Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.68 Durban 2015
http://dx.doi.org/10.17159/0379-4350/2015/v68a30
RESEARCH ARTICLE
New pyridazinium-based ionic liquids: An eco-friendly ultrasound-assisted synthesis, characterization and biological activity
Mouslim MessaliI, *; Mohammed N. AlmtiriI; Bousskri AbderrahmanII; Rachid SalghiII; Mohamed R. AouadI, III; Solhe F. AlshahateetIV; Adeeb A-S. AliI
IDepartment of Chemistry, Taibah University, 30002, Al-Madina Al-Mounawara, Saudi Arabia
IILaboratory of Environmental Engineering and Biotechnology, ENSA, Ibn Zohr University, Box 1136, 80000 Agadir, Morocco
IIIDepartment of Chemistry, (LCECM) USTO-MB, University of Sciences and Technology Mohamed Boudiaf, BP 1505 Oran, El M'nouar, Algeria
IVDepartment of Chemistry, Mutah University, Mutah 61710, Karak-Jordan
ABSTRACT
An eco-friendly ultrasound-assisted procedure was developed for the preparation of a series of novel pyridazinium ionic liquids (ILs) 1-8. The structures of the novel ILs were established based on their FT-IR, 1H, 13C NMR and mass spectra. Furthermore, the antimicrobial and anticancer activities of ILs 1-8 have been also investigated. The results of these screening experiments revealed that the ILs exhibited good to moderate antibacterial activity, such as IL 7, compared with standard drugs. Some of the newly synthesized ILs were also tested in vitro against human hepatocellular carcinoma (HEPG2), human breast adenocarcinoma (MCF7) and human colon carcinoma (HCT116) cell lines. Among the synthesized ILs, IL 8 was found to exhibit promising antiproliferative effects and produced the lowest IC50 values among the tested ILs.
Keywords: Eco-friendly procedure, ionic liquid, ultrasound irradiation, antibacterial, antifungal and anticancer activity
1. Introduction
The level of research interest directed towards ionic liquids (ILs) has grown considerably during the past two decades because of their many outstanding properties, including their negligible vapour pressure, great thermal stability, nonflamma-bility, good electrical conductivity, moderate viscosity and excellent solubility towards organic and inorganic compounds.1-2 Based on their attractive properties, ILs were extensively employed as an alternative green solvent to the common organic solvents.3 Furthermore, ILs have been used in a broad range of applications in a variety of different fields, including the electrodeposition of metals4 and the development of corrosion inhibitors,5-7 as well as numerous applications in polymer science and catalysis.8-9
On the basis of these findings, a series of novel functionalized pyridazinium-based ILs 1-7 is communicated using an efficient green ultrasound-assisted reaction. Our group recently reported the synthesis of another interesting IL known as 1-(2-(4-nitro-phenyl)-2-oxoethyl)pyridazinium bromide, which will be referred to hereafter as IL 8.7The antimicrobial and antitumor activities of ILs 1-8 were evaluated in the current study and the results of these experiments have been discussed in detail.
2. Results and Discussion
2.1. Chemistry
Numerous reports have recently been published pertaining to the use of ultrasonic irradiation to promote a variety of organic transformations. 10-11 With this in mind, ultrasonic irradiation was used in the current study as an eco-friendly approach for the successful construction of new pyridazinium-based ILs 1-8. Briefly, a solution of pyridazine was treated with several different alkyl halides under ultrasonic irradiation in a closed system (Scheme 1).
Conventional N-alkylation conditions generally require long reaction times and provide moderate to low yields of the desired products. However, the use of ultrasound technology can lead to a significant decrease in the reaction time (5 h) required for an N-alkylation reaction compared with conventional conditions.12 The yields obtained in the current study for the synthesis of ILs 1-8 under ultrasonic irradiation are shown in Table 1.
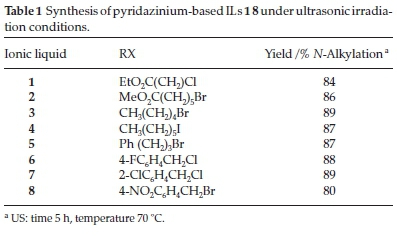
The newly synthesized ILs 1-8 were characterized by 1H NMR, 13C NMR, FT-IR and LCMS analyses. The characterization of IL 2 was discussed in detail as the most representative compound of this new family of ILs.
The N-alkylation of pyridazine with methyl 6-bromohexa-noate afforded 2, which result in the appearance of a singlet at 3.59 ppm due to the methyl protons of OCH3 group. The five methylene protons of the IL2 appeared respectively as a triplet at 4.88 ppm for NCH2CH2, a triplet at 2.32 ppm for COCH2CH2,a quintet at 2.03 ppm for COCH2CH2, a sextet at 1.58 ppm for CH2CH2CH2 and as a quintet at 2.03 for NCH2CH2 The 1H NMR spectrum for 2 displays also two type of signals related to the pyridazinium protons. Two doublets at 9.70 and 10.16 ppm represent CHN=NCH and two doublets of doublet at 8.69 and 8.80 originate from NCHCHCHCHN.
The 13C NMR spectra of ILs 2 contained signal is consistent with the presence of a carbonyl group (CO) at 173.2ppm. The signals of the five CH2 groups and a CH3 group appeared respectively at 64.2 ppm for (NCH2), 51.2 ppm for (OCH3), 32.9 ppm for (COCH2), and 23.7,24.7,29.0 ppm for CH2CH2CH2. The 13C NMR spectra also displayed the signals of the four tertiary carbons which appeared, respectively, at 150.0 and 154.4 ppm for the CHN=NCH, 136.0 and 136.5 ppm for the NCHCHCHCHN groups.
The structure of IL 2 was also confirmed by the FT-IR spectra in which an absorption band at 1731 cm-1 indicated clearly the presence of a carbonyl group (C=O) belonging to an ester. And finally, LCMS spectra of ILs 2 contained a peak at 209.3 ppm related the desired mass ions [M-Br]+.
Generally, the 1H NMR spectra of ILs 1 and 3-8 all contained signals in the range of 8.4-10.0 ppm, which were attributed to the pyridazinium protons. The phenyl protons of the pendent aromatic rings in ILs 5-8 were observed at 7.3-8.1 ppm. The characteristic ethyl ester protons of IL 1 appeared as a triplet and quartet at 1.25 and 4.25 ppm, respectively.
The 13C NMR spectra of ILs 1 and 8 contained signals consistent with the presence of a carbonyl group at dC 165.7 and 189.7 ppm, respectively. The 13C NMR spectra of ILs 1 and 3-8 all contained signals corresponding to aromatic carbons, including those of the pyridazinium ring, in the range of dC 116-162 ppm. Furthermore, the 13C NMR spectra of ILs 1 and 3-8 contained carbon signals corresponding to CH2 and CH3 groups with the usual chemical shifts.
The success of this new strategy for the N-alkylation of pyridazine was also confirmed by the FT-IR spectra of ILs 1 and 3-8. The FT-IR spectra of IL 1 contained an absorption band at 1730 cm-1, which indicated the presence of a carbonyl group (C=O) belonging to an ester. Furthermore, the FT-IR spectrum of IL 8 contained a peak at 1637 cm-1, which was consistent with the presence of a carbonyl group (C=O) belonging to a ketone. Finally, the LCMS spectra of ILs 1 and 3-8 contained peaks consistent with the desired mass ions [M-X]+.
2.2 Antimicrobial Properties
Several recent reports showed that ILs possess promising biological activities.13-15 Encouraged by these results, we proceeded to evaluate the antimicrobial activities of ILs 1-8 against four strains of bacteria, including two Gram-positive and two Gram-negative strains, and four strains of fungi.
2.2.1. Antifungal Properties
In recent years, extensive research appeared in literature regarding the design of new families of antifungal agents presumably due to the increasing resistance of fungi towards existing antifungal agents. The antifungal activities of ILs 1-8 were screened in vitro against Aspergillus fumigatus, Penicillium italicum, Candida albicans and Geotrichum candidum. The anti-fungal activities were measured using the agar diffusion method with Sabouraud's agar medium. The results of these experiments are summarized in Table 2 and represented graphi cally in Fig. 1.
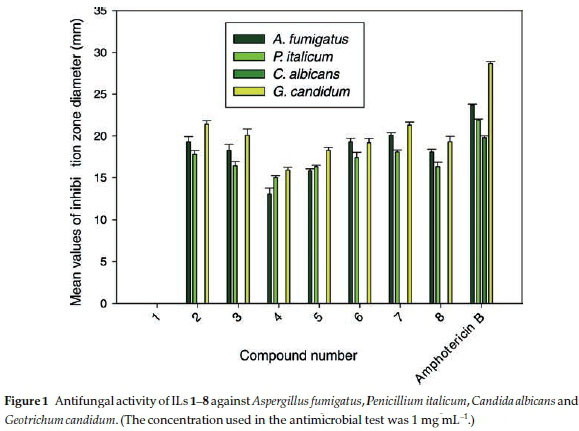
As shown in Table 2, the zones of inhibition fluctuated from 21.3 mm for IL 7 to 13.1 mm for IL 3, and were therefore comparable to those observed of the antibiotic Amphotericin B, which was used as a positive control.
2.2.2. Antibacterial Properties
The antibacterial activities of ILs 1-8 were tested in vitro against two Gram-positive (Staphylococcus aureus and Bacillus subtilis) and to Gram-negative (Pseudomonas aeruginosa and Escherichia coli) bacteria. The antibacterial activities of ILs 1-8 were determined using the agar diffusion method with Mueller-Hinton agar medium.
The results of these experiments are displayed in Table 3 and represented graphically in Fig. 2, and show that ILs 1-8 exhibited similar levels of antibacterial activity towards S. aureus, B. subtilis, P. aeruginosa and E. coli to both Ampicillin and Gentamicin.
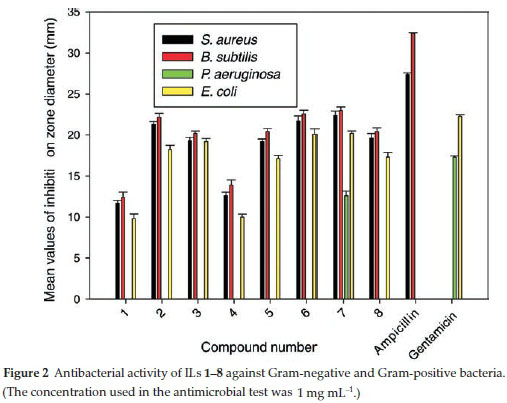
Furthermore, IL 7 exhibited the highest activity towards all of the microorganisms tested in the current study at a concentration of 30 mL-1. The higher activity of IL 7 was attributed to its 2-chlorobenzyl group.
2.2.3. Minimum Inhibitory Concentration (MIC)
Since ILs 2-3 and 5-8 showed good Inhibition zone, they were selected in order to test the in vitro Minimum Inhibitory Concentration (MIC) and the obtained results are summarized in Table 4.
The MIC of the tested ILs showed good to moderate activity against eight types of human pathogens comparing with drugs standard and the range of MIC levels varied from 0.99 to more than 260 μM.
2.2.4. Cytotoxic Activity of Compounds 1, 2 and 8 against Tumor Cell Lines
ILs 1, 2 and 8 were selected for further evaluation based on their in vitro activities, and their cytotoxic activities were evaluated towards a series of human cancer cell lines, including HEPG2 (human hepatocellular carcinoma), MCF-7 (human breast adenocarcinoma) and HCT116 (colon carcinoma) cells. Cell growth and cell viability measurements were recorded according to a previously reported procedure.16 Cytotoxicity was evaluated using various viability assays (HCT cytotoxicity Assay and MTT Metabolic impairment Assay), which were performed by the Regional Center for Mycology & Biotechnology at Al-Azhar University, Egypt. The results of these assays are displayed in Figs. 3-5. The used concentrations are the same for all tested ILs and are expressed in mg mL-1 and he results are the mean of three repetitions.
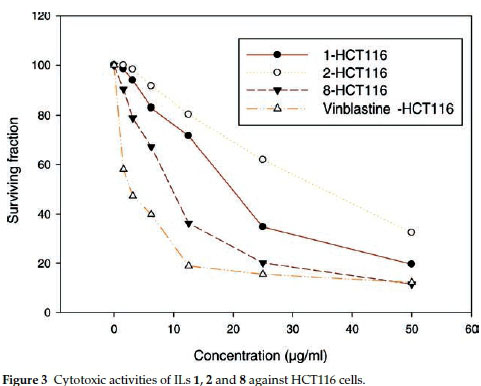
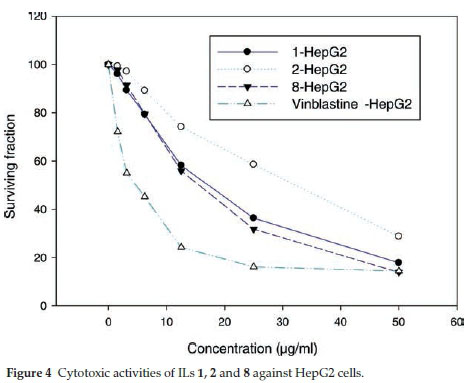
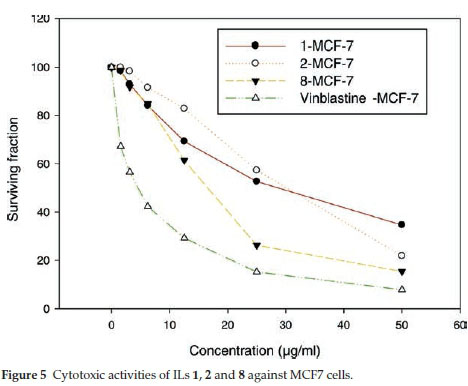
The results of the cytotoxicity assays showed that ILs 1,2 and 8 exhibited dose-dependent activities towards all three cell lines, with increases in the dose leading to a reduction in survival. However, the dose responses were less pronounced for concentrations in the range of 25-50 mg mL-1, but IL 8 was found to exhibit the higher antiproliferative activity in the range of 5 to 25 mg mL-1.
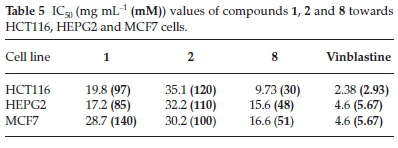
The IC50 values of ILs 1,2 and 8 were found to be in the range of 30 to 140 μΜ, with IL 2 showing the weakest activity of the three different compounds (Table 5). Notably, IL 8 showed the highest activities of the three ILs tested towards all three cell lines, with IC50 values in the range of 30 to 51 μΜ, although the activities of this compound was less than those of Vinblastine, which was used as a positive control.
3. Experimental
All new compounds were characterized by 1H NMR, 13C NMR and IR spectroscopy and LCMS. 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were measured in DMSO and D2Oat room temperature. Chemical shifts (d) were reported in ppm, with tetramethylsilane (TMS) as an internal standard. The Liquid chromatography-mass spectrometry LCMS (spectra were measured with a Micromass LCT (Liquid Chromatography Tandem) mass spectrometer (Model: Micromass LCT Classic, Agilent Technologies, Germany). IR spectra were recorded on a KBr disc with a Shimadzu 8201 PC FT-IR spectrophotometer (vmax in cm-1). Ultrasound-assisted reactions were performed with a high-intensity ultrasonic processor SUB Aqua 5 Plus-Grant with a temperature controller (750 W), microprocessor controlled-2004. The ultrasonic frequency of the cleaning bath used is 25 KHz.
3.1. Synthesis
3.1.1. General Procedure for the Synthesis of N-alkylpyridazinium Halides 1-8 with Ultrasonic Irradiation
Pyridazine (1.0 eq.) and an alkyl halide (1.1 eq.) were added to toluene (15 mL) and placed in a closed vessel and exposed to irradiation for5hat70°Cina sonicator until a precipitate was formed. The obtained solid was filtered and washed with ethylacetate to afford the desired pyridazinium IL
3.2. Characterization
3.2.1.1-(2-Ethoxy-2-oxoethyl)pyridazinium Chloride 1 This compound was obtained as brown solid; Mp 120-122 °C; yield 84 %; dH (DMSO-d 400 MHz,): 1.25 (t, J = 7.2 Hz, 3H), 4.26 (q, J = 7.2 Hz, 2H), 6.17 (s, 2H), 8.82-8.98 (2Ar-H), 9.82 (d, 1Ar-H), 10.42 (1Ar-H); dC (DMSO-d6,100 MHz,): 13.8 (CH3), 62.5 (CH2), 64.2 (CH2), 136.0 (CH), 137.8 (CH), 151.9 (CH), 154.7 (CH), 165.7 (CO); IR (nmaxcm-1) 3000 (C-H Ar), 1748 (C=O), 1572-1446 (c=C), 1240 (C-N), 1027 (C-O) cm-1; LCMS (M-Cl) 167 found for C8H11N2O2+. (Found: C, 47.35; H, 5.50; N, 13.76 %. Calc. for C8H11ClN2O2 (202.64); C, 47.42; H, 5.47; N, 13.82 %).
3.2.2.1-(6-Methoxy-6-oxohexyl)pyridazinium Bromide 2
This compound was obtained as dark semi-solid; yield 86 %; dH (DMSO-d6,400 MHz,): 1.34 (quint, J = 7.2 Hz, 2H),1.58 (sextet, J = 7.2 Hz, 2H), 2.03 (quint, J = 7.2 Hz, 2H), 2.32 (t, J = 7.2 Hz, 2H), 3.59 (s, 3H), 4.88 (t , J = 7.2 Hz , 2H), 8.69 (1Ar-H), 8.80 (1Ar-H), 9.70 (1Ar-H), 10.16 (1Ar-H); dC (DMSO, 100 MHz,): 23.7 (CH2), 24.7 (CH2), 29.0 (CH2), 32.9 (CH2), 51.2 (CH3), 64.2 (CH2), 136.0 (CH), 136.5 (CH), 150.0 (CH), 154.5 (CH),173.2 (CO); IR (νmax cm-1 ) 2 997 (C-H Ar), 1747 (C=O), 1595-1445 (C=C), 1195 (C-N), 998 (C-O) cm-1; LCMS (M-Br) 209 found for C11H17N2O2+. (Found: C, 45.60; H, 6.02; N, 9.74 %. Calc. for C11H17BrN2O2 (289.17); C, 45.69; H, 5.93; N, 9.69 %).
3.2.3.1-Pentylpyridazinium Bromide 3
This compound was obtained as brown solid; Mp 118-120 °C; yield 89 %; δH (400MHz, DMSO): 0.87 (t, 3H), 1.13 (m, 4H), 2.03 (quint, 2H), 4.89 (t, 2H), 8.68 (dd, 1Ar-H), 8.83 (dd, 1Ar-H), 9.71 (d, 1Ar-H), 10.20 (d, 1Ar-H); dC (100MHz, DMSO): 13.6 (CH3), 21.5 (CH2), 27.4 (CH), 29.1 (CH2), 64.4 (CH2), 136.0 (CH), 136.5 (CH), 149.8 (CH), 154.5 (CH). IR (νmax cm-1) 3032 (C-H, sp2), 1570-1471 (C = C), 1165(C-N); LCMS (M-Br) 151 found for C9H15N2+. (Found: C, 46.70; H, 6.48; N, 12.16 %. Calc. for C9H15BrN2 (231.13); C, 46.77; H, 6.54; N, 12.12 %).
3.2.4.1-Hexylpyridazinium Iodide 4
This compound was obtained as greenish brown solid; Mp 62-64 °C; yield 87 %; δH (400MHz, DMSO-d6): 0.85 (t, 3H), 1.30 (m, 6H), 2.02 (quint, 2H), 4.84 (t, 2H), 8.64 (1Ar-H), 8.77 (1Ar-H), 9.68 (1Ar-H), 10.04 (1Ar-H); dC (100MHz, DMSO-d6): 13.8 (CH3), 21.8 (CH2), 25.0 (CH2), 29.3 (CH2), 30.5 (CH2), 64.5 (CH2), 136.0 (CH), 136.5 (CH), 149.8 (CH), 154.5 (CH). IR (νmax cm-1) 3028 (C-H, sp2), 1567-1441 (c=C), 1143(C-N); LCMS (M-I) 165 found for C10H17N2+. (Found: C,41.05; H, 5.92; N, 9.53 %. Calc. for C1H17IN2 (292.16); C, 41.11; H, 5.86; N, 9.59 %).
3.2.5.1-(3-Phenylpropyl)pyridazinium Bromide 5
This compound was obtained as brown solid; Mp 100-102 °C; yield 87 %; δH (400MHz, DMSO-d6): 2.35 (quint, 2H), 2.72 (t, 3H), 4.91 (t, 2H), 7.19-7.31 (m, 5H), 8.67 (1Ar-H), 8.77 (1Ar-H), 9.68 (1Ar-H), 10.04 (1Ar-H); δH (100MHz, DMSO-d6): 30.9 (CH2), 31.5 (CH2), 64.2 (CH2), 126.1 (CH), 128.3 (CH), 128.4 (CH), 136.0 (CH), 136.5 (CH), 140.3 (C), 150.0 (CH), 154.4 (CH); IR (νmax cm-1) 2997 (C-H, sp 2 ), 1587-1473 (C=C), 1162(C-N); LCMS (M-Br) 199 found for C13H15N2+. (Found: C, 55.82; H, 5.49; N, 9.99 %. Calc. for C13H15BrN2 (279.18); C, 55.93; H, 5.42; N, 10.08 %).
3.2.6.1-(4-Fluorobenzyl)pyridazinium Chloride 6
This compound was obtained as beige solid; Mp 200-202 °C; yield 88 %; δH (400MHz, D2O): 5.96 (s, 2H), 7.75 (Ar-H), 8.13 (2Ar-H), 8.82 (1Ar-H), 8.94 (1Ar-H), 9.78 (1Ar-H), 10.05 (1Ar-H); dC (100MHz, D2O): 67.9 (CH2), 116.1 (CH), 116.3 (CH), 132.0 (CH), 132.1 (CH), 136.1 (CH), 137.0 (CH), 149.2 (CH), 154.7 (CH), 162.0 (C); IR (νmax cm-1) 3038 (C-H, sp2), 1588-1470 (c=C), 1188(C-N); LCMS (M-Cl) 189 found for C11H10FN2+. (Found: C, 58.72; H,4.55; N, 12.40 %. Calc. for C11H10ClFN2 (224.66); C, 58.81; H, 4.49; N, 12.47 %).
3.2.7.1-(2-Chlorobenzyl)pyridazinium Chloride 7
This compound was obtained as beige solid; Mp 176-178 °C; yield 89 %; δH (400MHz, D2O): 6.09 (s, 2H), 7.35-7.45 (m, 3Ar-H), 7.58 (1Ar-H), 8.47 (1Ar-H), 8.53 (1Ar-H), 9.37 (1Ar-H), 9.71 (1Ar-H); dC (100MHz, D2O): 66.3 (CH2), 128.0 (CH), 129.1 (C), 130.2 (CH), 132.1 (CH), 134.7 (C), 136.0 (CH), 137.1 (CH), 133.2 (C), 149.6 (CH), 154.7 (CH); IR (νmax cm-1) 3043 (C-H, sp2), 1572-1474 (C=C), 1167 (C-N); LCMS (M-Cl) 205 found for CnH10ClN2+; (Found: C, 54.71; H, 4.26; N, 11.59 %. Calc. for C11H10Cl2N2O2 (241.12); C, 54.79; H, 4.18; N, 11.62 %).
3.2.8.1-(2-(4-Nitrophenyl)-2-oxoethyl)pyridazinium Bromide 8
This compound was obtained as beige solid; Mp 225-227 °C; yield 80 %; δH (400MHz, DMSO): 6.87 (s, 2H), 7.75 (2Ar-H), 8.13 (dd, 2Ar-H), 8.82 (1Ar-H), 8.94 (1Ar-H), 9.78 (1Ar-H), 10.05 (1Ar-H); δC (100MHz, DMSO): 70.5 (CH2), 124.1 (CH), 129.9 (CH), 136.0 (CH), 137.6 (CH), 137.9 (C), 151.8 (CH), 154.7 (CH), 150.7 (C), 189.7 (CO); IR (νmax cm-1) 3112 (C-H, sp2), 1721 (C=O), 1591-1448 (C = C), 1112(C-N); LCMS (M-Br) 244 found for C12H10N3O3+; (Found: C, 44.39; H, 3.19; N, 12.93%. Calc. for C12H10BrN3O3 (324.13); C, 44.47; H, 3.11; N, 12.96%).
3.3. Determination of IZ and MIC
The antimicrobial potential was estimated in terms of inhibition zone (IZ) measurements by agar disc diffusion method. The test was performed by transferring 24 h fresh bacterial cultures onto the surface of Muller-Hinton agar plates to yield confluent growth. The antibacterial potential was assayed using filter paper discs (6 mm i.d.) loaded with 10 of tested ILs. Loaded discs were transferred onto the centre of inoculated Petri-plates which were then maintained for2hinarefrigerator at 4 C to allow for the diffusion of the bioactive compound. The diameter of the inhibition zone was measured (in mm) after 24 h incubation at 37 °C. Sterile distilled water was used as a control. Clinical samples of Escherichia coli, Enterobacter sp., Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus epidermidis, Bacillus cereus and Acinetobacter baumannii were used as test strains.
The minimum inhibitory concentration (MIC) of tested ionic liquids were determined by CLSI microdilution-based method.17 Test compound was dissolved in sterile, distilled water and diluted to a final concentration of 256 mL-1 in Mueller-Hinton broth (Becton Dickinson, USA).18 Two-fold serial dilutions were prepared in a 96-well microtitre plate. Bacterial suspension containing approximately 1 x 108 CFU mL-1 were prepared from 24 h agar plates with Mueller Hinton broth. Aliquots of 100 of each bacterial suspension was mixed with 100 serially diluted tested compound in microtitre plate.19 Uninoculated wells were prepared as control samples. Plates were incubated at 37 °C for 24 h. The MIC was defined as the lowest concentration of test compound producing no visible growth. All experiments were performed in triplicate.
4. Conclusion
A novel series of functionalized pyridazinium-based ILs has been successfully synthesized under ultrasonic irradiation conditions. The structures of these compounds were fully characterized by FT-IR, 1H-NMR, 13C-NMR and mass spectrometry. Compared with standard antibiotics, the results of several antimicrobial screening experiments showed that our newly synthesized ILs exhibited good to moderate antimicrobial and antifungal activities. Furthermore, the cytotoxic activities of three of these new ILs were tested in vitro against three different human carcinoma cell lines. The results revealed that IL 8 could be considered as a promising antiproliferative agent with low IC50 values against all three cell lines.
Supplementary Data
Supplementary data (1H NMR, 13C NMR, Dept NMR, IR and mass spectra) associated with this article are available in the online supplement.
References
1 H. Olivier-Bourbigou, L. Magna and D. Morvan, Ionic liquids and catalysis: recent progress from knowledge to applications, Appl. Catal. A, 2010, 373, 1-56. [ Links ]
2 R.P. Swatloski, S. K. Spear, J.D. Holbrey and R.D. Rogers, Dissolution of cellulose with ion icliquids,J.Am. Chem. Soc.,2002,124,4974-975. [ Links ]
3 R.A. Sheldon, R.M. Lau, M. J. Sorgedrager, F. van Rantwijk and K.R. Seddon, Biocatalysis in ionic liquids, Green Chem., 2002,4, 147-151. [ Links ]
4 F. Endres, Ionic liquids: solvents for the electrodeposition of metals and semiconductors, Port. Electrochim. Acta, 2011, 29, 375-389. [ Links ]
5 Ibrahim M.A.M. Ibrahim, M. Messali, Z. Moussa, A.Y. Alzahrani, S.N. Alamry and B. Hammouti, Corrosion inhibition of carbon steel by imidazolium and pyridinium cations ionic liquids in acidic environment, Port. Electrochim. Acta, 2011, 29, 375-389. [ Links ]
6 M. Messali, A green microwave-assisted synthesis, characterization and comparative study of new pyridazinium-based ionic liquids derivatives towards corrosion of mild steel in acidic environment, J. Mater. Environ. Sci., 2011, 2, 174-185. [ Links ]
7 M. Messali, A. Bousskri, A. Anejjar, R. Salghi and B. Hammouti, Electrochemical studies of new pyridazinium-based ionic liquid, (1-4-nitro phenyl-1-ethanone) pyridazinium bromide, on carbon steel corrosion in hydrochloric acid medium, Int. J. Electrochem. Sci., 2015,10, 4532-4551. [ Links ]
8 P. Kubisa, Application of ionic liquids as solvents for polymerization processes, Prog. Polym. Sci., 2004, 29, 3-12. [ Links ]
9 R.G. Kalkhambkar, S.N. Waters and K.K. Laali, Highly efficient synthesis of amides via Ritter chemistry with ionic liquids, Tetrahedron Lett., 2011, 52, 867-871. [ Links ]
10 J.D. Oxley, T. Prozorov and K.S. Suslick, Sonochemistry and sono- luminescence of room-temperature ionic liquids, J. Am. Chem. Soc., 2003,125, 11138-11139. [ Links ]
11 S. Zhao, E. Zhao, P. Shen, M. Zhao and J. Sun, An atom-efficient and practical synthesis of new pyridinium ionic liquids and application in Morita-Baylis-Hillman reaction, Ultrason. Sonochem., 2008, 15, 955-959. [ Links ]
12 M. Messali, An efficient and green sonochemical synthesis of some new eco-friendly functionalized ionic liquids, Arab. J. Chem., 2014, 7, 63-70. [ Links ]
13 M. Messali, Z. Moussa, A.Y. Alzahrani, M.Y. El Naggar, A.S. El Douhaibi and B. Hammouti, Synthesis, characterization, antimicrobial activity of new green-chemistry-friendly ionic liquids, Chemosphere, 2013, 91, 1627-1634. [ Links ]
14 M. Messali, M. R. Aouad, W.S. El sayed, A.A-S. Ali, T. Ben Hadda and B. Hammouti, New eco-friendly 1-alkyl-3-(4-phenoxybutyl) imidazolium-based ionic liquids derivatives: a green ultrasound- assisted synthesis, characterization, antimicrobial activity and POM analyses, Molecules, 2014,19, 11741-11759. [ Links ]
15 M. Messali, M.R. Aouad, A.A-S. Ali, N. Rezki, T. Ben Hadda and B. Hammouti, Synthesis, characterization, and POM analyses of novel bioactive imidazolium-based ionic liquids, Med. Chem. Res., 2015, 24, 1387-1395. [ Links ]
16 M.I. Atta-Ur-Rahman, W.J. Thomsen and W.J. Thomsen, Bioassay Techniques for Drug Development, Harwood Academic Publishers, Netherlands, 2001, p. 22. [ Links ]
17 Clinical and Laboratory Standards Institute (CLSI), Document M26-A, Methods of determiningbactericidal activity of antimicrobial agents for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved Guideline, Wayne, PA, 1999. [ Links ]
18 H. Nomura, Y. Isshiki, K. Sakuda, K. Sakuma and S. Kondo, The antibacterial activity of compounds isolated from oakmoss against Legionella pneumophila and other Legionella spp., Biol. Pharm. Bull., 2012, 35, 1560-1567. [ Links ]
19 Clinical and Laboratory Standards Institute (CLSI), Document M7-A5, Methods for antibacterial susceptibility test for bacteria that grow aerobically. Approved Standard, 5th edn., Wayne, PA, 2000. [ Links ]
Received 21 May 2015
Revised 25 June 2015
Accepted 26 June 2015
* To whom correspondence should be addressed. E-mail: mouslim@mail.be
Supplementary Data
The supplementary data is available in pdf: [Supplementary data]














