Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.68 Durban 2015
http://dx.doi.org/10.17159/0379-4350/2015/v68a29
RESEARCH ARTICLE
A simple polarimetry technique for predicting the absolute configuration of the preferred enantiomer in chiral host-guest inclusion complexes
Benita Barton*; Pieter Pohl
Department of Chemistry, PO Box 77000, Nelson Mandela Metropolitan University, Port Elizabeth, 6031, South Africa
ABSTRACT
A method for predicting the configuration of the preferred guest enantiomer in an inclusion complex with an optically pure hosl compound was developed. The method involves simply measuring the optical rotation of the host-guest inclusion complex as a whole, by means of polarimetry, and using this value in a calculation in order to obtain information about the guest configuration. The availability of standard optically pure guest materials is not required, nor is the isolation of the guest species from the host crystal, resulting in an attractive, inexpensive, rapid and simple procedure for this purpose.
Keywords: Host-guest chemistry, polarimetry, optical rotation, supramolecular chemistry, inclusion chemistry
Graphical Abstract
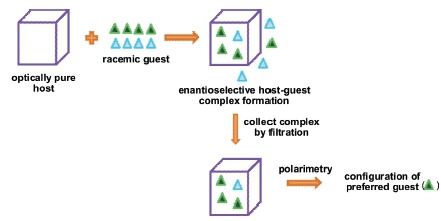
1. Introduction
Host-guest chemistry, or the chemistry of inclusion compounds, has previously been successfully employed for racemate resolutions by renowned host-guest chemists such as D. Seebach,1 F. Toda,2-5 K. Tanaka3,4 and L.R. Nassimbeni.2 In order for these separations to be successful, it is obligatory that the host compound is chiral and optically pure as well as crystalline. In a typical experiment, the host compound is recrystallized from the racemic guest. Due to the optical purity of the host species, the cavities in the host crystal have a particular shape, and this often leads to enantiodiscrimination between the two guest enantio-mers present, with one being better accommodated in these cavities than the other. The resultant host-guest complex which crystallizes out is then filtered off from the solution, thus effectively separating the two enantiomers from one another.
The host is oftentimes not completely selective in its preference for either enantiomer, and there is frequently a need to determine the enantiomeric excess (e.e.) of the entrapped guest species. This may be achieved in a number of ways. Chiral chro-matography on the inclusion complex results in two peaks on the chromatogram for the guest species, one for each of the enantiomers, and area comparisons of these peaks affords information on the relative amounts of these enantiomers.6 Alternatively, the guest may be recovered from the host crystal first by means of distillation, dissolved in an appropriate solvent, and injected directly onto the GC or HPLC column. The use of NMR analysis employing a chiral shift reagent such as tris[3-(hepta-fluoropropylhydroxymethylene)-d-camphorato]-europium(III) is also a viable method for determining e.e. values as applied by Toda et al.4in a similar chiral resolution experiment. Another method involves converting the liberated guest into diastereomers, and subsequent integration of the twinned singlets in the 13C-NMR spectrum for the mixture of diastereomers would provide the same e.e. information.78 Finally, measuring the optical rotation of the liberated guest by means of polarimetry, and knowing the theoretical value for the pure (R)- or (S)-enan-tiomer allows one to calculate the e.e. of the sample.
Chromatographic analysis techniques require that either the (R)- or (S)-enantiomer be available as a standard for comparative purposes, while the other methods mentioned involving guest release and/or derivatization are rather time-intensive and cost-prohibitive, and require a significant amount of the inclusion complex in order to obtain enough of the guest compound for e.e. determinations by these methods.
In our laboratories, a need arose to develop a method to predict not the e.e. but, more broadly, the configuration of the preferred guest enantiomer present in the chiral host crystal. Up until this stage, the authors were only aware of the aforementioned methods for determining the configuration (that is, through e.e. calculations). Since no standards were available to us, the optimal method had to be independent of chromatography. Furthermore, a simpler process that does not require that the guest first be liberated from the host cavities is, naturally, attractive from a time conservation point of view. Here we report a new and successful method based on polarimetry for predicting the configuration of the preferred guest compound in the host crystal. Though polarimetry has been used for determining the binding constants between cyclodextrin and guest molecules in solution,9 it has, to the best of our knowledge, not previously been used to provide information on the configuration of the guest species without first separating these species from the host crystal. The proposed method is simple and fast, and is independent of chromatography.
2. Experimental
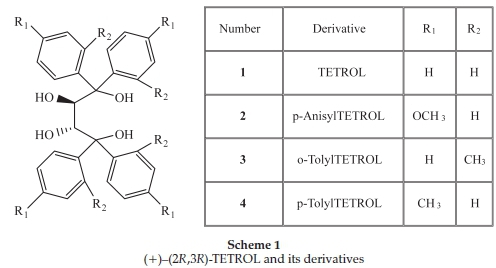
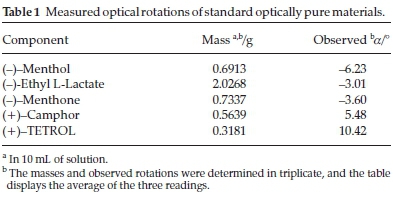
Five single solute solutions containing known amounts of the optically pure compounds (-)-menthone, (-)-menthol, (+)-camphor, (-)-ethyl L-lactate and the host compound (+)-(2R,3R)-1,1,4,4-tetraphenylbutane-1,2,3,4-tetraol (TETROL 1, Scheme 1) were prepared in 10 mL volumetric flasks using dichloromethane as the diluting solvent. The observed rotations (a) of these solutions were measured in triplicate using a polarimeter, and the data that were obtained is summarized in Table 1. Following this, binary solutions were then prepared by mixing known amounts of any two of the same standard materials listed above. Various combinations were prepared in this way, and the solutions were, once again, analyzed using the polarimeter. The results attained are given in Table 2.
From these experiments, therefore, it was confirmed that optical rotations are additive in nature, and we used this fact to predict the configuration of the preferred guest enantiomer in a host-guest complex without having to first liberate the guest. To this end, the optically pure host compound (+)-TETROL was recrystallized individually from racemic guest species 2-methyl-cyclohexanone, 3-methylcyclohexanone, methyl phenyl sulfoxide and 2-butanol by dissolving the host in the liquid racemic guest and allowing the latter to evaporate off slowly at ambient temperature and pressure. The resultant solid complexes that formed were recovered by vacuum filtration and washed thoroughly with petroleum ether. 1H-NMR spectroscopy showed that all four of these guests form 1:1 host:guest complexes with TETROL. In order to determine whether the host was able to discriminate between the guest enantiomers during complex formation, we prepared solutions with known masses of the so-obtained complexes and analyzed these using the polarimeter. The information (Table 3) was then used to determine whether there had been enantiodiscrimination and to predict, by calculation, the configuration of the preferred guest in each case.
3. Results and Discussion
Our research group has shown that the chiral host compound (+)-(2R ,3R)-1,1,4,4-tetraphenylbutane-1,2,3,4-tetraol (TETROL, 1) and its derivatives (2-4)(Scheme 1) are able to form inclusion complexes with a large number of guests.10 Some of the successful guests are chiral, and we were required to determine whether these hosts are able to discriminate between enantiomers of the racemic chiral guests during recrystallization experiments. More specifically, guests in this category of interest to us were 2-and 3-methylcyclohexanone, methyl phenyl sulfoxide and 2-butanol, all of which are included with 1:1 host:guest ratios by these hosts, as shown by 1H-NMR spectroscopy. Since we did not have enantiopure standards for the guests at our disposal, we could not use chromatographic analyses in order to obtain the required information. Furthermore, any experiments involving the initial liberation of the guests were considered tedious and time-consuming.
Initially, we made the natural assumption that the observed optical rotation of an optically pure compound with a specific concentration is the same whether the solution contains only this compound or a combination of optically active compounds. In essence, if a given quantity of compound X rotates plane polarized light by a certain number of degrees, the presence of another optically active species in the same solution will not alter this value for X (though the observed rotation will be different because of the presence of the other compound). In other words, in such a case, optical rotation follows a simple additive rule. This would, in effect, mean that direct optical rotation measurements of solutions of chiral host-guest complexes as a whole should provide information on the configuration of the preferred guest species since the optical rotation of the host is known (or can readily be determined in a separate experiment). In order to ensure that our assumption was valid, a series of polarimetry experiments were performed on various standard materials: the specific rotations of optically pure (-)-menthone, (-)-menthol, (+)-camphor, (-)-ethyl L-lactate and (+)-TETROL were determined individually, followed by measurements on binary mixtures of these components in which the quantities of each component were known accurately (to the nearest four decimal places). Knowing the experimental specific rotation as well as the mole fraction of each component present in the solution then allows for the calculation of the contribution that each component made to the overall observed rotation. If the optical rotation of a compound is independent of the presence of another optically active compound in the same solution (and the observed optical rotation of the mixture is equal to the sum of the contributions of the optical rotation of each component in the mixture) then, in a binary mixture, the specific rotation of one component will be equal to the specific rotation of that component as if it were on its own (Equation 1):

where [a] is the specific rotation of the chiral compound, a is the observed rotation (in degrees) measured on the polarimeter, l is the path length of the polarimeter cell (in decimetres) and c is the concentration of the compound in g mL-1. T and l, respectively, designate the temperature (in °C) and the wavelength of the polarimeter light source (in nanometres).
We were then able to equate the rotation of a component in a binary mixture to its observed rotation in a pure sample (Equation 2):

where the subscript'1' refers to the pure compound and the subscript '2' refers to the same compound in a binary mixture. Since the volume (V) and path length in each experiment are constant, Equation 3 may be derived (using c = m/V):

where m is the mass of the compound in grams. For example, if the observed rotation of (+)-camphor is determined to be +5.48 ° (the averaged value over three readings) when a mass of 0.5639 g is used, and a binary mixture containing (+)-camphor (0.3935 g) and (-)-menthol (0.4693 g) has a combined observed rotation of -0.50 °, the contribution of (+)-camphor to the observed rotation in the binary mixture can be determined using Equation 3 as shown below:
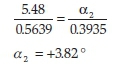
On the other hand, knowing that (-)-menthol has an observed rotation of -6.23 ° when a mass of 0.6913 g is used, its contribution to the observed rotation for the same mixture may be calculated as follows:
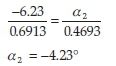
Now, adding together the contributions of (+)-camphor and (-)-menthol gives a combined calculated rotation of-0.41 °. This value is in reasonable agreement with the observed -0.50 °, implying that our previous assumption is valid.
One explanation for the slight discrepancy between the calculated and measured values may be due to the fact that the polarimeter measures rotations only to the nearest 0.05 degrees. For this reason, accurate e.e. values may not be reproducibly calculated using this method but it is, nonetheless, useful for providing information on the direction of rotation of plane polarized light by the major enantiomer present and hence for predicting its configuration.
Table 1 shows the calibrated observed rotations (an average of three readings) and masses (in 10 mL of solution) for the individual components, while Table 2 summarizes the observed rotations for binary mixtures of these components, the calculated contribution for each component in the mixture, and the deviation from the measured optical rotations extrapolated from values in Table 1.
The experiments summarized in Tables 1 and 2 therefore suggest that it is possible to predict with some confidence which guest enantiomer (of guests 2- and 3-methylcyclohexanone, methyl phenyl sulfoxide and 2-butanol) is preferred by the host in host-guest complexes of TETROL and its derivatives by simply measuring the optical rotation of the complex (dissolved in a suitable solvent). However, this method is not suitable for binary or higher solute-containing solutions that have relatively small expected combined optical rotations, since the error associated with these then becomes too large for high confidence levels. This is especially obvious when one notes the error associated with the (+)-camphor/(-)-menthone mixture where the expected combined optical rotation was only -0.15 (Table 2, entry 5).
Table 3 presents the polarimeter measurements of selected host-guest complexes and the required data in order to predict the configurations of the preferred enantiomers, considering that the H:G ratio was 1:1 for each complex, as determined by Ή-NMR spectroscopy.
4. Conclusion
Information about the configuration of the preferred guest species in host-guest inclusion compounds may be obtained without requiring standards of the optically pure guests, and without the need for liberating the guest first, simply by measuring the optical rotations of the complexes as a whole. This is because the optical rotation of one compound is not affected by the presence of other optically active compounds in the same mixture. The method is, however, not suitable for binary or higher solute-containing solutions that have low combined rotations since errors then become significant, and nor is it appropriate for the accurate determination of e.e. values for the same reason. The results presented in this paper, subsequently, greatly simplified our analyses of TETROL-derived complexes in the field of host-guest chemistry.
Acknowledgements
The Nelson Mandela Metropolitan University and the National Research Foundation are thanked for financial assistance.
References
1 D. Seebach, A.K. Beck and A. Heckel, TADDOLs, their derivatives, and TADDOL analogues: versatile chiral auxiliaries, Angew. Chem. Int. Ed., 2001,40, 92-138. [ Links ]
2 F. Toda, A. Sato, L.R. Nassimbeni and M.L. Niven, Optical resolution of amino acid and hydroxycarboxylic acid esters by complexation with optically active host compounds: a crystallographic result, J. Chem. Soc., Perkin Trans. 2, 1991, 1971-1975. [ Links ]
3 F. Toda and K. Tanaka, Design of a new chiral host compound, trans-4,5-bis(hydroxydiphenylmethyl)-2,2-dimethyl-1,3-dioxacyclo-pentane. An effective optical resolution of bicyclic enones through host-guest complex formation, Tetrahedron Lett., 1988, 29, 551-554. [ Links ]
4 F. Toda, K. Tanaka, T. Omata, K. Nakamura and T. Oshima, Optical resolution of 3-methylcycloalkanones and 5-methyl-g-butyrolactone by complexation with optically active 1,6-bis(0-halophenyl)- 1,6-diphenylhexa- 2,4-diyne-1,6-diol, J. Am. Chem. Soc., 1983, 150, 5151-1552. [ Links ]
5 F. Toda, Inclusion complex crystals formed by alcohol host compounds, Supramol. Sci., 1996, 3(1-3), 139-148. [ Links ]
6 S-Y. Lee, K-M. Park, S-H. Jo, H-G. Nam and S. Mun, Determination of chromatographic separation parameters of tryptophan enantiomers on a chirosil-SCA chiral stationary phase by using the inverse method based on the initial guesses estimated from elution by characteristic point method, J. Chromatogr. A, 2011,1218, 1195-1202. [ Links ]
7 B. Barton, M.R. Caira, E.C. Hosten, C.W. McCleland and S. Weitz, Clathrates of TETROL: selective inclusion of methylcyclohexanones in their energetically unfavorable axial methyl conformations, Chem. Commun, 2014, 50, 13353-13355. [ Links ]
8 B. Barton, M.R. Caira, E.C. Hosten, C.W. McCleland and S. Weitz, Clathrates of TETROL: further aspects of the selective inclusion of methylcyclohexanones in their energetically unfavorable axial methyl conformations, J. Org. Chem., 2015, 80, 7184-7192. [ Links ]
9 P.L. Meo, F. D'Anna, S. Riela, M. Gruttadauria and R. Noto, Polarimetry as a useful tool for the determination of binding constants between cyclodextrins and organic guest molecules, Tetra hedron Lett, 2006,47, 9099-9102. [ Links ]
10 B. Barton, M.R. Caira, E.C. Hosten and C.W. McCleland, A computational, X-Ray crystallographic and thermal stability analysis of TETROL and its pyridine and methylpyridine inclusion complexes, Tetrahedron, 2013, 69(41), 8713-8723. [ Links ]
Received 28 July 2014
Revised 28 August 2015
Accepted 30 August 2015.
* To whom correspondence should be addressed. E-mail: benita.barton@nmmu.ac.za














