Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Chemistry
versão On-line ISSN 1996-840X
versão impressa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.68 Durban 2015
http://dx.doi.org/10.17159/0379-4350/2015/v68a9
RESEARCH ARTICLE
Determination of Leachable Vanadium (V) in sediment
Lekgala A. Mampuru; Nikolay A. Panichev; Prince Ngobeni; Khakhathi L. Mandiwana; Makonga M. Kalumba*
Department of Chemistry, Tshwane University of Technology, P.O. Box 56208, Arcadia Pretoria, 0007, South Africa
ABSTRACT
A method for speciation of vanadium in solid samples was developed for quantification of vanadium( + 5) in solid samples of sediment Certified Reference Materials (CRM) PACS-2 and MESS-3 (Trace Elements in Sediments) of known total vanadium content. The method relies on a classical analytical chemistry procedure based on leaching water-insoluble vanadium( + 5) compounds from the solid state into solution using Na2CO3 followed by electrothermal atomic absorption spectrometry (ET-AAS) detection. The total amount content of vanadium was determined after complete digestion of the CRMs and found to be 132 ± 5 and 245 ±12 μg g-1 in PACS-2 and MESS-3, respectively, in good agreement with the certified values of 133±6 μg g-1 and 243 ± 12 μg g-1. The concentrations of vanadium(+5) were determined to be 25 ± 3 μg g-1 and 13 ± 2 μg g-1 in PACS-2 and MESS-3, respectively. These results were verified by the method of standard additions for which quantitative recoveries (100±2 and 97 ± 3 %, one standard deviation, n = 3) were obtained for spikes added to PACS-2 and MESS-3, respectively. Approximately 18.8 % of the total vanadium content of PACS-2 and 5.3 % in MESS-3 is in the form of vanadium(+5) species.
Keywords: Certified reference materials, vanadium(+5) speciation, electrothermal atomic absorption spectrometry.
1. Introduction
The measurement of the chemical species of elements, instead of the total element concentration, has become an irreversible trend in analytical chemistry.1-2 The motivation lies in the fact that the biochemical and geochemical behaviour of many elements is governed by its species. Because vanadium(+5) is more toxic, it is important to obtain valance speciation for this element in such environmental samples as soil, sediments and plants.3,4-5 Validation of the analytical procedures used for the speciation analysis requires the analysis of representative certified reference materials (CRMs) for the relevant species. However, the development of reliable methods for speciation of vanadium remains a challenging field of analytical chemistry, especially for solid samples.2-6
CRMs are very important tools for the verification of accuracy for an analytical procedure and they form an integral part of any quality control system for which validation of methodology is desired. CRMs with total amount content of vanadium are available, but not for vanadium(+5) speciation analysis. In the present study, a method for the separate determination of vanadium species in CRM marine sediments PACS-2 and MESS-3 having known total amount content of vanadium is described.7 The method is based on a classical analytical chemistry procedure of leaching of vanadium(+5) species, which are anionic, from solid samples using Na2CO3.8 Leaching is a procedure frequently applied for the extraction of metals or their species from environmental samples, e.g. soil, sediments and airborne particles. The leachable recoveries of analytes are generally lower than the total concentrations present. The treatment of solid samples with Na2CO3 permits conversion of the insoluble compounds of vanadium(+5) into soluble forms prior to their determination using electrothermal atomization atomic absorption spectrometry (ET-AAS).9 An earlier developed method used for the determination of vanadium (+5) in soil and plant samples, taken near a vanadium mine,7 indicates that leaching of vanadium (+5) can be achieved either with 0.1 M Na2CO3 or Na3PO4.10 However, such methods of leaching have never been tested and applied for the quantification of vanadium(+5) in solid samples of CRMs.
2. Experimental
2.1. Instrumentation and Measurements
A Perkin-Elmer atomic absorption spectrometer Model AAnalyst 600 with Zeeman-effect background correction was used for all measurements. The spectrometer was equipped with an AS-800 autosampler and the system was controlled by means of AAWinlab software running under Microsoft Windows.11 A vanadium hollow cathode lamp (Perkin-Elmer), operating at 40 mA, was employed, using the 318.4 nm resonance line and a spectral band pass of 0.7 nm. Transversely heated graphite tubes (THGA) with integrated Lvov platforms (Perkin-Elmer, part N B050-4033) were used as atomizers with Argon (Afrox, South Africa) as the sheath gas throughout.
2.2. Samples and Reagents
Vanadium (+5) standard solutions were prepared by appropriate dilution of Na3VO4 (Merck) stock solution, containing 1000 mg L-1 of V(V) with 0.1 M Na2CO3. For comparative studies, a 0.1 M solution of Na3PO4 was also prepared. Ultra-pure water (with a resistivity of 18.2 MQ cm) was obtained from a Milli-Q water purification system (Millipore, Bedford, MA, USA) and used for sample preparation and dilutions. Certified Reference Materials MESS-3, MESS-4, PACS-2 and PACS-3 (Marine Sediments for Trace Metals), obtained from the National Research Council Canada,12 were used for the validation of methodology for the determination of the concentration of total vanadium and as test samples for the determination of vanadium(+5) content. Hydrophilic 0.45 μm filters (PVDF, Millipore Millex, USA) were used for the filtration of sample solutions.
2.3. Sample Preparation for the Determination of Vanadium(+5)
Approximately 0.25 g test portions of MESS-3 or PACS-2 were accurately weighed into glass beakers and 25.0 mL 0.1 M Na2CO3 were added to each beaker. The contents of the beakers were boiled for 10 min. After cooling, the contents were transferred to plastic tubes without filtration and the volume of the solution was adjusted to 25.0 mL using ultra-pure water. The same procedure was followed for the leaching of V(V) compounds with Na3PO4. In order to ensure that total V(V) was extracted, multiple extractions were carried out from the same sample using fresh portions of both 0.1 M Na2CO3 and 0.1 M Na3PO4. It was found that a single treatment of the samples with Na2CO3 or Na3PO4 was sufficient as the efficiency of the leaching process using either compound was in the range 96 ± 5 % (n = 6).
2.4. Sample Preparation for the Determination of Total Vanadium Content
Because the CRMs may hold vanadium within the crystal lattice structure of specific minerals, complete acid digestion of samples was carried out in order to determine the total vanadium content. For this purpose, approximately 0.25 g test samples were transferred to a Teflon beaker and treated on a hot plate at about 110 °C with 5.0 mL of concentrated HF and 2.0 mL HClO4. If digestion is not complete, a further 2.0 mL of HF and 1.0 mL of HClO4 were added and again the mixture was heated to near dryness. Finally, the sample was treated with 1.0 mL of HClO4 and heated until the appearance of white fumes. The residue was then dissolved in 5.0 mL of 6.0 M HCl and diluted to 50.0 mL using ultra-pure water. The resulting solution was analyzed by ET-AAS (n = 6).
3. Results and Discussion
3.1. Analytical Results for Determination of Vanadium
Determination of vanadium is not a simple task using ET-AAS as it is a refractory element, requiring rapid heating and the use of fresh pyrolytic graphite coated tubes.13 Some problems, such as tailing of the absorbance signal, carbide formation and acid interferences have been encountered13-14. Atomization of vanadium from a matrix with a relatively high amount of Na2CO3 was investigated using the temperature programme presented in Table 1. It was noted that after the treatment of samples at 1600 °C for a relatively long time (20 s), atomization could be completed in less than 5 s13. Aomization of V from simple calibration standard solutions and solutions obtained after the treatment of soil or plant samples with Na2CO3 did not differ in their appearance time nor in the time required for complete atomization. This phenomenon may possibly be explained as the result of complete separation of V(V) compounds from all other metals leached from the sample solutions but subsequently precipitated as insoluble carbonates or hydroxides during treatment with Na2CO3.
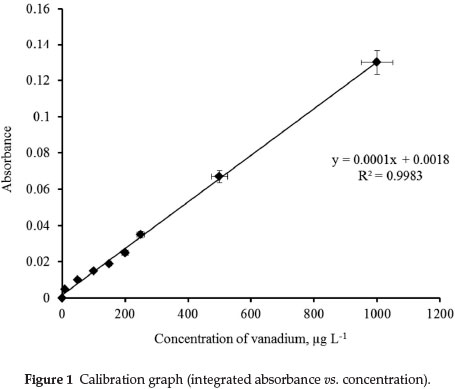
The calibration graph (integrated absorbance vs. concentration) is described by the equation y = 0.0001x + 0.0018 with regression coefficient R2 = 0.9983; linearity is maintained up to a concentration of 2500 μg L-1 of vanadium. The time evolution of the signals is presented in Fig. 1. The accuracy of this analytical technique was validated by the determination of total vanadium in the CRMs. Results are summarized in Table 2, from which it may be concluded that overall performance is very satisfactory.
The limit of detection (LOD) for determination of vanadium, calculated as the concentration corresponding to a signal equal to 3 times the standard deviation of the integrated absorbance from the blank solution, was established using solutions of 0.1 M Na2CO3 as a blank.15 The LOD was calculated to be about 5 μg L-1 for a 10 μL sample aliquot (n = 11). With respect to the solid samples, assuming that 0.25 g of material was used for digestion and the leachates diluted to a final volume of 25.0 mL, the LOD was estimated to be 0.5 μg g-1.
3.2. The effect of Leaching Time
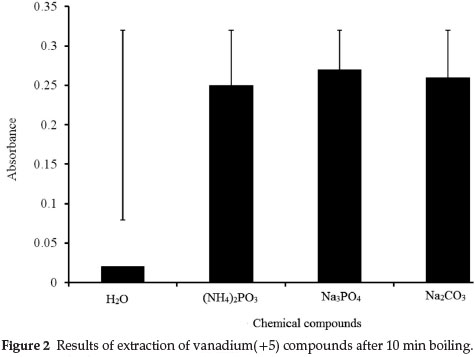
Leaching of vanadium(+5) compounds was tested by boiling 0.25 g test portions of the solid CRMs with 25.0 mL of 0.1 M Na2CO3for 30 min. The first test aliquot analysed (1.0 mL of the solution) was taken immediately after the boiling point was reached and the following samples, also 1.0 mL volume, were taken every 5 min in such a manner that the ratio between mass of solid sample and the volume of extracting solution was kept constant (by addition of a fresh portion of 0.1 M solution of Na2CO3). The same procedure was followed for the leaching of V(V) compounds using Na3PO4. The results presented in Fig. 2 show that extraction of vanadium(+5) compounds from both CRMs could be completed after 10 min of boiling.
3.3. Efficiency of Leaching
In order to ensure that the total amount of vanadium(+5) were extracted, multiple extractions were carried out on the same sample using fresh portions of either 0.1 M Na2CO3 or 0.1 M Na3PO4 For this purpose, mixtures of 0.25 g of solid CRM and 25.0 mL of Na2CO3 were boiled for 10 min. After filtration, the solid residue was treated with a fresh portion of 25.0 mL 0.1 M Na2CO3 for the second time and, once again, for a third time. The efficiency of the leaching process based on the ration of results for the first extraction to those of the sum of all extractions using either 0.1 M Na2CO3 or 0.1 M Na3PO4 was in the range 97 ± 5 % as shown in Fig. 3. The second extraction step did not yield more than 2 % of the remaining total V in the samples.
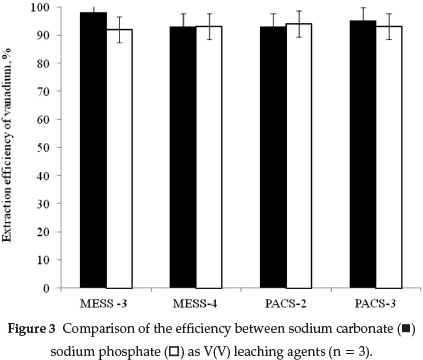
3.4. Validation of Results
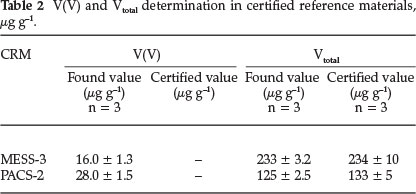
In order to validate this methodology for the determination of vanadium (+5), 12 sub-samples of each CRM were prepared. Three samples were treated with 0.1 M Na2CO3 solution without addition of spikes and the average quantity of vanadium obtained was taken as a base value14. Subsequently, increasing quantities of V(V) were added to other aliquots and vanadium (+5) was determined according to the recommended procedure. Quantitative spike recoveries of 100 ± 3 and 98 ± 4 % (one standard deviation, n = 3) were obtained for PASC-2 and MESS-3, respectively. The concentrations of vanadium (+5) in PACS-2 and MESS-3, summarized in Table 3, were determined to be 25 ± 3 and 13 ± 2 μg g-1, respectively.
4. Conclusion
A simple, fast, sensitive and accurate method for vanadium (+5) speciation in solid samples of PACS-2 and MESS-3 marine sediment CRMs has been developed. The method is based on leaching of V(+5) with either 0.1 M Na2CO3 or 0.1 M Na3PO4 followed by determination using ET-AAS. The results show that 18.8 % of the total amount of vanadium present in PACS-2 and 5.3 % in ESS-3 are in the form of vanadium (+5) species.
References
1 R. Cornelis, H. Crews, O.F.X. Donard and L. Ebdon, Trends in certified reference materials for the speciation of trace elements, Fresenius J. Anal. Chem,. 2001, 370, 120-125. [ Links ]
2 J. Feldmann, A. Elgazali, M.F. Ezzeldin, Z. Gajdosechova, E. Krupp, F. Aborode, M.M. Lawan, A. Raab, A.H. Petursdottir and K. Amayo, Microwave-Assisted Sample Preparation for Trace Element Analysis, Elsevier, Amsterdam, 2014, pp. 281-312. [ Links ]
3 J. Nriagu (Ed), Vanadium in the Environment: Part 2, Health Effects, John Wiley & Sons, New York, Chichester, Weinheim, Brisbane, Singapore, Toronto, 1998. [ Links ]
4 B. Mukerjee, B. Patra, S. Mahapatra, P. Banerjee, A. Tiwari and M. Chatterjee Vanadium - an element of atypical biological significance, Tox. Lett., 2004,150, 135-143. [ Links ]
5 F.L. Assem and A. Oskarsson, Handbook on the Toxicology of Metals, vol. 2, 4th edn., Elsevier, London, 2015, pp. 1347-1367. [ Links ]
6 P. Quevauviller, Operationally defined extraction procedures for soil and sediment analysis I. Standardization, Trends Anal. Chem., 1998, 17, 289-298 [ Links ]
7 N. Panichev, K. Mandiwana, D. Moema, R. Molatlhegi and P. Ngobeni, Distribution of vanadium(V) species between soil and plants in the vicinity of vanadium mine, J. Hazard. Mater., 2006,137, 649-653 [ Links ]
8 L. Ebdon, L. Pitts, R. Cornelis, H. Crews, O.F.X. Donard and P. Quevauviller, Trace Element Speciation for Environment and, Food and Health, Royal Society of Chemistry, Cambridge, 2001. [ Links ]
9 K. Mandiwana and N. Panichev, Electrothermal atomic absorption spectrometric determination of vanadium (V) in soil after leaching with Na2CO3, Anal. Chim. Acta, 2004, 517, 201-206. [ Links ]
10 K.L. Mandiwana, N. Panichev and R. Molathlegi, The leaching of V (V) with PO43- in the speciation analysis of soil, Anal. Chim. Acta, 2005, 545, 239-243. [ Links ]
11 N. Panichev, K. Mandiwana, M. Kataeva and S. Siebert, Determination of Cr(VI) in plants by electrothermal atomic absorption spectrometry after leaching with sodium carbonate, Spectrochim. Acta, B, 2005, 60, 699-703 [ Links ]
12 National Research Council Canada, http://www.nrccnrc.gc.ca/eng/solutions/advisory/crm/inorganic/list_products.html, accessed 14 July 2014 [ Links ]
13 B. Welz and M. Sperling, Atomic Absorption Spectrometry, 3rd edn., Wiley's Publishers, Weinheim, 1999, pp. 323-332. [ Links ]
14 D.C. Manning and W. Slavin, Factors influencing the atomization of vanadium in graphite furnace AAS, Spectrochim. Acta Part, B, 1985,40, 461-473. [ Links ]
15 J.N. Miller and J.C. Miller, Statistics and Chemometrics for Analytical Chemistry, Pearson Education, Edinburgh Gate, Harlow, UK, 2005. [ Links ]
Received 11 November 2014
Revised 2 February 2015
Accepted 4 February 2015
* To whom corresponence should be addressed. E-mail: kalumbam@tut.ac.za














