Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.68 Durban 2015
http://dx.doi.org/10.17159/0379-4350/2015/v68a4
RESEARCH ARTICLE
http://dx.doi.org/10.17159 /0379-4350/2015/v68a4
Kinetics and mechanism of the ligand exchange reaction between tetradentate schiff base N,N'-ethylen-bis (salicylaldimine) and Ni(N,N'-propylen-bis(salicylaldimine))
Rasoul Vafazadeh*; Maryam Bagheri
Department of Chemistry, Yazd University, Yazd, Iran
ABSTRACT
Visible spectrophotometry is used to study the kinetics of ligand exchange in the system Ni(salpn)/H2salen with or without triethylamine (NEt3) and H2O in dimethylformamide (DMF) solvent at 25 ± 0.1 °C and 0.01 M NaNO3 [H2salen and H2salpn are N,N'-ethylen-bis(salicylaldimine) and N,N'-propylen-bis(salicylaldimine), respectively]. It was found that the reaction rate is of the first-order with respect to Ni(salpn). In addition, the effect of NEt3 and H2O on the rate of the reaction was examined. The rate of the ligand exchange reaction was accelerated by adding NEt3 to the reaction mixture. However, the ligand exchange rate was not changed by adding H2O to the mixture reaction. The effects of NEt3 and H2O on the ligand exchange rate show that deprotonation/protonation of the H2salen ligand and anionic form of H2salen is important. On the basis of these results, the reaction mechanism is discussed.
Keywords: Kinetic, mechanism, Schiff base, Ni complexes, ligand exchange.
1. Introduction
Ligand substitution reaction on planar four-coordinate metal complexes with central metal ions of d8 electronic configuration, such as Ni(II), have been generally studied using replaceable monodentate ligands.1,2 The results of these studies have almost universally suggested an associative mechanism with a five-coordinate intermediate.1,3
Polydentate ligand exchange reactions are very interesting from a mechanistic point of view due to their importance in coordination chemistry.4,5 However, the kinetics of polydentate ligands exchange reactions have not been studied in the same detail as monodentate ligands. In general, such systems proceed through intermediates, where the incoming polydentate ligand is partially coordinated and the leaving ligand is partially dissociated.5-7 The driving force for ligand exchange reactions in metal-complexes with polydentate ligands is the formation of a more stable complex.8-10
The structures of the Ni(II) and Cu(II) complexes of multiden-tate ligands such as salen Schiff base ligands and its derivatives (Scheme 1) have been studied by various groups.11-17 These studies have indicated that the increase in the number of CH2 groups allows sufficient flexibility to the structure of complexes by changing their structure from a planar to distorted tetrahedral form or by forming five- or six-coordinate species in the presence of additional donors.17-21 In addition, the metal complex stability decreases as the alkyl chain length (number of CH2 groups) increases.22,23
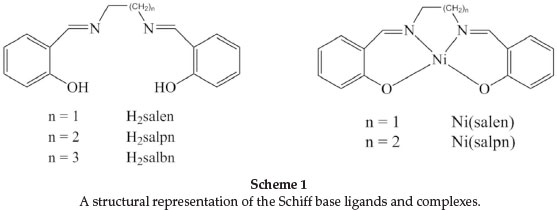
In our previous work, we reported the kinetics of ligand exchange in the systems Cu(salpn)/H2salen, Cu(salbn)/H2salen and Cu(salbn)/H2salpn, where H2salen, H2salpn and H2salbn are N,N'-ethylen-bis(salicylaldimine), N,N'-propylen-bis(salicyl-aldimine) and N,N'-butlen-bis(salicylaldimine), respectively (reactions 1-3).9,10 In order to investigate the effect of the metal on the kinetics of ligand exchange, we carried out a kinetic study for the ligand exchange reaction salpn with the Ni(salen) complex, Ni(N,N'-propylen-bis(salicylaldimine)), reaction 4).
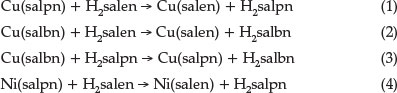
2. Experimental
2.1. Materials
The Schiff bases H2salen and H2salpn were prepared from a general method,9,10 namely the condensation reaction between two equivalents of salicylaldehyde and one equivalent of the appropriate diamine (1,2-ethylendiamine and 1,3-propan-diamine), in ethanol. The yellow products were obtained in yields typically 70 % or better. Purity of products was verified by comparing with literature melting points (m.p.), 124 and 53 °C for H2salen and H2salpn, respectively.19,20,23
H2salen: Yield = 80 %, IR (cm-1): n(O-H) 3000-3500, v(C=N) 1633, v(C-O) 1281, v(C=C) 1578. Electronic spectra in DMF solvent: λmax(log ε, M-1 cm-1) = 403 (2.23), 316 (3.49), 256 (4.20) nm.
H2salpn: Yield = 70%,IR(cm-1): v(O-H) 3000-3500, v(C=N) 1629, v(C-O) 1276, v(C=C) 1580. Electronic spectra in DMF solvent:λmax(log ε,M-1 cm-1) = 404 (2.36), 313 (4.02),253 (4.37) nm.
The nickel complexes were prepared by a general method, using the reaction solution of nickel(II) acetate with the Schiff base ligand (1:1 molar ratio).24,25
Ni(salen): Red needle-shaped crystals, m.p. >250, Yield = 61 %, IR (cm-1): v(C=N) 1619, v(C-O) 1345, v(C=C) 1530. Electronic spectra in DMF solvent: λmax(log ε,M-1 cm-1) = 540 (2.18), 345 (3.98), 270 (4.79) nm. Found: C, 59.13; H, 4.34; N, 8.62 %. Calc. for C16H14N2NiO2 (324.99); C, 58.99; H, 4.27; N, 8.75 %.
Ni(salpn): Brown needle-shaped crystals, m.p. >250, Yield = 70 %, IR (cm1): n(C=N) 1606, v(C-O) 1355,v(C=C) 1540. Electronic spectra in DMF solvent: λmax (log e,M-1 cm-1) = 650 (1.86), 350 (3.81), 275 (4.81) nm. Found: C, 60.01; H, 4.66; N, 8.38 %. Calc. for C17H16N2NiO2 (339.02); C, 60.23; H, 4.76; N, 8.26 %.
2.2. Kinetics Measurements
To measure the ligand exchange reaction rate, the absorbance changes of reaction mixtures were recorded as a function of time using a GBC UV-Visible Cintra 101 spectrophotometer. The greatest change in molar absorbance between reactants and products was observed at 530 nm. Therefore, all kinetic measurements of absorbance versus time were made at this wavelength. Reaction mixtures were made in dimethylfor-mamide, DMF, an aprotic polar solvent, ionic strength 0.1 M (adjusted with NaNO3) and the Ni(salpn) complex concentration was held constant at 5.0 x 10-3 M, while concentration of H2salen ligand (with and without triethylamine, NEt3 and/or Η2θ) was varied from 5.0 x 10-3 to 1.0 x 10-1 M at 25 ± 0.1 °C Under these conditions, pseudo-first-order kinetic behaviour was observed for reaction 4.
To initiate the reaction, equal volumes of Ni(salpn) and H2salen were mixed and the absorbance of reaction product at 530 nm was followed as a function of time. At least three runs at each concentration were recorded. The absorbance A versus time t data were computer-fitted with the sigmaplot 12.0 software using Equation 5 (irreversible first-order reaction), 6 (biphasic reaction), and 7 (triphasic reaction) to find the best fit and kobs:
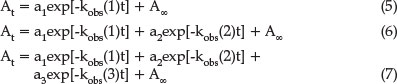
where a1 and a2 comprise rate constants and molar absorptivities, respectively:1
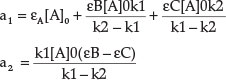
3. Results and Discussion
3.1. Kinetics study
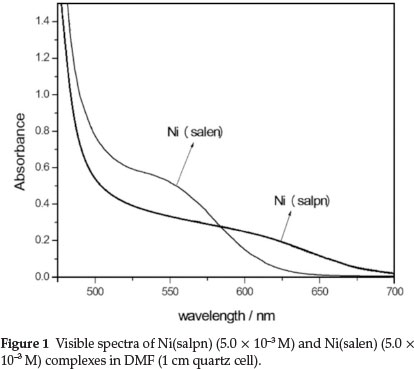
The visible absorption spectra of Ni(salen) and Ni(salpn) complexes in DMF solvent show a maximum absorption due to d-d transition at 540 and 600 nm, respectively (Fig. 1). The UV-Vis absorption spectra recorded after mixing Ni(salpn) and H2salen in DMF (Fig. 2) shows a consecutive series of spectra recorded in DMF for the Ni(salpn)/H2salen system, indicating that the Ni(salpn) complex is converted to the Ni(salen) complex by adding H2salen ligand. The spectrum of Ni(salen) in DMF (Fig. 1), was similar to the last spectrum shown in Fig. 2. This similarity confirms the conversion of the Ni(salpn) to the Ni(salen) complex (reaction 4).
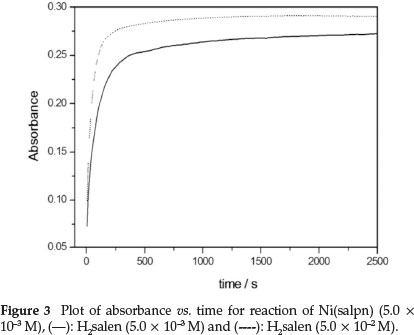
The rate constants for the ligand exchange reaction were obtained by measuring the absorbance changes at 530 nm under pseudo-first-order conditions, [H2salen] >> [Ni(salpn)] (Figs. 3 and 4). The absorbance at 530 nm was found to increase with time, due to formation of Ni(salen) complex (Fig. 3). Under these conditions, the best fit for the absorbance versus time data was obtained utilizing Equation 6 (biphasic reaction). Therefore, two rate constants kobs(1) and kobs(2) were obtained. The plots of the two observed constants, kobsvs. [H2salen], are shown in Fig. 4. The figure indicates that the first reaction step was fast and dependent on the [H2salen], while the second reaction step was slow and independent of the concentration of H2salen ligand. These results can be described by Equations 8 and 9.

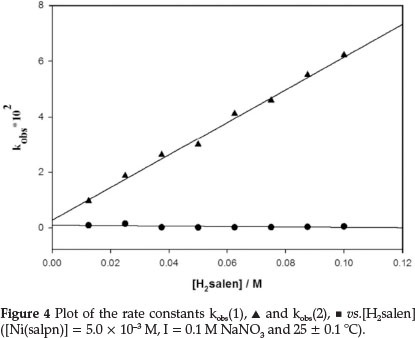
The k1 (second-order rate constant) value was obtained from the slope of line kobs(1) vs. [H2salen], k1 = 5.80 ± 0.28 x 10-1 M-1 s-1 and k2 = 2.56 ± 0.89 x 10-3 s-1 was determined as the mean of the experimentally obtained data for kobs(2) (Fig. 4), which was independent of [H2salen]. The rate constant for the second step of the ligand exchange reaction of the Ni(salpn)/H2salen system is similar to the analogous reactions of the Cu(salpn)/ H2salen system (3.11 1.06 x 10-3 s-1).9
The kinetic study of the ligand exchange reaction was investigated in the presence of H2O (protic solvent) and/or NEt3.
3.2. Effect of Triethylamine
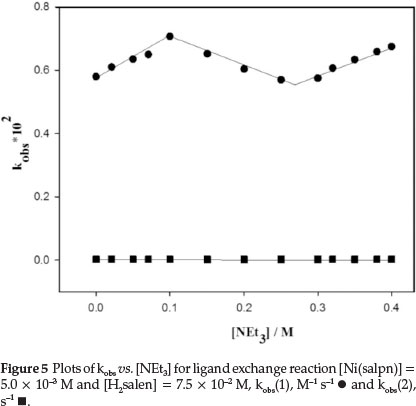
The rate of the ligand exchange reaction was observed to be accelerated by addingNEt3 to the reaction mixture. Consequently, the effect of [NEt3] on the rate of the reaction was examined. The absorbance at 530 nm was found to increase with time, due to formation of Ni(salpn) complex (Fig. 3). The rate of the ligand exchange reaction at different NEt3 concentrations indicated that the rate of reaction strongly depends on [NEt3]. The plots of k1 and k2 values vs. [NEt3] are shown in Fig. 5. As shown in Fig. 5, the value of k1 initially increased by increasing the NEt3 concentration, while the k2 value was independent of the concentration of NEt3 amine. However, as shown in Fig. 5, for the plot of k1vs. [NEt3] there is an obvious break between the 0.1-0.3 M concentrations of NEt3 - this could be related to a change of the reaction species at these concentrations of NEt3. This effect on the reaction rate is also found in the salen-type copper(II) complexes.9,10
3.3. Effect of H2O
Like the analogous reaction of copper(II) complexes,9,10 the ligand exchange rate of H2salen for salpn in Ni(salpn) complex does not significantly change with the addition of H2Otothe mixture reaction. The values of k1 and k2 in the presence and the absence of H2O were of the same order (Table 1). This result allowed us to propose that reactive species were similar in both the reaction conditions (vide infra).
3.4. Reaction in Presence of H2O and NEt3
The rate of the exchange reaction Ni(salpn) with H2salen in presence of NEt3 and H2O was studied for three different conditions: 1) [NEt3] < 0.1 M (before break in plot in Fig. 5); 2) [NEt3]> 0.3 M (after break in plot in Fig. 4); 3) 0.1 < [NEt3] < 0.3 M (break limits in plot in Fig. 5). In the first and second condition, the ligand exchange reaction rate was considerably decreased byaddingH2O(0.4M).Inthelastcondition,0.1 < [NEt3] < 0.3M, the rate of reaction was decreased by adding H2O (0.4 M) to the reaction mixture (Table 1). In spite of this, the reaction rate in this condition (0.1 < [NEt3] < 0.3 M) was increased by adding of H2O up to 0.05 M. The effects of NEt3 and H2O show the importance of protonation/deprotonation on the rate of the ligand exchange reaction (see proposed mechanism).
3.5. Proposed Mechanism
The ligand exchange reaction Ni(salpn) with H2salen was found to be a two-step process (biphasic reaction) as described by the experimental rate constants kobs(1) and kobs(2). The kobs(1), is depended on the concentration of the H2salen ligand, according to Equation 8. The kobs(2) is H2salen concentration independent (Fig. 4).
A similar effect on reaction rate was also found for the salen-type ligands copper(II) complexes, suggesting a common mechanism for the ligand exchange reactions (vide infra).9,10
As observed from the experimental data, the ligand exchange rate in the absence of NEt3 did not change with addition of H2O (protic solvent) to the reaction mixture, it is reasonable to assume that Hsalen- and salen- ion concentrations are negligible, so that H2salen can be treated as the major reactive species. The acidity of H2salen and its family of ligands enable us to assume that under these reaction conditions neutral H2salen ligand was the original species.26
These observations lead us to suggest a possible mechanism for the reaction when a Ni(salpn) complex reacts with H2salen ligand (Equations 10 and 11):
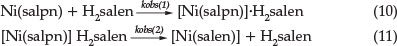
It is clear from Equation 10 that rate constant kobs(1) should increase linearly with [H2salen], according to Equation 8. The formation of the adduct [Ni(salpn].H2salen in Equation 10 is in agreement with a number of studies on the polydentate ligand exchange reaction.5-7 The lone pairs electron on the phenolic oxygen atoms of H2salen provide a reasonable basis to propose the formation of adduct [Ni(salpn].H2salen as the first step in the reaction.
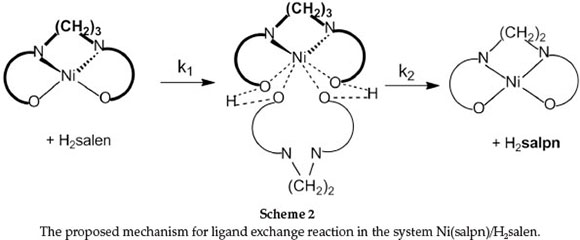
The second reaction step is first-order and independent of [H2salen] (Equation 11) with rate constant k2. As indicated in Scheme 2, substitution of salpn from the Ni(II) complexes involves initial coordination of phenolic oxygen atoms of H2salen to the nickel centre in the Ni(salpn) complex followed by protons-transfer from H2salen to salpn, with bond cleavage of two-ends of salpn. The ligand exchange reaction is completed by substituting salpn with salen.
As shown in Fig. 5, the reaction rate increases by adding NEt3. The effect of NEt3 could be due to its interaction with either Ni(salpn) or with Schiff base ligand. The UV-Vis spectrum of the Ni(salpn) complex in DMF solvent does not change with the addition of NEt3 to the solvent, therefore, the formation of the adduct Ni(salpn) and NEt3 is not observed. In our previous study,9,10 we have explained that interaction between H2salen and NEt3 could lead to the formation of Hsalen- and salen2- ions (dependent on [NEt3]) by the deprotonation phenolic group(s) of H2salen (reactions 12 and 13).
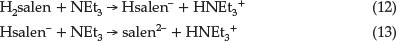
It is clear that in the presence of NEt3, the major reactive species are Hsalen- and salen2- ions. At relatively low [NEt3], the Hsalen-ion and at high [NEt3] the salen2- ion is the original reactive species. This suggests that a plausible mechanism in the presence of NEt3 is that presented in Scheme 3. In the first step, NEt3 quickly produced labile Hsalen- and salen2- ions. Then, Hsalen- ion (path 1) or salen2- ion (path 2) coordinated to a nickel centre in Ni(salpn) complex by lone pairs electron on the pheno-lic oxygen atoms. This is followed by intramolecular proton transfer from the coordinated Hsalen- to one of the phenolic oxygen atoms coordinated to the salpn ligand with bond-cleavage of one end of salpn (path 1).
As shown in Fig. 5, the slope of the plot at high [NEt3] (0.97 ± 0.07 M-1.s-1, salen2- is the major reactive species, Equation 13) is less than with low [NEt3] (1.18 ± 0.13 M-1.s-1, Hsalen- is the major reactive species, Equation 12). Therefore, we can assume that the Hsalen- ion is a more active species than the salen2- ion in the ligand exchange reaction.9,10 At high concentration of [NEt3] (> 0.3 M), although the salen2- ion is similar to the Hsalen- ion and can be quickly coordinated to a Ni(II) centre in the Ni(salpn) complex, the salen2- ion has no available proton for intramolecular proton transfer and thus cannot quickly undergo bond cleavage of salpn in the Ni(salpn) complex. Therefore, the rate of the reaction increases less under these conditions. At concentration 0.1<[ NEt3]<0.3 M there is equilibrium between Hsalen- and salen2- ions.
To ensure the validity of the mechanism shown in Scheme 3, the reactions have been performed in the presence of H2O and NEt3. These experiments are studied with three different concentrations of NEt3:
1. In the first case, [NEt3] < 0.1 M (before the break in the plot in Fig. 5), the ligand exchange rate was decreased by adding H2O. The decrease of the reaction rate could be due to protonated NEt3 and Hsalen- ion. The protonated Hsalen-ion gave rise to the formation of neutral H2salen ligand and the rate of reaction decreased.
2. In the second case, [NEt3] > 0.3 M (after break in plot in Fig. 5), the reaction rate was decreased by adding H2O (0.4 M). In this condition, salen2- ion was protonated by adding H2O, H2salen was formed and the reaction rate deceased.
3. In 0.1 <[NEt3] < 0.3 M (break limits in plot in Fig. 5), the effect of H2O on the rate of reaction was dependent on the concentration of H2O. The reaction rate of ligand exchange was increased by increasing the concentration of H2Oupto 0.05 M. The increase of the reaction rate at low [H2O] could be due to the formation of Hsalen- ion by protonated salen2- ion. However, the reaction rate was decreased by adding more H2O(>0.4 M) due to complete protonation of salen2 and Hsalen- ions and formation of neutral H2salen.
4. Conclusion
The distortion of Ni(II) in the Ni(salpn) complex due to the formation the larger chelate ring cause a less stable complex than the Ni(salen) complex. This is the driving force for replacing salen with salpn in Ni(salpn) complex. In the presence of NEt3, the ligand exchange reaction rate will be increased due to the formation of Hsalen- ion. However, at high concentration of NEt3, the reaction rate partially decreases in comparison with low [NEt3], due to the formation of salen2- ion. These observations confirm that, as with similar reactions of salen-type Cu(II) complexes, the deporotonation/protonation H2salen ligand and the anionic form of H2salen are important for the ligand exchange reaction.
Acknowledgements
The authors are grateful to the Yazd University for partial support of this work.
References
1 R.G. Wilkins, Kinetics and Mechanism of Reactions of Transition Metal Complexes, 2nd edn., Wiley, New York, USA, 2002. [ Links ]
2 S. Mallick, B.K. Bera, P. Karmakar, S. Mondal, A. Mandal and A.K. Ghosh, kinetics and mechanism of the ligand substitution reaction of the di-μ,-hydroxobis(bipyridyl)dipalladium(II) ion with some bio-relevant ligands, J. Solution Chem., 2011, 40, 532-544. [ Links ]
3 D. Reddy, K.J. Akerman, M.P. Akerman and D. Jaganyi, A kinetic investigation into the rate of chloride substitution from chloro terpyridine platinum(II) and analogous complexes by a series of azole nucleophiles, Transition Met. Chem., 2011, 36, 593-602. [ Links ]
4 M.A. Brownback, R.K. Murmann and C.L. Barnes, Kinetic, thermo-dynamic and X-ray crystal studies on N-alkyl-a-amineoxime ligand exchange between planar nickel(II) complexes, Polyhedron, 2011, 20, 2505-2515. [ Links ]
5 Z. Asadi, Kinetic studies of the interaction between organotin(IV) chlorides and tetraaza Schiff bases: synthesis and characterization of some novel tin(IV) Schiff base complexes, Int. J. Chem. Kinet., 2011, 43, 247-254. [ Links ]
6 R. M. Naik, Multidentate ligand exchange kinetics: Substitution reaction of polyaminocarboxylatoferrate (III) complex, [FeHPDTA (OH)]2- with 4-(2-pyridylazo)resorcinol, Int. J. Chem. Kinet., 2005, 37, 333-340. [ Links ]
7 G.V Rao, R. Bellam and N.R. Anipindi, Kinetics and mechanism of substitution of bis(2,4,6-tripyridyl 1,3,5-triazine)iron(II) by 2,2',6,2"-terpyridine, Transition Met. Chem., 2012,37,189-196. [ Links ]
8 S. Busse, H. Elias, J. Fischer, M. Poggemann and K.J. Wannowius, Kinetics and mechanism of metal substitution and the Irving-Williams Series:? Anion-catalyzed substitution of nickel for copper in Cu(amben) [=(N,N'-ethylenebis(2-aminobenzaldiminato))copper(II)], Inorg. Chem., 1998, 37, 3999-4005. [ Links ]
9 R. Vafazadeh and S. Bidaki, Kinetics of the ligand exchange reaction between tetradentate Schiff base N,N'-ethylen-bis (salicylaldimine) and Cu(N,N'-propylen-bis(salicylaldimine)), Acta Chim. Slov., 2010, 57, 310-317. [ Links ]
10 R. Vafazadeh and S. Bidaki, Kinetics and mechanism of ligand exchange reaction of copper(II) complexes with tetradentate Schiff base ligands, Acta Chim. Slov., 2014, 61,153-160. [ Links ]
11 Y. Fan, W. You, W. Huang, J.L. Liu and Y.N. Wang, Salen-type nickel(II), palladium(II) and copper(II) complexes having chiral and racemic camphoric diamine components, Polyhedron, 2010, 29, 1149-1155. [ Links ]
12 A.O. Sobola, G. M. Watkins and B. V. Brecht, Synthesis, characterization and antimicrobial activity of copper(II) complexes of some ortho-substituted aniline Schiff bases; crystal structure of bis(2-methoxy-6-imino)methylphenol copper(II) complex, S. Afr. J. Chem., 2014, 67, 45-51. [ Links ]
13 R. Vafazadeh, R. Esteghamat-Panah, A.C. Willis and A.F. Hill, Synthesis and structure studies of mono- and di-nuclear Cu(II) complexes with a ONO donor Schiff base ligand: self-assembly and sulfato-bridged, Polyhedron, 2012, 48,51-57. [ Links ]
14 K. Singh, Y. Kumar and M.S. Barwa, Synthesis, characterization and thermal studies of Co(II), Ni(II), Cu(II) and Zn(II) complexes of some Schiff bases derived from 4-amino-3-mercapto-6-methyl-5-oxo-1,2,4 triazine, S. Afr. J. Chem., 2010, 63, 169-174. [ Links ]
15 R. Vafazadeh, A. Gorji,S. AnsariandA.C. Willis,Synthesis,characterization and crystal structure studies of nickel(II) complexes with NO donor Schiff base ligands, Acta Chim. Slov., 2012, 59, 897-903. [ Links ]
16 R. Vafazadeh, B. Khaledi and A.C. Willis, Synthesis and crystal structure of a new hetro-nuclear Cu2Sr complex of salen type ligand, Acta Chim. Slov., 2012, 59, 954-958. [ Links ]
17 R. Vafazadeh, B. Khaledi, A.C. Willis and M. Namazian. Synthesis, crystal structure and DFT analysis of a new trinuclear complex of copper, Polyhedron, 2011, 30, 1815-1819. [ Links ]
18 M. Kondo, K. Nabari, T. Horiba, Y. Irie, M.K. Kabir, R.P. Sarker, E. Shimizu, Y. Shimizu and Y. Fuwa, Synthesis and crystal structure of [Ni{bis(2,5-dihydroxysalicylidene)ethylenediaminato}]: ahydro-gen bonded assembly of Ni(II)salen complex, Inorg. Chem. Commun., 2003, 6, 154-156. [ Links ]
19 J. Reglinski, S. Morris and D.E. Stevenson, Supporting conformational change at metal centres. Part 1: octahedral systems, Polyhedron, 2002, 21, 2167-2174. [ Links ]
20 J. Reglinski, S. Morris and D.E. Stevenson, Supporting conformational change at metal centres. Part 2: four and five coordinate geometry, Polyhedron, 2002,21, 2175-2182. [ Links ]
21 M. Dieng, I. Thiam, M. Gaye, A.S. Salla and A.H. Barryb, Synthesis, crystal structures and spectroscopic properties of a trinuclear [Cu3(HL)2(NO3)2](H2O)(CH2CH2OH) Complex and a [Mn(HL) (CH3COO)]n polymer with H3L= N,N'-(2-hydroxypropane-1,3-diyl)-bis-(salicylaldimine), Acta Chim. Slov., 2006, 53,417-423. [ Links ]
22 R.H. Holm, Studies on Ni(II) Complexes. I. Spectra of tricyclic Schiff base complexes of Ni(II) and Cu(II), J. Am. Chem. Soc., 1960, 82, 5632-5636. [ Links ]
23 L.C. Nathan, J.E. Koehne, J.M. Gilmore, K.A. Hannibal, W.E. Dewhirst and T.D. Mai, The X-ray structures of a series of copper(II) complexes with tetradentate Schiff base ligands derived from salicylaldehyde and polymethylenediamines of varying chain length, Polyhedron, 2003, 22, 887-894. [ Links ]
24 M.K. Taylor, J. Reglinski and D. Wallace, Coordination geometry of tetradentate Schiff's base nickel complexes: the effects of donors, backbone length and hydrogenation, Polyhedron, 2004,23,3201-3209. [ Links ]
25 R.H. Holm and T. M. McKinney, Magnetic observations of some substituted nickel(II) salicylaldimine complexes, J. Am. Chem. Soc., 1960, 82, 5506-5507. [ Links ]
26 N. Hirayama, I. Takeuchi, T. Honjo, K. Kubono and H. Kokusen, Ion-pair extraction system for the mutual separation of lanthanides using divalentquadridentateSchiffbases, Anal. Chem., 1997,69,4814-4818. [ Links ]
Received 14 October 2014
Revised 17 December 2014
Accepted 18 December 2014
* To whom correspondence should be addressed. E-mail: rvafazadeh@yazd.ac.ir / rvafazadeh@gmail.com





![Synthesis and characterization of new bis-symmetrical adipoyl, terepthaloyl, chiral diimido-di-L-alanine diesters and chiral phthaloyl-L-alanine ester of tripropoxy p-tert-butyl calix[4]arene and study of their hosting ability for alanine and Na+](/img/en/next.gif)








