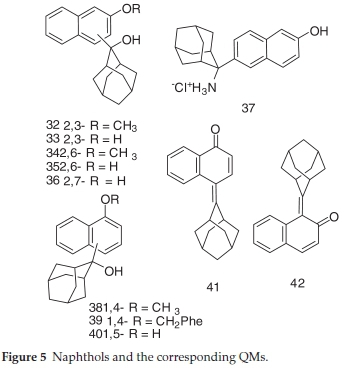
2.1.5. Synthesis of Naphthols 32-40 and the Corresponding QM Derivatives 41 and 42
2-(2-Hydroxy-2-adamantyl)-3-methoxynaphthalene (32) was prepared in a Grignard reaction from 2-bromo-3-methoxy-naphthalene and 2-adamantanone in 87 % yield according to the published procedure.23 Characterization of product 32 compares well with literature (mp = 208-210 °C).231H NMR (CDCl3, 300 MHz) δ/ppm: 7.86 (s, 1H), 7.76 (d, 1H, J = 8.0 Hz), 7.69 (d, 1H, J = 8.0 Hz), 7.42 (dt, 1H, J = 1.2Hz, J = 8.0 Hz), 7.34 (dt, 1H, J = 1.2 Hz, J = 8.0 Hz), 7.17 (s, 1H), 3.97 (s, 3H, OCH3), 3.56 (s, 1H, OH), 2.78 (br s, 2H), 2.55 (br s, 2H), 1.60-2.08 (m, 10H).
2-(2-Hydroxy-2-adamantyl)-3-hydroxynaphthalene (33) was obtained from 32 with BBr3 in 64 % yield according to the published procedure.23 Characterization of product 33 compares well with literature (mp = 226-228 °C).23 1H NMR (DMSO-d6,600 MHz) δ/ppm: 9.69 (s, 1H, OH), 7.85 (s, 1H), 7.80 (d, 1H, J = 8.1 Hz), 7.63 (d, 1H, J = 8.1 Hz), 7.36 (t, 1H, J = 8.1 Hz), 7.25 (t, 1H, J = 8.1 Hz), 7.16 (s, 1H), 5.07 (s, 1H, OH), 2.76 (br s, 2H), 2.50 (br s, 1H, covered by DMSO), 2.47 (br s, 1H), 1.80-1.85 (m, 3H), 1.72-1.80 (m, 3H), 1.67 (br s, 2H), 1.57 (d, 2H, J = 11.6 Hz).
2-(2-Hydroxy-2-adamantyl)-6-methoxynaphthalene (34) was prepared in a Grignard reaction from 2-bromo-6-methoxy-naphthalene and 2-adamantanone in 81 % yield according to the published procedure.23 Characterization of product 34 compares well with literature (mp = 162-163 °C).23 1H NMR (CDCl3, 300 MHz) d/ppm: 7.86 (d, 1H, J = 1.1 Hz), 7.68-7.76 (m, 2H), 7.63 (dd, 1H, J = 1.8 Hz, J = 8.8 Hz), 7.09-7.16 (m, 2H), 3.90 (s, 3H, OCH3), 2.66 (br s, 2H), 2.47 (br s, 1H), 2.43 (br s, 1H), 1.92 (br s, 1H), 1.68-1.80 (m, 10H).
2-(2-Hydroxy-2-adamantyl)-6-hydroxynaphthalene (35) was obtained from 34 with BBr3 in 62 % yield according to the published procedure.23 Characterization of product 35 compares well with literature (mp = 196-198 °C).23 1H NMR (DMSO-d6,300 MHz) δ/ppm: 9.63 (s, 1H, OH), 7.80 (br s, 1H), 7.74 (d, 1H, J = 8.6 Hz), 7.63 (d, 1H, J = 8.8 Hz), 7.53 (dd, 1H, J = 1.2 Hz, J = 8.8 Hz), 7.01-7.08 (m, 2H), 4.57 (s, 1H, OH), 2.57 (br s, 2H), 2.44 (br s, 1H), 2.40 (br s, 1H), 1.82 (br s, 1H), 1.45-1.65 (m, 9H).
2-(2-Hydroxy-2-adamantyl)-7-hydroxynaphthalene (36) was obtained from 2-(2-hydroxy-2-adamantyl)-7-methoxynaphthalene with BBr3 in 63 % yield according to the published proce-dure.23 Characterization of product 36 compares well with literature (mp = 250-252 °C).231H NMR (DMSO-d6,300 MHz) d/ppm: 7.66-7.74 (m, 3H), 7.40 (d, 1H, J = 8.8 Hz), 7.11 (d, 1H, J = 2.2 Hz), 7.03 (dd, 1H, J = 2.2 Hz, J = 8.7 Hz), 2.58 (br s, 2H), 2.44 (br s, 1H), 2.40 (br s, 1H), 1.82 (br s, 1H), 1.56-1.72 (m, 9H).
2-(2-Amino-2-adamantyl)-6-hydroxynaphthalene hydrochloride ( 37) was obtained from 2-(2-azido-2-adamantyl)-6-hydroxynaphthalene by reduction with Raney Ni in 19 % yield according to the published procedure.23 Characterization of product 37 compares well with literature (mp = 256-258 °C).23 1H NMR (DMSO-d6,300 MHz) d/ppm: 9.92 (s, 1H), 8.12 (br s, 3H), 7.98 (br s, 1H), 7.75-7.85 (m, 2H), 7.56-7.62 (m, 1H), 7.11-7.16 (m, 2H), 2.86 (br s, 2H), 2.34 (br s, 1H), 2.29 (br s, 1H), 1.63-1.97 (m, 10H).
1-(2-Hydroxy-2-adamantyl)-4-methoxynaphthalene (38) was prepared in a Grignard reaction from 1-bromo-4-methoxy-naphthalene and 2-adamantanone in 68 % yield according to the published procedure.32 Characterization of product 38 compares well with literature (mp = 211-212 °C).321H NMR (CDCl3, 300 MHz, 20 °C) d/ppm 8.78 (d, 1H, J = 7.8 Hz), 8.31 (d, 1H, J = 7.8 Hz), 7.55 (d, 1H, J = 8.2 Hz), 7.40-7.48 (m, 2H), 6.70 (d, 1H, J = 8.2 Hz), 3.99 (s, 3H), 2.64 (d, 2H, J = 12.0 Hz), 1.93 (br s, 1H), 1.84 (br s, 1H), 1.68-1.80 (m, 6H).
1-Benzyloxy-4-(2-hydroxy-2-adamantyl)naphthalene (39) was prepared in a Grignard reaction from 1-benzyloxy-4-bromo-naphthalene and 2-adamantanone in 58 % yield according to the published procedure.23 Characterization of product 39 compares well with literature (mp = 152-153 °C).231H NMR (CDCl3, 600 MHz) d/ppm: 8.78-8.81 (m, 1H), 8.39-8.48 (m, 1H), 7.52-7.57 (m, 3H, with a discernible doublet at 7.55, J=8.3 Hz), 7.41-7.49 (m, 4H), 7.35-7.38 (m, 1H), 6.79 (d, 1H, J = 8.3 Hz), 5.26 (s, 2H), 2.67 (br s, 1H), 2.65 (br s, 1H), 1.95 (br s, 1H), 1.85 (s, 1H), 1.70-1.82 (m, 6H).
1-Hydroxy-5-(2-hydroxy-2-adamantyl)naphthalene (40) was prepared by catalytic hydrogenation of 1-benzyloxy-5-(2-hy-droxy-2-adamantyl)naphthalene in 82 % yield according to the published procedure.23 Characterization of product 40 compares well with literature (mp = 218-220 °C).231H NMR (CD3OD) δ/ppm: 8.26 (d, 1H, J = 8.8 Hz), 8.17(d, 1H, J = 8.5 Hz), 7.65 (d, 1H, J = 7.4 Hz), 7.32 (dd, 1H, J = 8.2, J = 7.5 Hz), 7.16 (dd, 1H, J = 8.8, J = 7.5 Hz), 6.75 (d, 1H, J = 7.5 Hz), 2.70 (s, 1H), 2.69 (s, 1H), 1.87 (br s,1H), 1.62-1.81 (m, 6H);
1-(2-Adamantylidene)naphthalene-2(1fi)-one (41)was obtained in a reaction of 1-bromo-2-hydroxynaphthalene with BuLi and 2-adamantanone in 48 % yield according to the published procedure.23 Characterization of product 41 compares well with literature (mp = 152-153 °C).23 1H NMR (DMSO-d6,600 MHz) δ/ppm: 7.48-7.52 (m, 2H), 7.30-7.37 (m, 3H), 6.22 (d, 1H, J = 9.8 Hz), 4.10 (br s, 1H), 3.43 (br s, 1H), 1.94-2.02 (m, 6H), 1.84-1.90 (m, 4H), 1.77 (d, 2H, J = 12.2 Hz).
4-(2-Adamantylidene)naphthalene-1(4H)-one ( 42)was obtained by hydrogenation of 39 in 83 % yield according to the published procedure.23 Characterization of product 42 compares well with literature (mp = 158-160 °C).23 1H NMR (DMSO-d6,600 MHz) δ/ppm: 8.14 (d, 1H, J = 10.3 Hz), 8.04 (dd, 1H,J = 7.8 Hz, J = 1.4 Hz), 7.71 (d, 1H, J = 7.8 Hz), 7.64 (dt, 1H, J = 1.3 Hz, J = 8.0 Hz), 7.51 (dt, 1H, J = 0.9 Hz, J = 7.4 Hz), 6.3 (d, 1H, J = 10.3 Hz), 3.73 (s, 1H), 3.61 (s, 1H), 1.85 2.17 (m, 12H).
2.2. Antimicrobial Testing
Preliminary antimicrobial screening of the compounds was determined in duplicate using a modification of the Kirby-Bauer disc diffusion method. All compounds were dissolved in dimethyl sulfoxide (DMSO) and tested against Staphylococcus aureus ATCC 25923 (Gram-positive), Bacillus subtilis ATCC 6633 (Gram-positive), Candida albicans ATCC 10231 (fungus) Esche-richia coli ATCC 25922 (Gram-negative) and Klebsiella pneumonia ATCC 31488 (Gram-negative). The microbial cultures were grown overnight at 37 °C on Nutrient Agar plates (Biolab, South Africa), adjusted to 0.5 McFarlands standard using distilled water and lawn inoculated onto Mueller-Hinton agar (MHA) plates (Biolab, South Africa). 30 uL of each sample was inoculated onto antibiotic assay discs (6 mm diameter) and placed on the MHA plates which were incubated overnight at 37 °C and zones of inhibition were measured. DMSO was used as a control. Thereafter, minimum inhibitory concentrations (MIC) were determined in triplicate with compounds displaying antimicrobial activity using the broth dilution method. Serial dilutions (10000-19.56 ug/mL) of the compounds (with the exception of compounds 16 [4200-8.20 ug/mL) and 37 [4700-9.18 ug/mL]) were prepared from the stock solutions and tested against the cultures used in the preliminary antimicrobial activity studies.
3. Results and Discussion
MICs as determined by the broth dilution method are presented in Table 1. Generally, the compounds are not active against Gram-negative bacteria. The only exception was compound 37 that exhibited very weak activity against E. coli. Similarly, only compounds 3 and 41 exhibited antifungal activity, but only at higher concentrations. On the contrary, compounds were found to be more potent against Gram-positive bacteria. This finding suggests that the antimicrobial activity is not due to the non-specific ability to alter membranes, but rather an unknown mechanism of action. The activity against Gram-positive bacteria has been reported also for the adamantyl derivatives of oxa-diazoles, thioureas and thiadiazoles.25,26,29 However, the activity data are not comparable since different activity tests were employed.33 Adamantyl derivative of Platensimycin was found also as extremely potent against Gram-positive methicillin-resistant Staphylococcus aureus (MRSA) exhibiting activity in the range 1-2 ug/mL.27,28
With respect to the SAR it is difficult to make a straightforward conclusion, since compounds from each library were found to be active. However, the findings of this study suggest that only compounds with two aromatic rings exert activity against Gram-positive bacteria. Both 2-hydroxy-2-adamantyl and diphenylmethanol derivatives were found to be active against the tested strains, suggesting that incorporation of the adamantyl group does not enhance the activity. With respect to the substitution on the biphenyl skeleton, better activities were observed for derivatives of 3- and 4-, than for 2-hydroxy-biphenyl. From the naphthalene derivatives only compound 37 was found to be active. The data suggest that the ammonium functional group at the benzylic position (compounds 14 and 37) instead of the hydroxyl (compounds 13 and 35) probably contributes to the enhancement of the antimicrobial effect. Since the ammonium is a better leaving group, this finding suggests that the activity could be correlated with the ability of these compounds to be metabolically transformed to QMs. The most active compound is QM 41. However, the structure of QM is also important since the other QM derivative 42 exerts no activity. To verify the proposed action mechanism of the investigated compounds it would be of significant importance to detect QMs formed in a metabolic process inside the living cells. However, such a detection is not warranted since all QMs except 41 and 42 are transient species, reactive intermediates with submillisecond lifetimes.19-24
4. Conclusion
Five groups of compounds containing in total 42 phenol and naphthol derivatives were synthesized and screened for antimicrobial activity. Generally, compounds were found to be more active against Gram-positive bacteria suggesting some new mechanism of action. From the SAR studies it can be concluded that the mode of action can be correlated with the ability of compounds to from QMs. This hypothesis is to be further tested, and if shown to be true, will have an impact in the further design of antimicrobial agents.
Acknowledgements
These materials are based on work financed by the Croatian Foundation for Science (HRZZ grant no. 02.05/25 and IP-2014-09-6312), the Ministry of Science Education and Sports of the Republic of Croatia (grant No. 098-0982933-2911), The National Research Foundation (South Africa), Aspen Pharmacare and the University of KwaZulu-Natal.
Supplementary Material
The online supplement contains a table with zones of inhibition (in mm) of the compounds (n = 3).
References
1 J.J. Lucchini, J. Corre and A. Cremieux, Antibacterial activity of phenolic compounds and aromatic alcohols. Res. Microb., 1990, 141, 499-510. [ Links ]
2 C. Cueva, M.V. Moreno-Arribas, P. Martín-Álvarez, G. Bills, M.F. Vicente, A. Basilio, C. López Rivas, T. Requena, J. M. Rodriguez and B. Bartolomé, Antimicrobial activity of phenolic acids against commensal, probiotic and phatogenic bacteria. Res. Microb., 2010, 161, 372-382. [ Links ]
3 W.B. Hugo, Phenols: a review of their history and development as antimicrobial agents. Microbios., 1978, 23, 83-85. [ Links ]
4 M. Lang and R.M. Rye, The uptake by E. coli and growth inhibitory properties of benzyl alcohol and phenethyl alcohol. J. Pharm. Pharmacol., 1972, 24, 219-226. [ Links ]
5 O'Neal and T.M. Buttle, Effects of alcohols on micro-organisms. Adv. Microb. Physiol., 1984, 25, 253-300. [ Links ]
6 J. Corre, J.J. Lucchini, G.M. Mercier and A. Cremieux, Antibacterial activity of phenethyl alcohol and resulting membrane alterations. Res. Microb., 1990, 141, 483-97. [ Links ]
7 S. Silver and L. Wendt, Mechanism of action of phenethyl alcohol: breakdown of cellular permeability barrier. J. Bact. 1967,93,560-566. [ Links ]
8 M.T. Silva, J.C.F. Sousa, M.A.E. Macedo, J. Polonia and A.M. Parente, Effects of phenethyl alcohol on Bacillus and Streptococcus. J. Bact., 1976,127, 1359-1369. [ Links ]
9 P. Gilbert, E.G. Beveridge and P.B. Corne, Effect of phenoxyethanol on the permeability of E. coli NCTC 5933 to inorganic ions. Microbios, 1977,19, 17-26. [ Links ]
10 R.G. Kroll and G.D. Anagnostopoulos, Potassium fluxes on hyper-osmotic shock and the effect of phenol and bronopol (2-bromo-3-nitropropan-1,3-diol) on deplasmolysis of P aeruginosa. J. Appl. Bact., 1981, 51, 313-323. [ Links ]
11 M.L. Higgins, T.J. Shaw, M.C. Tillman and F.R. Leach, Effect of phenethyl alcohol on cell culture growth. Exp. Cell Res., 1969, 56, 24-28. [ Links ]
12 L. Jurd, A.D. King Jr., K. Mihara and W.L. Stanley, Antimicrobial properties of natural phenols and related compounds. Appl Microb., 1971, 21, 507-510. [ Links ]
13 L.J. Nohynek, H.-L. Alakomi, M.P. Kahkönen, M. Heinonen, I.M. Helander, K.-M. Oksman-Caldentey and R.H. Puupponen-Pimiä, Berry phenolics: antimicrobial properties and mechanisms of action against severe human pathogens. Nutrition Cancer., 2006, 54, 18-32. [ Links ]
14 C. Papadopoulou, K. Soulti and I.G. Roussis, Potential antimicrobial activity of red and white wine phenolic extracts against strains of Staphylococcus aureus, Escherichia coli and Candida albicans. Food Technol. Biotechnol., 2005, 43, 41-46. [ Links ]
15 S.O. Salawu, A.O. Ogundare, B.B. Ola-Salawu and A.A. Akinda-hunsi, Antimicrobial activities of phenolic containing extracts of some tropical vegetables. African J. Pharm. Pharmacol., 2011, 5, 486-492. [ Links ]
16 M.J. Alves, I.C.F.R. Ferreira, H.J.C. Froufe, R.M.V. Abreu, A. Martins and M. Pintado, Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J. Appl. Microb., 2013,115, 346-357. [ Links ]
17 Y. Shen, M.H. Hwang, S. Roffler and C.F. Chen, Cytotoxicity and antimicrobial activity of some naphthol derivatives. Arch. Pharm., 1995, 328, 197-201. [ Links ]
18 M. Reboud-Ravaux and M. Wakselman, Quinone methides and aza-quinone methides as latent alkylating species in the design of mechanism-based inhibitors of serine protease and /?-lactamase, in S. Rokita, Quinone Methides, John Wiley and Sons, Hoboken, USA, 2009, p 357-383. [ Links ]
19 N. Basaric,I. Zabcic, K. Mlinaric-Majerski and P. Wan, Photochemical formation and chemistry of long-lived adamantylidene quinone methides and 2-adamantyl cations. J. Org. Chem., 2010, 75, 102-116. [ Links ]
20 N. Basaric, N. Cindro, Y. Hou, I. Zabcic, K. Mlinaric-Majerski and P. Wan, Competing photodehydration and excited state intramolecular proton transfer (ESIPT) in adamantyl derivatives of 2-phenylphenols, Can. J. Chem., 2011, 89, 221-234. [ Links ]
21 N. Basaric, N. Cindro, D. Bobinac, Mlinaric-Majerski, L. Uzelac, M. Kralj and P. Wan, Sterically congested quinone methides in photo-dehydration reactions of 4-hydroxybiphenyl derivatives and investigation of their antiproliferative activity. Photochem. Photobiol. Sci., 2011.10, 1910-1925. [ Links ]
22 N. Basaric, N. Cindro, D. Bobinac, L. Uzelac, K. Mlinaric-Majerski, M. Kralj and P. Wan, Zwitterionic biphenyl quinone methides in photodehydration reactions of 3-hydroxybiphenyl derivatives: laser flash photolysis and antiproliferation study, Photochem. Photobiol. Sci., 2012.11, 381-396. [ Links ]
23 J. Veljkovic, L. Uzelac, K. Molcanov, K. Mlinaric-Majerski, M. Kralj, P. Wan and N. Basaric, Sterically congested adamantylnaphthalene quinone methides. J. Org. Chem., 2012, 77, 4596-4610. [ Links ]
24 D. Skalamera, K. Mlinaric-Majerski, I. Martin-Kleiner, M. Kralj and P. Wan, Near-visible light generation of a quinone methide from 3-hydroxymethyl-2-anthrol, J. Org. Chem., 2014, 79, 4390-4397. [ Links ]
25 R.E. Lee, M. Protopopova, E. Crooks, R.A. Slayden, M. Terrot and C.E. Barry, Combinatorial lead optimization of [1,2]-diamines based on ethambutol as potential antituberculosis preclinical candidates. J. Comb. Chem., 2003, 5, 172-187. [ Links ]
26 M. Protopopova, C. Hanrahan, B. Nikonenko, R. Samala, P. Chen, J. Gearhart, L. Einck and C.A. Nacy, Identification of a new antituber-cular drug candidate, SQ109, from a combinatorial library of 1,2-ethylenediamines. J. Antimicrob. Chemother., 2005, 56, 968-974. [ Links ]
27 K.C. Nicolaou, T. Lister, R.M. Denton, A. Montero and D.J. Edmons, Adamantaplatensimycin: a bioactive analogue of platensimycin. Angew. Chem. Int. Ed., 2007, 46, 4712-4714. [ Links ]
28 K.C. Nicolaou, A.F. Stepan, T. Lister, A. Li, A. Montero, G. Scott Tria, C.I. Turner, Y. Tang, J. Wang, R.M. Denton and D.J. Edmons, Design, synthesis, andbiological evaluation of platensimycin analogues with varying degrees of molecular complexity. J. Am. Chem. Soc., 2008,130, 13110-13119. [ Links ]
29 L. Wanka, K. Iqbal and P.R. Schreiner, The lipophilic bullet hits the targets: medicinal chemistry of adamantane derivatives. Chem Rev., 2013,113, 3516-3604. [ Links ]
30 L. Diao and P. Wan, Chemistry of photogenerated a-phenyl-substi-tuted o-, m-, and p-quinone methides from phenol derivatives in aqueous solution. Can. J. Chem., 2008, 86, 105-118. [ Links ]
31 N. Cindro, I. Antol, K. Mlinaric-Majerski, I. Halasz, P. Wan and N. Basaric, Reactivity of cations and zwitterions formed in photochemical and acid-catalyzed reactions from m-hydroxycycloalkyl-substituted phenol derivatives. J. Org. Chem., 2015, 80, 12420-12430. [ Links ]
32 J. Veljkovic, I. Antol, N. Basaric, V. Smrecki, K. Molcanov, N. Müller and K. Mlinaric-Majerski, Atropisomerism in 1-(2-adamantyl)naph-thalene derivatives, J. Mol. Struct. 2013,1046, 101-109. [ Links ]
33 A.A. Kadi, N.R. El-Brollosy, O.A. Al-Deeb, E.E. Habib, T.M. Ibrahim and A.A. El-Amam, Synthesis, antimicrobial, and anti-inflammatory activities of novel 2-(1-adamantyl)-5-substituted-1,3,4-oxadiazoles and 2-(1-adamantylamino)-5-substituted-1,3,4-thiadiazoles. Eur. J. Med. Chem., 2007, 42, 235-242 [ Links ]
Received 11 August 2015
Revised 20 January 2016
Accepted 5 February 2016
* To whom correspondence should be addressed. E-mail: nbasaric@irb.hr
Supplementary Data
The supplementary data is available in pdf: [Supplementary data]




![Synthesis and characterization of new bis-symmetrical adipoyl, terepthaloyl, chiral diimido-di-L-alanine diesters and chiral phthaloyl-L-alanine ester of tripropoxy p-tert-butyl calix[4]arene and study of their hosting ability for alanine and Na+](/img/pt/prev.gif)









