Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Chemistry
versão On-line ISSN 1996-840X
versão impressa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.68 Durban 2015
http://dx.doi.org/10.17159/0379-4350/2015/V68A15
RESEARCH ARTICLE
Study of preparation and properties on polymer-modified magnetite nanoparticles
Ruili TongI, II; Yonggang WangI; Guang YangIII; Aiqing MaIV; Keji SunIV; Hui YangII, *; Jinben WangII
ISchool of Chemical and Environmental Engineering, China University of Mining and Technology (Beijing), Beijing 100083, People's Republic of China
IIKey Laboratory of Colloid, Interface and Chemical Thermodynamics, Institute of Chemistry, Chinese Academy of Science, Beijing 100190, People's Republic of China
IIICNOOC Research Institute, State Key Laboratory of Offshore Oil Exploitation, Beijing 100027, People's Republic of China
IVOil Production Technology Research Institute, Shengli Oilfield Branch Company, Sinopec, Dongying, Shandong 257000, People's Republic of China
ABSTRACT
In this paper, polyacrylamide (PAM)-modified magnetite (Fe3O4) nanoparticles were prepared by in situ polymerization in aqueous solution. The particle size, morphology, crystal phase and magnetic properties were measured utilizing scanning electron microscopy (SEM), transmission electron microscopy (TEM), Fourier-transform infrared spectroscopy (FTIR), X-ray diffraction (XRD) and vibrating sample magnetometer (VSM), respectively. The size distribution and stability of the nano-particles in aqueous solution were evaluated using the laser particle size analyzer and ultraviolet-visible spectroscopy (UV-vis). The influences of the dose of acrylamide (AM) and the pH value on the particle size and stability were also examined. The results showed that the Fe3O4 nanoparticles possessed superparamagnetic property and super dispersion stability in aqueous solution after PAM modification.
Keywords: Magnetite (Fe3O4), nanoparticles, superparamagnetism, polymer modification, colloidal stability
1. Introduction
Magnetic nanoparticles have attracted much more attentions for its wide-ranging application, including magnetic fluids, catalysis, biotechnology, data storage and environmental pro-tection.1-5 Amongst various magnetic nanoparticles, Fe3O4 nanoparticles have been recognized as a promising candidate for its good biocompatibility, strong superparamagnetism, low toxicity and easy preparation process.6,7 However, pure Fe3O4 nanoparticles are likely to aggregate for their large specific surface area and strong magnetic dipole-dipole attractions between nanoparticles, resulting in aggregation in aqueous solution and the change of magnetic properties which should be avoided in applications.8
To avoid the aggregation of pure Fe3O4 nanoparticles during the application processes as mentioned above, modification on the surface of Fe3O4 nanoparticles with a surfactant or hydro-philic polymer is one of the most effective methods.9,10 Furthermore, another significant function of the modification on the nanoparitcles is that the polymer or surfactant provides chemical groups for further grafting to satisfy further applications.11
Many researchers have discussed the modification of magnetic nanoparitcles. Yang et al. reported that the iron oxide nanoparticles were modified by various poly(amino acid)s for use as magnetic resonance probes.12 Yu et al. investigated the hydroxypropyl-β-cyclodextrin/polyethylene glycol 400 modified on Fe3O4 nanoparticles for congo red removal.13 Cui et al. studied perfluoro-polyether carboxylic acid surfactant modified Fe3O4 magnetic nanoparticles and the modified layer could withstand high temperature.14 Wang et al. presented modified magnetic nanoparticles using 3-aminopropyltriethoxy silane and subsequently activated by glutaraldehyde and then proteins were immobilized on the activated nanoparticles.15 Several other materials were also reported on the modification of magnetic nanoparticles. For example, some reports confirmed that magnetic nanoparticles were coated by chitosan for applications in biotechnology.16-18 Oleic acid was used to modify the surface of magnetite nanoparticles.19,20 PEG/PVA and Poly(4-MS-DVB-GMA) matrix grafted with poly(amidoamine) PAMAM dendrimer were also suggested to modify magnetic nanoparticles.21,22
However, few studies on the modification of polyacrylamide (PAM) on the surface of magnetite nanoparticles appear in literature. PAM can be utilized to protect the particles' original properties and stabilize them in aqueous solution. In addition, this kind of magnetic composite can be moved effectively and simply by using applied magnetic field. In this paper, we prepared PAM-modified Fe3O4 nanoparticles via direct polymerization using the frequently-used bottom-up approach which was reported previously.23-26 The Fe3O4 nanoparticles filler was encapsulated before and during the synthesis of the polymer.27 The particle size distribution and the morphology of PAM-modified Fe3O4 nanoparticles were investigated. Other properties, such as crystal phase, magnetic properties and the mass lost value in TGA were also determined. Most importantly, the ζ-potential and stability of the PAM-modified nanoparticles in aqueous solution were illustrated.
2. Experimental
2.1. Materials
The chemicals used in this work were all analytical reagents. Ferric chloride (FeCl3) was purchased from Sinopharm Chemical Reagent Beijing Co., Ltd., iron dichloride tetrahydrate (FeCl2.4H2O) from Shanghai Gongxuetuan No. 2 Experiment Factory, NaOH from Beijing Chemical Works, potassium persul-fate (K2S2O8) and acrylamide (AM) from Beijing Yili Fine Chemicals Co., Ltd.
2.2. Preparation of Fe3O4 Nanoparticles
3.25 g of FeCl3 and 3.38 g of FeCl2.10H2O were successively dissolved in the 25 mL of deoxygenated water, which was obtained by bubbling nitrogen gas for 15 min, and then the solution was stirred and filtrated. The resulted solution was added dropwise into 200 mL of 1 M NaOH solution under vigorous stirring (600 r min-1) under N2 atmosphere, followed by the generation of Fe3O4 precipitate.
The total precipitate was isolated using a magnetic field, and the supernatant was removed by decantation. Then the precipitate was washed using purified deoxygenated water for several times with the same method, until no more Cl- was detected upon addition of Ag+. Finally, purified deoxygenated waterwas added to 1.7 g Fe3O4 precipitate until the total volume was 100 mL to form the required solution.
2.3. Preparation of Polymer-modified Magnetite Nanoparticles
The polymer modified magnetite nanoparticles were formed by bottom-up approach.27 First, another 100 mL purified water was added to the Fe3O4 suspension prepared previously, to give 200 ml solution. Subsequently, 0.02 mol % of K2S2O8 was added as initiator. After 10 minutes of ultrasonic vibration, AM (34 % by weight) solution was added dropwise into the above 200 mL of mixed solution under stirring (600 r min-1) and ultrasonic vibration during the whole process. The solution was kept under N2 atmosphere during the whole process.
Three kinds of products with different ratio of nanoparticles to AM were prepared, namely, PAM + 10%Fe3O4, PAM + 20%Fe3O4, PAM+50%Fe3O4. The pure Fe3O4 nanoparticles were also prepared for contrast. After polymerization, the samples PAM+10%Fe3O4 and PAM+20%Fe3O4 were semi-liquid jelly and dried in electric blast drying oven. For sample PAM+50% Fe3O4, the product was liquid, it was lyophilized. All the dried samples could be dispersed into water to form a homogeneous, transparent colloid. The results are summarized in Table 1.
2.4. Characterization
The sample phases and particle sizes were determined by X-ray diffraction (XRD) (Rigaku-D/max-2500, Japan). The morphology of the Fe3O4 Particles, before and after coated by PAM, were measured via micrographs obtained by scanning Electron Microscopy (SEM) (JSM-6700 JEOL, Japan) and transmission electron microscopy (TEM) (TECNAI-12, Philip Apparatus Co., USA). The size distribution and the stability of the PAM modified nanoparticles in aqueous solution were measured with a laser particle size analyzer (Horiba LB-550, Japan) and ultraviolet-visible spectroscopy (UV-vis) (UV2102, UNICO USA), respectively.
The infrared measurements in the 4000-100 cm-1 range on powder specimens dispersed on a pressed KBr disk, using a Fourier-transform infrared spectroscopy (FTIR) (Tensor27, Bruker Germany). The magnetic properties were carried out using a vibrating sample magnetometer (VSM) (MPMSXL, Quantum Design USA). The composition particles were also determined with thermogravimetric analysis (TGA) (Pyris 1, PerkinElmer USA).
3. Results and Discussion
3.1. Electron Microscopy
Figure 1 shows SEM and TEM images of the Fe3O4 particles before and after modification. The pure Fe3O4 particles are nearly spherical and have uniform morphology with the diameter about 10 nm. After modification, the particles became larger and the size varies from 30 to 100 nm. Particularly, the TEM image (b-2) shows that the particle is separated from its neighbours and the boundary is very clear.
3.2. X-ray Diffraction
Diffraction patterns of the samples (pure Fe3O4, PAM+10% Fe3O4, PAM+20%Fe3O4, PAM+50%Fe3O4) are shown in Fig. 2. The characteristic peaks of Fe3O4 cubic space group (20 = 35.4 °, 62.74 °, 56.98 °, 30.34 °, 43.12 ° and 53.46 °) were observed. It is consistent with the standard pattern for Fe3O4 (JCPDS 19-629).4,28,29 The average particle size calculated from the Debye-Scherrer formula is 9 nm, which is consistent with SEM/TEM results.
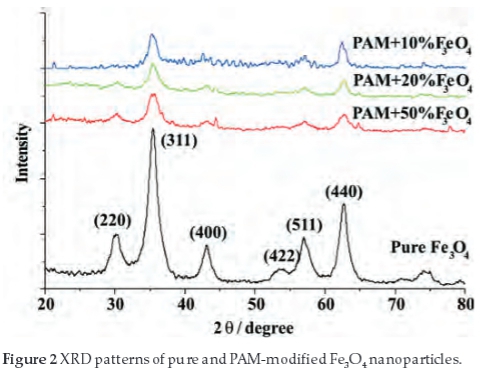
Compared to the pure Fe3O4, the characteristic peaks of the PAM-modified Fe3O4 particles are weak, but the position does not shift, which indicate that the PAM modified on the surface of the Fe3O4 particles does not change the size and crystal phase of the Fe3O4.
3.3. IR Results
The IR spectrum of the four samples is shown in Fig. 3. The peak near 574 cm-1 of the pure Fe3O4 curve belongs to the characteristic absorption band of the Fe-O bond,4,7,30 and the absorption bands near 1632 cm-1 and 3418 cm-1 refers to the O-H stretching mode and H-O-H bending mode, indicating the presence of interstitial water in the samples.7,28
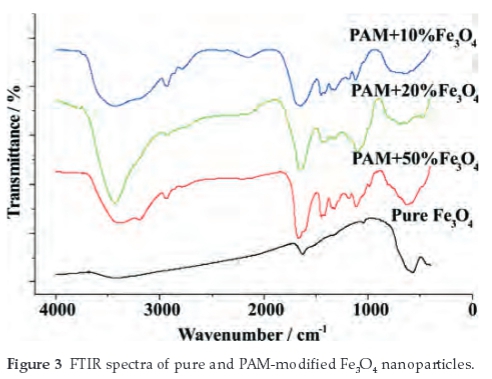
In the absorption curves of PAM-modified Fe3O4 nano-particles, the 3450 cm-1 peak belongs to H-O bond vibrations of water,3,28 and 3139 cm-1 peak belongs to N-H stretching vibra-tions.23 2783-2986 cm-1 can be assigned to the stretch vibrations of the C-H bonds which originates from the PAM modified on the surface of the particles.3,28 The absorption bands near 1655 cm-1 shows common characteristic of (NH2)C=O23 and 1420 cm-1 represents the stretching vibration of the CN group.30 In addition, the characteristic absorption band of Fe-O (around 580 cm-1) still can be found in the PAM modified Fe3O4 nanoparticles.28,30 Both of the absorption peaks from the PAM and Fe3O4 indicates that the magnetite nanoparticles have been well modified.
3.4. Magnetic Measurement
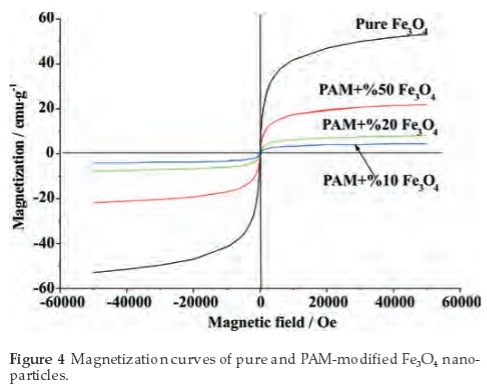
In order to investigate the magnetic properties of the samples, VSM was employed. Figure 4 shows the magnetization curves of samples at ambient temperature. Measurable hysteresis or coercivity were not observed in magnetization curves, indicating that all the samples show typical superparamagnetic behaviour.8 The superparamagnetic behaviour also indicates that the particles' size range below 20 nm,31 which is consistent with the morphological characteristics from the SEM/TEM in Fig. 1. The saturation magnetization was reached at a field of approximately 50 000 Oe. Figure 4 shows that the saturation measure-ments of the pure and PAM-modified Fe3O4 samples are approximately 53.5, 20.7, 7.9, 4.3 emu g-1, respectively. The saturation magnetization of the nanoparticles is significantly smaller than that of bulk magnetite which is 84 emu g-1.8 It is believed that the decrease of measured saturation magnetization can be attributed to the reduction of size and the PAM coating.1,7,8,12
3.5. TGA
Four dried samples were subjected to TGA in the 30-650 °C range under an N2 atmosphere (shown in Fig. 5). The pure Fe3O4 lost 13.3 % of its total weight, which is probably due to the absorbed water from the environment.8 The PAM-modified samples show that there are three stages of mass loss for the thermal degradation of PAM. The first stage involves a slight weight loss around 9 % at temperature below 250 °C. It can be attributed to the presence water absorbed in PAM-modified Fe3O4 nanoparticles.23 The second stage appears in the range of 250-380 °C for the decomposition of pendant amide groups and the intactness of polymer main chains.23 The observed values of weight loss are around 24.5 %, 34.5 %, and 39.7 % for the three samples, respectively. The third stage appears in the range of 380-510 °C with 8 %, 10 %, and 11.5 % of weight loss for the different samples, respectively, due to the breakdown of the polymer backbones.23

3.6. The Aggregation of PAM-modified Nanoparticles in Aqueous at Different pH Values
The aggregation variety of the nanoparticles dispersed in water under different pH values are shown in Fig. 6. For pure Fe3O4 nanoparticles, at low pH value, the particles size is about 150 nm. As the pH rises, the aggregation becomes significant, for the size of the aggregate becomes larger and the maximum is up to 550 nm and after which the aggregation decreases as the pH value continues to rise. For the polymer-modified Fe3O4 nanoparticles, however, the slope of the aggregation curve is much more flat which means that polymer modified on the surface of Fe3O4 nanoparticles can effectively decrease their sensitiveness to pH. In order determine the principles involved for this phenomenon, the ζ -- potentials of samples at different pH values were studied.

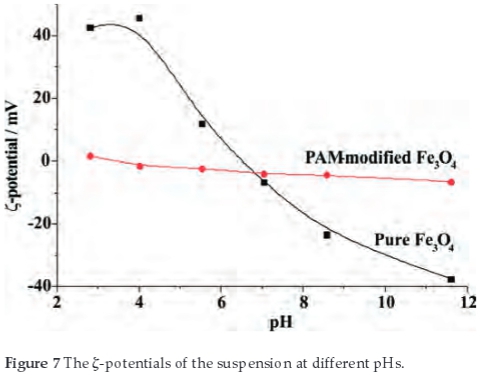
Figure 7 shows the relationship between the pH and the ζ-potential of pure and PAM-modified Fe3O4 nanoparticles. For pure Fe3O4 nanoparticles, at low pH value, the surface charge of the particles is initially positive (more than 40 mV) at pH~4. As a result, the particles aggregate less (shown in Fig. 6) due to the repulsive Coulombic force. At an intermediate pH value near the isoelectric point (IEP), where the surface charge density of particles is very low, the aggregation of magnetite particles becomes significant (shown in Fig. 6) due to the attractiveness of Van der Waals force.32,33 At higher pH value far from the IEP, the overall charge is reversed and the repulsive Coulombic interactions among negative charged particles could again minimize the aggregation. So the ζ-potential of nanoparticle plays a vital role in aggregation of nanoparticles. But after modification of the particles with PAM, Fig. 7 shows that the nanoparticles' ζ-potential distribution is quite narrow which indicating that the ζ-potential of nanoparticles is not sensitive to the pH value.
Aggregation of PAM-modified Fe3O4 nanoparticles at different pH is not as significant as the case for the pure Fe3O4(shown in Fig. 6).
3.7. The Stability of the PAM-modified Nanoparticles in Water
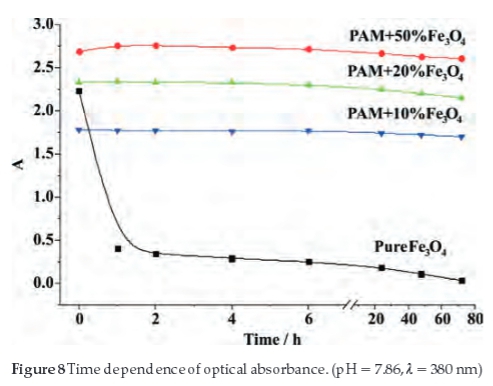
The suspension stability in water of the pure and PAM-modified Fe3O4 was also studied using UV-vis measurement, as the aggregation and deposition rates can be reflected by the intension of UV-vis absorbance.31,34 Figure 8 shows the absor-bance intensity of the four samples, which were measured at different retention times. The absorbance of the pure Fe3O4 nanoparticles decreased sharply in the first hour and nearly becomes zero after 72 hours, which indicated that the pure Fe3O4 is not stable in the water and prone to aggregation and precipitation due high electromagnetic attractive forces between pure Fe3O4 nanoparticles. But after modification by the PAM, the absorbance intensity decreased only slightly after 72 hours, which indicates that PAM largely improves the stability of the nanoparticles in water. It can be attributed to the water-soluble PAM molecular chains that surround the particles and form a hydration shell to prevent them from aggregation and precipitation.
4. Conclusions
In this paper, the PAM modified superparamagnetic Fe3O4 nanoparticles were prepared. SEM shows that the single particle size is about 10-25 nm. XRD spectrum indicates that the produced nanoparticles are consistent with the standard pattern for Fe3O4. In addition, the FTIR spectrum demonstrated that PAM completely coated the surface of Fe3O4 nanoparticles. The magnetic measurement proves that the nanoparticles show superpara-magnetic property. The absorbance intensity of UV-vis implies that the suspension stability of the nanoparticles in aqueous solution can be remarkably improved after being modified by PAM.
Acknowledgements
The authors are grateful to the Second Batch of Open Projects Funding from the State Key Laboratory of Offshore Oil Exploitation (CCL2013RCPS0244GNN) and the Important National Science and Technology Specific Project of China (2011ZX05024-004-03).
References
1 B. Kaboudin and A. Ghaderian, A novel magneto-fluorescent microsphere: preparation and characterization of polystyrene-supported Fe3O4 and CdS nanoparticles, Appl. Surf. Sci., 2013, 282, 396-399. [ Links ]
2 S. Zhang, F. Ren, W. Wu, J. Zhou, L. Sun, X. Xiao and C. Jiang, Size effects of Ag nanaoparticles on plasmon-induced enhancement of photocatalysis of Ag-a-Fe2O3 nanocomposites, J. Colloid Interface Sci., 2014,427, 29-34. [ Links ]
3 N. Wu, L. Fu,M. Su,M. Aslam, K.C. Wong and VP. Dravid, Interaction of fatty acid monolayers with cobalt nanoparticles, Nano Lett.,2004,4, 383-386. [ Links ]
4 M.N. Esfahani, S.J. Hoseini, M. Montazerozohori, R. Mehrabi and H. Nasrabadi, Magnetic Fe3O4 nanoparticles: efficient and recoverable nanocatalyst for the synthesis of polyhydroquinolines and Hantzsch 1,4-dihydropyridines under solvent-free conditions, J. Mol. Catal. A: Chem., 2014, 382, 99-105. [ Links ]
5 Y. Liu, Y. Chi, S. Shan, J. Yin, J. Luo and C. Zhong, Characterization of magnetic NiFe nanoparticles with controlled bimetallic composition, J. Alloys Comp, 2014, 587, 260-266. [ Links ]
6 H. Qin, C.M. Wang, Q.Q. Dong, L. Zhang, X. Zhang, Z.Y. Ma and Q.R. Han, Preparation and characterization of magnetic Fe3O4 - chitosan nanoparticles loaded with isoniazid, J. Magn. Magn. Mater., 2015,381, 120-126. [ Links ]
7 G. Zhao, J. Feng, Q. Zhang, S. Li and H. Chen, Synthesis and characterization of prussian blue modified magnetite nanoparticles and its application to the electrocatalytic reduction of H2O2, Chem. Mater., 2005, 17, 3154-3159. [ Links ]
8 H. Shang, Wo. Chang, S. Kan, S. A. Majetich and G.U. Lee, Synthesis and characterization of paramagnetic microparticles through emul-sion-templated free radical polymerization, Langmuir, 2006, 22, 2516-2522. [ Links ]
9 S. Buendia, G. Cabanas, G. Alvarez-Lucio, H. Montiel-Sanchez, M.E. Navarro-Clemente and M. Corea, Preparation of magnetic polymer particles with nanoparticles of Fe(0), J. Colloid Interface Sci., 2011, 354,139-143. [ Links ]
10 M.Z. Rong, M.Q. Zhang, H.B. Wang and H.M. Zeng, Surface modification of magnetic metal nanoparticles through irradiation graft polymerization, Appl. Surf. Sci., 2002, 200, 76-93. [ Links ]
11 Q. Zhang, L. Luan, S. Feng, H. Yan and K. Liu, Using a bifunctional polymer for the functionalization of Fe3O4 nanoparticles, React. Funct. Polym., 2012, 72, 198-205. [ Links ]
12 H.M. Yang, C.W. Park, T. Ahn, B. Jung, B.K. Seo,J.H. ParkandJ.D. Kim, A direct surface modification of iron oxide nanoparticles with various poly(amino acid)s for use as magnetic resonance probes, J. Colloid Interface Sci., 2013, 391, 158-167. [ Links ]
13 L. Yu, W. Xue, L. Cui, W. Xing, X. Cao and H. Li, Use of hydroxy - propyl-β-cyclodextrin/polyethylene glycol 400, modified Fe3O4 nanoparticles for congo red removal, Int. J. Biol. Macromol., 2014, 64, 233-239. [ Links ]
14 H. Cui, D. Li and Z. Zhang, Preparation and characterization of Fe3O4 magnetic nanoparticles modified by perfluoropolyether carboxylic acid surfactant, Mater. Lett., 2015,143, 38-40. [ Links ]
15 W. Wang, Y. Jing, S. He, J. P. Wang and J. P. Zhai, Surface modification and bioconjugation of FeCo magnetic nanoparticles with proteins, Colloids Surf. B, 2014,117, 449-456. [ Links ]
16 M. Ziegler-Borowska, D. Chelminiak, T. Siódmiak, A. Sikora, M.P Marszall and H. Kaczmare, Synthesis of new chitosan coated magnetic nanoparticles with surface modified with long-distanced amino groups as a support for bioligands binding, Mater. Lett., 2014, 132, 63-65. [ Links ]
17 M. Ziegler-Borowska, T. Siódmiak, D. Chelminiak, A. Cyganiuk and M. P. Marszall, Magnetic nanoparticles with surfaces modified with chitosan-poly [N-benzyl-2-(methacryloxy)-N,N-dimethylethana-minium bromide] for lipase immobilization, Appl. Surf. Sci., 2014,288, 641-648. [ Links ]
18 Y. Ding, S.Z. Shen, H. Sun, K. Sun, F. Liu, Y. Qi and J. Yan, Design and construction of polymerized-chitosan coated Fe3O4 magnetic nano-particles and its application for hydrophobic drug delivery, Mater. Sci. Eng. C, 2015, 48, 487-498. [ Links ]
19 P.B. Shete, R.M. Patil, B.M. Tiwale and S.H. Pawar, Water dispersible oleic acid-coated Fe3O4 nanoparticles for biomedical applications, J. Magn. Magn. Mater, 2015, 377, 406-410. [ Links ]
20 X. Zhang, L. Xue, J. Wang, Q. Liu, J. Liu, Z. Gao and W. Yang, Effects of surface modification on the properties of magnetic nanoparticles / PLA composite drug carriers and in vitro controlled release study, Colloids Surf. A, 2013, 431, 80-86. [ Links ]
21 P. Tancredi, S. Botasini, O. Moscoso-Londono, E. Méndez and L. Socolovsky, Polymer-assisted size control of water-dispersible iron oxide nanoparticles in range between 15 and 100 nm, Colloids Surf. A, 2015,464, 46-51. [ Links ]
22 E. Murugan and J.N. Jebaranjitham, Dendrimer grafted core-shell Fe3O4-polymer magnetic nanocomposites stabilized with AuNPs for enhanced catalytic degradation of Rhodamine B - A kinetic study, Chem. Eng. J., 2015, 259, 266-276. [ Links ]
23 M. Chen, L. Wang, J. Han, J. Zhang, Z. Li and D. Qian, Preparation and study of polyacryamide-stabilized silver nanoparticles through a one-pot process, J. Phys. Chem. B, 2006,110, 11224-11231. [ Links ]
24 A. Chatterjee and S. Mishra, Novel synthesis with an atomized microemulsion technique and characterization of nano-calcium carbonate (CaCO3)/poly(methyl methacrylate) core-shell nano-particles, Particuology, 2013, 11, 760-767. [ Links ]
25 R. Joksimovic, S. Prévost, R. Schweins, M.S. Appavou and M. Gradzielski, Interactions of silica nanoparticles with poly(ethylene oxide) and poly(acrylic acid): effect of the polymer molecular weight and of the surface charge, J. Colloid Interface Sci., 2013, 394, 85-93. [ Links ]
26 A. Fedorczyka, J. Ratajczakb, A. Czerwinski and M. Skompska, Selective deposition of gold nanoparticles on the top or inside a thin conducting polymer film, by combination of electroless deposition and electrochemical reduction, Electrochim. Acta, 2014, 122, 267-274. [ Links ]
27 T. Banert and U.A. Peuker, Preparation of highly filled super-para-magnetic PMMA-magnetite nano composites using the solution method, J. Mater. Sci., 2006, 41, 3051-3056. [ Links ]
28 L.Yu, L. Zheng and J. Yang, Study of preparation and properties on magnetization and stability for ferromagnetic fluids, Mater. Chem. Phys., 2000, 66, 6-9. [ Links ]
29 A. Cosultchi, J.A. Ascencio-Gutierrez, E. Reguera, B. Zeifert and H. Yee-Madeira, On a probable catalytic interaction between magnetite (Fe3O4) and petroleum, Energy Fuels, 2006, 20, 1281-1286. [ Links ]
30 T. Madrakian, A. Afkhami, H. Mahmood-Kashani and M. Ahmadi, Superparamagnetic surface molecularly imprinted nanoparticles for sensitive solid-phase extraction of tramadol from urine samples, Talanta, 2013, 105, 255-261. [ Links ]
31 S. Peng, C. Wang, J. Xie and S. Sun, Synthesis and stabilization of monodisperse Fe nanoparticles, J. Am. Chem. Soc., 2006, 128, 10676-10677. [ Links ]
32 F. Bouyer, A. Robben, W. Yu and M. Borkovec, Aggregation of colloidal particles in the presence of oppositely charged polyelectrolytes: effect of surface charge heterogeneities, Langmuir, 2001,17,5225-5231. [ Links ]
33 E. Illés and E. Tombácz, The effect of humic acid adsorption on pH-dependent surface charging and aggregation of magnetite nanoparticles, J. Colloid Interface Sci., 2006, 295, 115-123. [ Links ]
34 S.A. Gomez-Lopera, J.L. Arias, V Gallardo and A.V Delgado, Colloidal stability of magnetite/poly(lactic acid) core/shell nanoparticles, Langmuir, 2006, 22, 2816-2821. [ Links ]
Received 17 June 2014
Revised 4 March 2015
Accepted 1 April 2015.
* To whom correspondence should be addressed. E-mail: yanghui@iccas.ac.cn














