Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Chemistry
versão On-line ISSN 1996-840X
versão impressa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.68 Durban 2015
http://dx.doi.org/10.17159/0379-4350/2015/V68A18
RESEARCH ARTICLE
Application of ultrasound-assisted emulsification microextraction followed by gas chromatography for determination of oxadiazon in water and soil samples
Abolfazl SemnaniI, II; Hedayat HaddadiI; Mohammad RezaeeIII, *; Faezeh KhalilianIV
IDepartment of Chemistry, Faculty of Sciences, Shahrekord University P.O. Box 115, Shahrekord, Iran
IICenter of Excellence for Mathematics, Shahrekord University P.O. Box 115, Shahrekord, Iran
IIINuclear Fuel Cycle Research School, Nuclear Science & Technology Research Institute, Atomic Energy Organization of Iran, P.O. Box 14395-836, Tehran, Iran
IVDepartment of Chemistry, College of Basic Science, Yadegar -e- Imam Khomeini (RAH) Branch, Islamic Azad University, Tehran, Iran
ABSTRACT
In this study, a simple and efficient ultrasound-assisted emulsification microextraction (USAEME) method combined with gas chromatography (GC) was developed for the preconcentration and determination of oxadiazon in water and soil samples. In this method, fine droplets of toluene were formed and dispersed in the sample with the help of ultrasonic waves which accelerated the formation of a fine cloudy solution without using disperser solvents. Several factors influencing the extraction efficiency, such as the nature and volume of organic solvent, extraction temperature, ionic strength and centrifugation time, were investigated and optimized. Using optimum extraction conditions a detection limit of 0.1 μg L-1 and a good linearity in a calibration range of 0.25-250 μg L-1 were achieved for the analyte in a river water sample. This proposed method was successfully applied to the analysis of oxadiazon in water and soil samples.
Keywords: Utrasound-assisted Emulsification Microextraction, Oxadiazon, Gas Chromatography, Water Samples, Soil Samples.
1. Introduction
Oxadiazon, 5-tert-butyl-3-(2,4-dichloro-5-isopropoxy-phenyl)-1,3,4-oxadiazol-2(3H)-one, is an effective herbicide for control of obnoxious grasses and broad-leaf weeds in a wide variety of crops, e.g. citrus fruit, vines, cotton, rice, soya beans and onions.1 The chemical structure of oxadiazon is shown in Fig. 1.
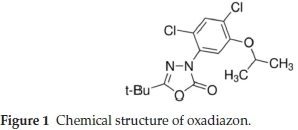
Oxadiazon has been used since the 1970s. Many mono and multiresidue chromatographicmethods foroxadiazonareavail-able in the litreature.2-5 Several methods for the determination of trace amounts of pesticides require the concentration of large volumes of sample by liquid-liquid extraction (LLE) or solid-phase extraction (SPE). Solid-phase microextraction (SPME) is an alternative technique that involves direct extraction of the analytes with the use of a small diameter, optical fibre coated with a polymeric stationary phase and housed in a syringe assembly for protection.6-7
SPME eliminates the separate concentration step from the SPE and LLE methods, and analytes diffuse directly into the coating of the SPME fibre and are concentrated there. This fibre is then transferred directly into the injection port of the GC where all analytes are thermally desorbed and deposited at the head of the GC column.6 LLE needs large amounts of toxic solvent and time-consuming procedures. SPE is less time-consuming than LLE but still needs column conditioning and elution with organic solvents; another drawback of SPE is cost. SPME has been applied for determination of oxadiazon.8 However, SPME also has some problems such as high cost, sample carry-over and a decline in performance with time.
A more recent technique, introduced by Rezaee et al., which does not involve the use of either a fibre or a syringe has been termed dispersive liquid-liquid microextraction (DLLME).9 As the name suggests, it is based on a ternary component solvent system similar to homogeneous liquid-liquid extraction and cloud point extraction.10,11 In DLLME a cloudy solution is formed when an appropriate mixture of extraction solvent and disperser solvent is quickly injected into the sample. Thus a high turbulence is produced. This turbulent regimen gives rise to the formation of small droplets, which are dispersed throughout the aqueous sample. Emulsified droplets have a large interfacial area. Only water-immiscible extraction solvents with higher density than water are used, which facilitates their collection as they settle below the aqueous phase after centrifugation. Organic solvents (such as carbon tetrachloride, chloroform or chlorobenzene) are generally used as the extractants in DLLME and are toxic.12-18
Ultrasound-assisted emulsification microextraction (USAEME)19 is based on the application of ultrasonic radiation for accelerating the emulsification phenomenon. On application of ultrasonic radiation the solution becomes turbid due to the dispersion of extraction droplets into the aqueous phase. The emulsification process favours the mass transfer of analytes from aqueous phase into the organic phase which leads to enhanced extraction efficiency of analytes in a minimum amount of time, thereby combining the benefits of microextraction and ultrasonic radiation. USAEME is a fast and efficient microextraction technique for extractions of trace analytes from liquid media.20 For the first time, Saleh et al. applied a low-density organic solvent in USAEME.21
To the best of our knowledge, none of the published papers reports the use of USAEME for the extraction and determination of oxadiazon in water and soil samples. The aim of this study was the application of the USAEME technique combined with GC-FID for the extraction and determination of oxadiazon in water and soil samples. A series of parameters influencing the extraction recovery were investigated.
2. Experimental
2.1. Chemicals and Reagents
All reagents were of analytical-reagent grade unless stated otherwise. Oxadiazon and sodium chloride of the highest purity available from Merck (Darmstadt, Germany) were used in this study. A stock standard solution of oxadiazon (99.5 %) (1000 mg L-1) was prepared in methanol. A fresh 10 mg L-1 standard solution containing the analyte was prepared in methanol every week and stored at 4 °C. The working standard solutions were prepared in doubly distilled water, stored at 4 °C in a fridge, and brought to ambient temperature prior to use. Toluene, 1-octanol, 1-undecanol, 1-dodecanol were obtained from Merck. The water used was purified on an Aqua Max-Ultra Youngling ultra pure water purification system (370 series, Dongan-gu, Korea).
2.2. Instrumentation
A 40 kHz and 0.138 kW ultrasonic water bath with temperature control (Tecno-Gaz SpA, Strada Cavalli, Parma, Italy) was applied to emulsify the organic solvent. 100 and 25 μL Hamilton syringes (Bonaduz, GR, Switzerland) were used to inject the organic solvent into samples. Home-designed centrifuge glass vials were used for the extraction and collection procedure (Fig. 2). Separation and quantification of oxadiazon were carried out using an Agilent 7890 gas chromatograph, equipped with a FID detector and a DB-5 fused-silica capillary column (30 m x 0.32 mm i.d. x 0.25 μm film thickness). The injection was performed in splitless mode, and helium gas with high purity was used as carrier at a constant flow rate of 1.5 mL min-1. The injector and detector temperatures were 250 and 280 °C, respectively. The column temperature programme was as follows: 75 °C for 3 min, increased to 270 °C at 10 °C min-1, and then held for 1 min, The analytical signal was taken as the peak area of the analyte. A model 2010 D centurion scientific centrifuge (Chichester,West Sussex, United Kingdom) was used for the separation of the floated phase from the sample solution.
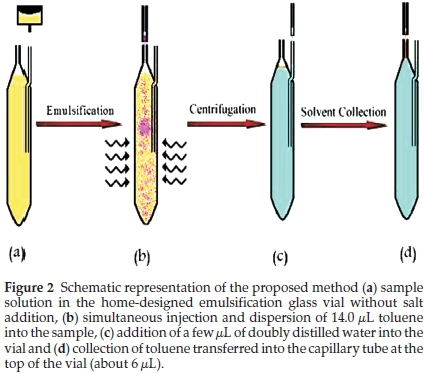
2.3. Ultrasound-assisted Emulsification Microextraction Procedure
Ten mL of sample was placed in a home-designed centrifuge glass vial (Fig. 1 a). 14.0 toluene was injected into the solution and the sample was sonicated for thirty second at 25 °C in the ultrasonic bath (Fig. 1b). As a result, oil-in-water emulsions of toluene in water were formed. After centrifuging at 3500 rpm for 5 min, the organic solvent droplet separated on the surface of the aqueous solution due to its lower density. A few microlitres of doubly distilled water were added to the vial through the glass tube fixed on the side of the vial (Fig. 1c). The organic solvent rose up the capillary tube attached to the top of the vial and could be collected in a gas-tight syringe (Fig. 1d). The final extract phase was injected into the GC-FID instrument.
3. Results and Discussion
In the present study, an ultrasound-assisted emulsification microextraction (USAEME) method was investigated for the preconcentration and determination of oxadiazon in the water and soil samples. The influences of the various parameters such as the kind and the volume of the extraction solvent, ionic strength, extraction temperature and centrifugation time on the extraction efficiency were studied and then the optimum conditions were selected. The optimization of the above mentioned variables was performed using one at a time variable method. All experiments were replicated three times.
3.1. Selection of Extraction Solvent
The selection of a suitable extraction solvent is critical for the USAEME process. In USAEME, the extraction solvent should have the following characteristics:211) lower density than that of water, 2) low solubility in water, 3) the ability to extract analytes of interest. Based on these requirements, four organic solvent candidates, toluene, 1-undecanol, 1-dodecanol and 1-octanol were investigated. The results (Fig. 3) revealed that the extraction recovery obtained for the analyte using toluene was higher than recoveries obtained with the other solvents. Therefore, toluene was selected as the extraction solvent for the study.
3.2. Influence of Centrifugation Time
Centrifugation is essential to separate extraction solvent from aqueous solution in USAEME. Centrifugation time may affect the volume of the organic phase. The effect of the centrifugation time on the extraction efficiency was examined from 2 to 20 min at 3500 rpm. The experimental results showed that the best performance was obtained at 3500 rpm for 10 min, At higher centrifugation times, the volume of collected solvent decreased.
3.3. Influence of the Volume of the Extracting Solvent
The effect of the volume of the extracting solvent on amount of analyte extracted was investigated in the range of 12.0-50.0 μL. As shown in Fig. 4, increasing the volume of toluene, decreases the preconcentration factor, because the volume of collected solvent increases. Hence, highest preconcentration factors are obtained using 12.0 μL volume of extraction solvent. However, problems with the collection of 2 of toluene meant that better precision was observed when 14.0 was used. Consequently, 14.0 was selected as the optimum volume of toluene.
3.4. Influence of Ionic Strength
The salting out effect has been universally used in SPME and LLE methods.7,9 The addition of salt to an analytical sample can potentially increase the analyte extraction recovery in micro-extraction procedures. The effect of the ionic strength on the extraction efficiency was evaluated by increasing NaCl concentrations in the range of 0-8 % (w/v) in the samples containing 100 L-1 of oxadiazon. The results show that increasing the concentration of NaCl, does not change the extraction efficiency of oxadiazon significantly. This is possibly because of two opposing effects of salt addition. One is to increase the volume of organic phase and decrease the dispersion efficiency, which reduces the extraction efficiency; the other is the salting-out effect, which increases the extraction efficiency. By increasing the salt concentration, the volume of organic phase increases, because of the decrease of solubility of the extraction solvent in the presence of salt. Therefore, further extractions were performed without addition of salt.
3.5. Influence of Extraction Temperature
Temperature affects organic solvent solubility in water as well as the emulsification phenomenon. Consequently, this affects the mass-transfer process and the extraction efficiency. To determine the influence of the extraction temperature, extraction producers were performed at different temperatures such as 20, 25, 35, 40 and 50 °C. The results are shown in Fig. 5. It was observed that the highest extraction efficiency was obtained in the range 20-25 °C, but at higher temperature (35-50 °C), extraction recoveries decrease. This is possibly because of the decrease in distribution coefficient (KD) at higher temperature. Hence, 25 °C was used for further experiments.
3.6. Influence of Extraction Time and Ultrasound Time
The effect of extraction time on the extraction efficiency was examined in the range 0-40 min, The results show that extraction time has no significant effect on the extraction efficiency of oxadiazon. This showed that the contact surface area between extraction solvent and the sample solution was large and the equilibrium state was achieved quickly. The effect of ultrasound time on the extraction efficiency of oxadiazon was examined in the range 15-180 seconds. The results are shown in Fig. 6. At less than 30 s, extraction efficiency is low, because the ultrasound time is not sufficient for dispersion phenomenon, and the surface area between extraction solvent and sample solution is lower. After 30 s the extraction efficiency does not change significantly, as an equilibrium state has been achieved. Therefore, 30 s was selected as the optimum value of ultrasound time for further experiments.
3.7. Quantitative Analysis
The characteristics of the calibration curve are shown in Table 1 which was obtained under the optimized conditions. Linearity was observed in the range of 0.25-250 μL-1 for oxadiazon with correlation coefficient (r2) of 0.9992 in river water. The relative standard deviation (RSD) was 7.1 % (n = 8) in river water at the concentration level of 5.0 μg L-1. The limit of detection (LOD), based on signal-to-noise (S/N) of three was 0.1 μL-1 in the river water.
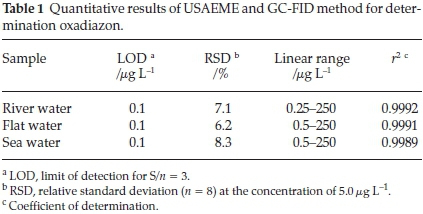
Table 2 compares the proposed method with other extraction methods for the determination of oxadiazon. The comparison of extraction time for the proposed method with that forheadspace solid-phase microextraction (SPME)22 and solid-phase micro-extraction23 indicates that this novel method has a very short equilibrium time compared to the mentioned methods and the extraction time needed for the proposed method is only a few seconds. Quantitative results for the proposed method are better than for headspace solid-phase microextraction22 and solid-phase microextraction.23 The comparison of the proposed method with the electroanalytical determination of oxadiazon24 indicates that the quantitative results for the proposed method are better, and the proposed method is simple and inexpensive by comparison. Direct comparison of the proposed method with a method not using the ultrasound procedure in the range 20-500 dm-3 shows that with the ultrasound procedure the preconcentration factor increases, because of the large surface area between extraction solvent and analyte. Also, the comparison of the proposed method with DLLME indicates that consumption of disperser solvent in DLLME leads to disadvantages such as decreasing the partition coefficients of the analyte into the extracting solvent, and increasing the cost as well as environmental pollution, moreover the variety of solvents that can be used with DLLME is limited.
3.8. Extraction of Oxadiazon from Water Samples
During the present investigation, matrix effects on the extraction performance were also evaluated by investigating the applicability of the proposed method to determine oxadiazon concentration in river, flat and sea water samples. Physicochemical characteristics are as follow: river water (T: 23.1 °C; pH: 6.4; EC: 652μscm-1 and TDS: 410); flat water (T: 23 °C; pH: 7.3 and EC: 11.5 ds m-1) and sea water (T: 25 °C; pH: 7 and EC: 23 ds m-1). These samples were extracted using the USAEME method and analyzed by GC-FID. The results from river, flat and sea water samples showed that they were free of oxadiazon contamination. These samples were spiked with an oxadiazon standard to assess matrix effects. Figure 7 shows the chromatograms obtained for river water and spiked river water.
Relative recoveries were between 90 to 94 %. These results (Table 3) demonstrate that the flat, sea and river water matrices, in our present context, had little effect on the USAEME method. Validation for spiked water samples was carried out by using a one-sample test (Student's t-test).25 Samples were spiked with different levels of oxadiazon and analyzed by the proposed method. Table 3 shows the results obtained. The P-values calculated in all cases were >0.05 and the null hypothesis can be accepted.
3.9. Extraction of Oxadiazon from Soil Samples
Soil samples were collected from the north of Iran (Mazandaran, Iran). The physical and chemical properties of soil samples were as follows. Flat soil:silty-clay texture, organic matter: 1.3 %, pH: 7.2 and maximum water capacity: 22 %. Citrous soil: silty-loam texture, organic matter: 1.57 %, pH 7.1 and maximum water capacity: 19.3 %. The proposed method combined with the ultrasonic assisted extraction was applied to extract oxadiazon from these matrices. Soil samples were pulverized and passed through a 1 mm sieve. 2.0 g of the samples accurately weighed and put into a 20 mL centrifuge tube, to which 3.0 mL of methanol was added. The resultant samples were ultrasonically extracted for 5 min, After the sonication, the extracts were centrifuged at 5000 rpm for 3 min and the supernatant liquid was passed through a PTFE syringe filter (13 mm, 0.22 μm) (Sigma-Aldrich) to remove particles. For USAEME, a 2.0 mL aliquot of the residual filtrate and 10.0 mL of water was placed in a 12 mL home-designed centrifuge glass vial and ultrapure water was added to fill the tube. Then the sample was submitted to USAEME as described previously.
The recoveries of the analyte from soil samples spiked at 0.1 and 0.05 mg kg-1 levels using the proposed method are given in Table 4. The relative recoveries for the method for the oxadiazon were in the range 89.0-92 % for the soil samples, indicating good performance of the described method for the determination of the oxadiazon in soil matrices. Validation of the proposed method for soil samples was made using comparison with an ultrasonic extraction method reported by Sanchez-Brunete et al.4 as the reference method. Spiked soil samples with concentrations of 0.1 and 0.05 mg kg-1 of oxadiazon were analyzed by both methods. Similar results were obtained with both methods. The statistical comparison of these results by means of a Student's t-test showed no significant difference (P-value of 5 %).
4. Conclusion
A simple and reliable new ultrasound-assisted emulsification microextraction method was developed for the rapid concentration and determination of oxadiazon in water and soil samples. An ultrasound-assisted process was applied to accelerate the formation of a cloudy solution, which markedly increased the extraction efficiency and reduced the equilibrium time. The developed method was sensitive, reproducible and linear over a wide range. The performance of this procedure in oxadiazon extraction from soil samples was excellent and no matrix effect was observed. Finally, it can be concluded that the broad linear dynamic range combined with the low detection limit suggests a high potential for monitoring oxadiazon in water and soil samples using the USAEME-GC-FID method.
Acknowledgements
This work has been supported by Shahrekord University through a research fund. The authors were also partially supported by the Center of Excellence for Mathematics, Shahrekord University.
References
1 C. Tomlin, The Pesticide Manual: a World Compendium, British Crop Protection Council, 11th edn., Farnham, UK, 1997. [ Links ]
2 D.J. George, Chemical contaminants monitoring, Assoc. Off. Anal. Chem., 1982, 65, 1160-1163. [ Links ]
3 A. Tanabe, H. Mitobe, K. Kawata and M. Sakai, Monitoring of herbicides in river water by gas chromatography-mass spectrometry and solid-phase extraction, J. Chromatogr. A, 1996, 754, 159-163. [ Links ]
4 C. Sanchez-Brunete, R. Perez, E. Miguel and J. Tadeo, Multiresidue herbicide analysis in soil samples by means of extraction in small columns and gas chromatography with nitrogen-phosphorus and mass spectrometric detection, J. Chromatogr. A, 1998, 823, 17-24. [ Links ]
5 M.B. Riley and R.J. Keese, Comparison of solid phase extraction techniques for herbicides, Weed Sci., 1996, 44, 689-693. [ Links ]
6 C.L. Arthur and J. Pawliszyn, Solid phase microextraction with thermal desorption using fused silica optical fibres, Anal. Chem., 1990, 62, 2145-2148. [ Links ]
7 C.L. Arthur, L.M. Killam, K.D. Bucholz, J. Pawliszyn and J.R. Berg, Automation and optimization of solid-phase microextraction, Anal. Chem., 1992, 64, 1960-1966. [ Links ]
8 A. Navalon, A. Prieto, L. Araujo and J.L. Vilchez, Determination of oxadiazon residues by headspace solid-phase microextraction and gas chromatography-mass spectrometry, J. Chromatogr. A 2002, 946, 239-245. [ Links ]
9 M. Rezaee, Y. Assadi, M.R. Milani Hosseini, E. Aghaee, F. Ahmadi and S. Berijani, Determination of organic compounds in water using dispersive liquid-liquid microextraction, J. Chromatogr. A, 2006, 1116, 1-9. [ Links ]
10 S. Oshite, M. Furukawa and S. Igarashi, Homogeneous liquid-liquid extraction method for the selective spectrofluorimetric determination of trace amounts of tryptophan, Analyst, 2001,126, 703-706. [ Links ]
11 J.L. Manzoori and G. Karim-Nezhad, Development of a cloud point extraction and preconcentration method for Cd and Ni prior to flame atomic absorption spectrometric determination, Anal. Chim. Acta, 2004, 521, 173-177. [ Links ]
12 R. Rahnama Kozani, Y. Assadi, F. Shemirani, M.R. Milani Hosseini and M.R. Jamali, Part-per-trillion determination of chlorobenzenes in water using dispersive liquid-liquid microextraction combined gas chromatography-electron capture detection, Talanta, 2007, 72, 387-393. [ Links ]
13 M.A. Farajzadeh, M. Bahram and J.A. Jonsson, Dispersive liquid-liquid microextraction followedby high-performance liquid chroma-tography-diode array detection as an efficient and sensitive technique for determination of antioxidants, Anal. Chim. Acta, 2007, 591, 69-79. [ Links ]
14 A. Bidari, E. Zeini Jahromi, Y. Assadi and M.R. Milani Hosseini, Monitoring of selenium in water samples using dispersive liquid-liquid microextraction followed by iridium-modified tube graphite furnace atomic absorption spectrometry, Microchem. J., 2007, 87, 6-12. [ Links ]
15 N. Shokoufi, F. Shemirani and Y. Assadi, Fibre optic-linear array detection spectrophotometry in combination with dispersive liquid-liquid microextraction for simultaneous preconcentration and determination of palladium and cobalt, Anal. Chim. Acta, 2007, 597, 349-356. [ Links ]
16 M. Rezaee, Y. Yamini, S. Shariati, A. Esrafili and M. Shamsipur, Dispersive liquid-liquid microextraction combined with high-performance liquid chromatography-UV detection as a very simple, rapid and sensitive method for the determination of bisphenol A in water samples, J. Chromatogr. A, 2009, 1216, 1511-1514. [ Links ]
17 M. Rezaee, Y. Yamini and M. Faraji, Evolution of dispersive liquid- liquid microextraction, J. Chromatogr. A, 2010,1217, 2342-2357. [ Links ]
18 Y. Yamini, M. Rezaee, A. Khanchi, M. Faraji and A. Saleh, Dispersive liquid-liquid microextraction based on the solidification of floating organic drop followed by inductively coupled plasma-optical emission spectrometry as a fast technique for the simultaneous determination of heavy metals, J. Chromatogr. A, 2010, 1217, 23582364. [ Links ]
19 M.D. Luque de Castro and F. Priego-Capote, Ultrasound-assisted preparation of liquid samples, Talanta 2007, 72, 321-334. [ Links ]
20. J. Regueiroa, M. Llomparta, C. Garcia-Jaresa, J.C. Garcia-Monteagudob and R. Celaa, Ultrasound-assisted emulsification-microextraction of emergent contaminants and pesticides in environmental waters, J. Chromatogr. A, 2008,1190, 27-38. [ Links ]
21 A. Saleh, Y. Yamini, M. Faraji, M. Rezaee and M. Ghambarian, Ultrasound-assisted emulsification microextraction method based on applying low density organic solvents followed by gas chromatography analysis for the determination of polycyclic aromatic hydrocarbons in water samples, J. Chromatogr. A, 2009, 1216, 6673-6679. [ Links ]
22 A. Navalon, A. Prieto, L. Araujo and J.L. Vilchez, Determination of tebufenpyrad and oxadiazon by solid-phase microextraction and gas chromatography-mass spectrometry, Chromatographia, 2001, 54, 377-382. [ Links ]
23 A. Navalon, A. Prieto, L. Araujo and J.L. Vilchez, Determination of oxadiazon residues by headspace solid-phase microextraction and gas chromatography-mass spectrometry, J. Chromatogr. A, 2002, 946, 239-245. [ Links ]
24 E.M. Garrido, J.L.F.C. Lima, C. Delerue-Matos, M.F.M. Borges and A.M. Oliveira Brett, Electroanalytical determination of oxadiazon and characterization of its base-catalyzed ring-opening products, Electroanalysis, 2001,13, 199-203. [ Links ]
25 J.C. Miller and J.N. Miller, Statistics for Chemical Analysis, Addison- Wesley Iberoamericana, Wilmington, DE, 1993. [ Links ]
Received 27 January 2015
Revised 28 April 2015
Accepted 29 April 2015
* To whom correspondence should be addressed. E-mail: chem.rezaee219@gmail.com














