Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.67 Durban Jan. 2014
RESEARCH ARTICLE
Synthesis, crystal structure and anti-ischaemic activity of (E)-1-{4-[Bis(4-methoxy-phenyl)methyl]piperazin-1-yl}-3-(4-chlorophenyl)-prop-2-en-1-one
Yan ZhongI; Zhaoying XuII; Yao WangII; Yi XuII; Ping LiIII; Bin WuII, *
ISchool of Chemistry and Chemical Engineering, Southeast University, Nanjing, China
IISchool of Pharmacy, Nanjing medical University, Nanjing, China
IIISchool of Basic Medical Sciences, Nanjing Medical University, Nanjing, China
ABSTRACT
The title compound (E)-1-{4-[bis(4-methoxyphenyl)methyl]piperazin-1-yl}-3-(4-chloro- phenyl)prop-2-en-1-one (C28H29ClN2O3, Mr = 476.98) (5) was synthesized and studied by the single crystal X-ray diffraction method. Its structure was confirmed by 1H NMR, 13C NMR, HRMS and X-ray single crystal structure determination. Compound 5 crystallized in the monoclinic system, space group P21/c with a = 10.392(2), b = 7.9180(16), c = 30.474(6) Å, β = 97.78(3) °, V = 2484.4(9) Å3,Z = 4, Dc = 1.275 g cm-3, F(000) = 1008, μ = 0.186 mm-1,MoKa radiation (λ = 0.71073 Å), R = 0.0692 and wR = 0.1469 for 2046 observed reflections with I 2σ(I). The title compound was screened for the anti-ischaemic activity in vivo. The results showed that compound 5 significantly prolonged the survival time of mice subjected to acute cerebral ischaemia at all doses tested and exhibited potent neuroprotective activity.
Keywords: Cinnamide, crystal structure, synthesis, anti-ischaemic activity.
1. Introduction
In China, stroke is the second-highest cause of mortality in all diseases. Particularly, ischaemic strokes account for 60-80 % of these strokes. With the rapid increase of aging population in China, stroke has become a critical cause for the health of the old, and stroke is becoming a major burden facing the government and health providers.1-6 Therefore, there is an urgent need of effective neuroprotective agents to treat stroke-related brain damage.
We reported the syntheses of novel cinnamide derivatives containing bis(4-fluorophenyl)methyl moiety starting from commercially available materials. Previous work suggested that cinnamide scaffold often affords neuroprotective compounds.7
The reaction of cinnamic acid chloride with 1-[bis(4-methoxyphenyl)methyl]piperazine in the presence of triethylamine in dichloromethane at room temperature afforded the corresponding new cinnamide derivative with a total yield of 73.5 % (Scheme 1), which was expected to be a potent neuroprotective agent. Cinnamic acids are one of the key intermediates for the synthesis of this series of compounds, which are usually obtained by the Perkin reaction,8 the Knoevenagel condensation,9 the Claisen condensation,10 and the Heck reaction.11 In this context, we chose a modification of the procedure of a literature method.7 In this article, we present our results on the synthesis, crystal structure and anti-ischaemic activity of new cinnamide derivative of (E)-1-{4-[bis(4-methoxyphenyl)methyl]piperazin-1- yl}-3-(4-chlorophenyl)prop-2- en-1-one (5).
2. Experimental
2.1. Materials and Instruments
All chemicals, reagents, and solvents for synthesis of the compound were commercially available and used without further purification. Melting points were determined on an electrothermal digital apparatus model WRR-401 (Shanghai, China) without correction. 1H NMR and 13C NMR spectra were recorded on a Bruker ACF-300 MHz instrument (Bruker) with CDCI3 as the solvent and tetramethylsilane as an internal standard (chemical shifts are expressed as d values, J in hertz). High-resolution mass spectra (HRMS) were recorded on a MALDI Micro MX instrument (Waters).
2.2. {.E)-1-{4-[bis(4-methoxyphenyl)methyl]piperazin-1-yl}-3-(4-chlorophenyl)prop-2-en-1-one (5).
The synthetic route of the title compound is illustrated in Scheme 1 and is based on a modification of a procedure in the literature7. Intermediate 1-[bis(4-methoxyphenyl)-methyl]pipe-razine 2 was synthesized from bis(4-methoxyphenyl)methanone 1 according to the literature.12
(E)-3-(4-chlorophenyl)acrylic acid 4 was prepared from 4-chlorobenzaldehyde 3 by the Knoevenagel reactions.13 The title compound (E)-1-{4-[bis(4-methoxyphenyl)methyl]piperazin-1-yl}-3-(4-chlorophenyl)prop-2-en-1-one 5 was obtained as follows:thionylchloride (2mL) and piperadine (1 drop) were added to a stirred solution of (E)-3-(4-chlorophenyl)acrylic acid (4,4 mmol) in dichloromethane (15 mL) at room temperature. The reaction mixture was stirred at ambient temperature for 6 hours. After completion, the solvent was removed under reduced pressure. The residue was dissolved in acetone (15 mL) and reacted with the solution of 1-[bis(4-methoxy-phenyl)methyl]piperazine (2, 6 mmol) and triethylamine (5 mL) in dichloromethane (30 mL). After being stirred for 12 hours at room temperature, the solvent was removed under reduced pressure. The residue was purified bysilica gel column chromatography using ethyl acetate/hexane (V/V2:1) as mobile phase to afford target compound 5 as a white solids (1.402 g, 74 %), m.p. 114.2-116.0 °C.
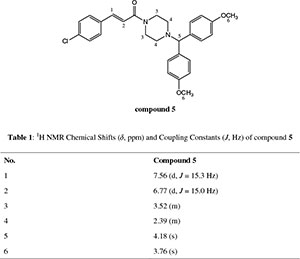
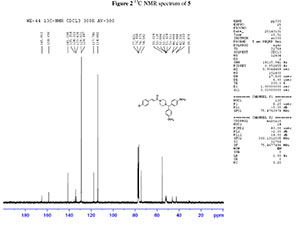
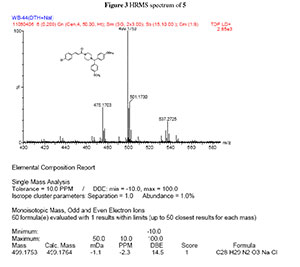
dH (300 MHz, CDCl3): 2.39 (4H, m, N(CH2)2), 3.52 (4H, m, CON(CH2)2), 3.76 (6H, s, 2CH3O), 4.18 (1H, s, CH(Ph)2), 6.77 (1H, d, J 15 Hz, = CHCO), 6.78-7.43 (12H, m, Ar-H), 7.56 (1H, d, J 15.3 Hz, Ph-CH=,); dC (75 MHz, CDCl3): 42.3,46.0, 52.0, 55.2, 74.5, 114.0, 117.7, 128.9, 129.0, 133.8, 134.2, 135.4, 141.2, 158.7, 165.0; HRMS (ESI, m/z): Calcd. for C28H29ClN2NaO3 [M + Na] + 499.1764. Found: 499.1753.
Single crystals suitable for X-ray diffraction were obtained by slowevaporationfromanethanolsolutionofthetitlecompound at room temperature.
2.3. X-ray Crystal Structure Determination
A colourless single crystal of the title compound (0.20 mm x 0.10 mm x 0.10 mm) was selected and mounted on the top of a glass fibre. Diffraction data was collected on an Enraf-Nonius CAD4/PC four-circle diffractometer equipped with a graphite-monochromatic MoKa radiation (l = 0.71073 Å) using an ω/2θ scan mode in the range of 1.35 <θ< 25.38 °(0 < h < 12,0 < k < 9, -36 < l < 36) at293(2) K.Atotal of 4840 reflections were collected, of which 4571 were independent (Rint = 0.0551) and 2046 were observed with I <2σ(I). The structure was solved by direct methods and refined against F2 by full-matrix least-squares using SHELXTL-97.14 The nonhydrogen atoms were located in successive difference Fourier synthesis. The hydrogen atoms were added theoretically and riding on the concerned atoms. The final refinement gave R = 0.0692, wR = 0.1469 (w = 2(F02) + (0.0700P)2], where P = F02+ 2Fc2)/3), S = 1.004, (Δp)max = 0.180, (Dp)mhl = -0.234 e/ Å3 and (Δ/σ)max < 0.001.
3. Results and Discussion
As shown in Scheme 1, the target cinnamide compound 5 was readily synthesized by coupling the corresponding cinnamic acid chloride with 1-[bis(4-methoxyphenyl)- methyl] piperazine at room temperature with a total yield of 74 %. The structure of the product was characterized with 1H NMR, 13C NMR and HRMS. The structure of compound 5 was finally confirmed utilizing single-crystal X-ray structure determination.
The molecular structure of the title compound with atomic numbering is shown in Figs 1 and 2 and depict the molecular packing in the unit cell. The selected bond distances, bond angles and torsion angles are listed in Table 1, and the corresponding lengths and angles of hydrogen bonds are given in Table 2.
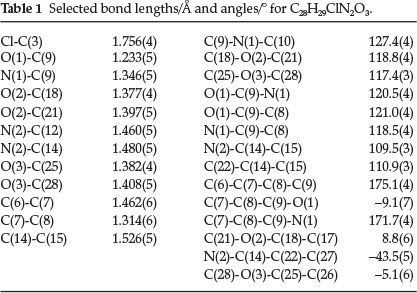

Within the molecule, the bond length of C(7)-C(8) is 1.314(6) Å, shorter than that of typical C=C(1.34 A),15 due to the aromatic conjugation; while the single bond lengths of C(9)-N(1), C(6)-C(7) and C(8)-C(9) are 1.346(5), 1.462(6) and 1.473(6) A, respectively, significantly shorter than the typical C(sp2)-N (1.426 A)16 and C-C (1.53 A) due to the same reason, showing a large conjugated system among the carbonyl group, ethene bond, and the benzene ring.
The molecule exists in an E configuration with respect to the C7=C8 ethene bond [1.314(6)]. The piperazine ring adopts a chair conformation with puchering parameters Q = 0.578(4) Å, theta = 169.7(4) °, phi = 8(3)°.Inthe title compound, the molecule contains three essentially planar phenyl rings: atoms of C(1)-C(6) form the 4-chlorophenyl plane (I), C(15)-C(20) generate the 4-methoxyphenyl plane (II) and C(22)-C(27) yield the other 4-methoxyphenyl plane (III). The planes II and III form dihedral angles of 83.6 (2) ° and 76.4 (2) °, respectively, with the 4-chloro-phenyl ring (I). The two 4-methoxyphenyl rings are inclined at an angle of 67.2 (2)° with respect to one other.
There are two weak intramolecular hydrogen bonds, C(7)-H(7A)-O(1) and C(13)-H(13A)-O(1), forming five-membered rings. At the same time, intermolecular hydrogen bonds are also present, as shown in Table 2. Atom C(1) in the molecule acts as a donor, via the H(1A) atom, to the O(1) of an adjacent molecule (symmetry code: 1-x, -y, 1-z). These interaction forces forces determine the three-dimensional structure.
4. Anti-ischaemic Activity
The anti-ischaemic activity of the title compound 5 was tested using bilateral common carotid artery occlusion.17 Kunming mice of both sexes were randomly divided into groups (10 mice per group). The title compound was dissolved in aqueous 0.5 % sodium carboxy methyl cellulose (CMCNa) solution before use and administered intraperitoneally (i.p.). Nimodipine was i.p. treated with 80 mg kg-1 as a positive control. The negative control group received normal saline (NS) in the same volume as other groups. All groups were given drugs twice a day for 3 days. Sixty minutes after the last administration, all mice were anaesthetized with ether. All groups underwent the operation for common carotid artery and vagus nerves ligation.18 Then the survival time of mice were recorded and given in Table 3.
As shown in Table 3, the title compound 5 was shown to significantly prolong the survival time of mice subjected to acute cerebral ischemia and decrease the mortality rate at all doses tested, which indicate that the title compound 5 exhibits the potent neuroprotective activity.
5. Conclusion
The title compound 5 was synthesized via a facile approach and the structure assignment was supported by 1H NMR data, 13C NMR, HRMS and crystallographic studies. In the preliminary screening of the anti-ischaemic activity study, the title compound 5 was shown to significantly prolong the survival time of mice subjected to acute cerebral ischaemia and decrease the mortality rate at all doses tested, which suggest that the title compound 5 exhibits the potent neuroprotective activity.
Supplementary Material
X-ray crystallography data of (E)-1-{4-[bis(4-methoxy-phenyl)methyl]piperazin-1-yl}-3-(4- chlorophenyl)prop-2-en-1-one has been deposited with the Cambridge Crystallographic Data Centre (CCDC) and can be obtained free of charge on request at http://www.ccdc.cam.ac.uk/conts/retrieving.html or from the Cambridge Crystallographic Data Centre (CCDC), 12 Union Road, Cambridge CB2 1EZ, UK; fax:. +44(0)1223-336033; e-mail: deposit@ccdc.cam.ac.uk, quoting the CCDC number, 921041. The IR, MS and NMR spectra of this compound are also provided with supplementary material.
Acknowledgements
This research was supported by the Natural Science Foundation of China Grant No. 81371451 and the Natural Science Foundation of Jiangsu Province Grant No. BK20131390.
References
1 H. Liu, X. F. Tian; Y.B. Zhang, C.S. Wang and H.E. Jiang, J. Ethno-pharmacol., 2013,146, 278-286. [ Links ]
2 T. Li and T. Peng, Antiviral. Res., 2013, 97, 1-9. [ Links ]
3 X.M. Li, J. Allergy Clin. Immunol, 2007,120, 25-31. [ Links ]
4 F. P. Chen, Y. Y. Kung, Y. C. Chen, M. S. Jong, T. J. Chen, F. J. Chen and S. J. Hwang, J. Ethnopharmacol, 2008,117, 84-91. [ Links ]
5 Y. Motoo, I. Arai, I. Hyodo and K. Tsutani, Complement. Ther. Med, 2009, 17, 147-154. [ Links ]
6 S.P. Wang, X. Wu, M. Tan, J. Gong, W. Tan, B.L. Bian, M.W. Chen and Y.T. Wang, J. Ethnopharmacol, 2012,140, 33-5. [ Links ]
7 B. Wu, L. Zhou and H.H. Cai, Chin. Chem. Lett., 2008,19, 1163-1166. [ Links ]
8 L.F. Fieser and M. Fieser, Advanced Organic Chemistry, Rheinhold Publishing Corporation, New York, 1961, p. 464. [ Links ]
9 H.O. House, Modern Synthetic Reactions, Benjamin, Inc., New York, 1965, p. 225. [ Links ]
10 L.F. Fieser and M. Fieser, Advanced Organic Chemistry, Rheinhold Publishing Corporation, New York, 1961, p. 470. [ Links ]
11 L.E. Overman and A. Dounay, Chem. Rev, 2003,103, 2945-2964. [ Links ]
12 L.S. Wang, H.Y. Jiang, Y.H. Zhou, B.L. Liu and Z.Z. Ji, Chin. J. Med. Chem., 2002,12, 125-129. [ Links ]
13 K. Tanaka, K. Matsuo, A. Nakanishi, T. Hatano, H. Izeki, Y. Ishida and W. Mori, Chem. Pharm. Bull., 1983, 31, 2810-2819. [ Links ]
14 G.M. Sheldrick, SHELXL97 and SHELXS97, 1997, Institut fur Anorganische Chemie University of Gottingen, Gottingen, Germany. [ Links ]
15 M. Li, L.R. Wen, W J. Fu, F.Z. Hu and H.Z. Yang, Chin. J. Struct. Chem., 2004, 23, 11-14. [ Links ]
16 L.S. Bartell, E.A. Roth and C.D. Hollowed, J. Chem. Phys., 1965, 42, 2683-2686. [ Links ]
17 Y.Y. Zhang, X.Y. Wang, X.R. Wang, Z.H. Xu, Z. Liu, Q. Ni, X.P. Chu, M.F. Qiu, A.H. Zhao and W Jia, J. Ethnopharmacol.,2006,108,355-360. [ Links ]
18 S.U. Yanpallewar, D. Hota, S. Rai, M. Kumar and S.B. Acharya, Pharmacol. Res., 2004,49, 143-150. [ Links ]
Received 6 September 2014
Revised 26 November 2014
Accepted 28 November 2014
* To whom correspondence should be addressed. E-mail: wubin@njmu.edu.cn
Supplementary Information, S. Afr. J. Chem.
Synthesis, Crystal Structure and Anti-ischemic Activity of (£)-1-(4-(Bis(4-methoxy-phenyl)methyl)piperazin-1-yl)-3-(4-chlorophenyl)-prop-2-en-1-one
Yan ZhongI, Zhaoying XuII, Yao WangII, Yi XuII, Ping LiIII and Bin WuII, *
ISchool of Chemistry and Chemical Engineering, Southeast University, Nanjing, China.
IISchool of Pharmacy, Nanjing medical University, Nanjing, China.
IIISchool of Basic Medical Sciences, Nanjing medical University, Nanjing, China.
Table of Contents
1. Table 1: 1H NMR Chemical Shifts (δ, ppm) and Coupling Constants (J, Hz) of 5
2. 1H, 13C NMR spectrum of compound 5
3. HRMS spectrum of compound 5
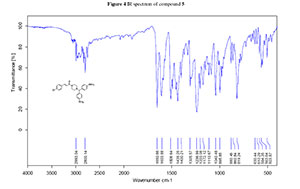
4. IR spectrum of compound 5
Material and Methods
1H NMR and 13C NMR were recorded on a Bruker ACF-300 MHz instrument (Bruker) with CDCl3 as the solvent and tetramethylsilane as an internal standard (chemical shifts are expressed as δ values, J in hertz). High resolution mass spectra (HRMS) was recorded on a MALDI Micro MX instrument (Waters). IR spectra was recorded on a Bruker Tensor 27 FT-IR instrument (Bruker).




![Polyaniline/SiO2 catalyzed one-pot synthesis of tetrahydrobenzo[b]pyran derivatives](/img/en/prev.gif)