Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Chemistry
versão On-line ISSN 1996-840X
versão impressa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.67 Durban Jan. 2014
RESEARCH ARTICLE
An efficient green synthesis of 3-Amino-1H-chromenes catalyzed by ZnO Nanoparticles thin-film
Shahrzad AbdolmohammadiI, *; Maryam AfsharpourII; Shima Keshavarz-FatidehI
IDepartment of Chemistry, Faculty of Science, East Tehran Branch, Islamic Azad University, P.O. Box 33955-163, Tehran, Iran
IIChemistry & Chemical Engineering Research Center of Iran, P.O. Box 14335-186, Tehran, Iran
ABSTRACT
A very simple and environmentally benign approach for the synthesis of 3-amino-1ff-chromenes is described using ZnO nanoparticles thin-film as an efficient heterogeneous catalyst in green media, namely water. The mild reaction conditions, reusability of the catalyst, easy work-up and high yields of products make the present protocol sustainable and advantageous compared to conventional methods.
Keywords: 3-Amino-1H-chromene, aqueous medium, hydrothermal solution method, reusability of the catalyst, ZnO nanoparticles thin-film.
1. Introduction
Chromenes are a common structural motif in variety of natural and non-natural products.1,2 Their derivatives have been known to exhibit a wide range of biological and pharmacological activities such as antioxidant,3,4 anticancer,5-8 antimicrobial,9-12 hypotensive13 and local anesthetic.14 In addition, they can be used as cognitive enhancers,15,16 for the treatment of neurodegenera-tive diseases, including Alzheimer's disease17 and schizophrenia disorder.18
Traditional methods have been reported for the synthesis of 3-amino-1H-chromenes by condensation of b-naphthol, aldehyde, and malononitrile in the presence of an organic base, such as K2CO3,19 DABCO,20 basic ionic liquids,21,22 CuO-CeO2,23 and Rochelle salt.24 However, these procedures provide their effectiveness; most of them have some drawbacks including low yields of the products, harsh reaction conditions, use of organic solvents or environmental disposal problems. Thus, it is evident that there is still a need to continue research to find improved catalysts for the synthesis of these biologically active derivatives.
Nanostructure metal oxides are a class of materials that have been widely used in different catalytic reactions.25-30 Among the numerous metal oxides, ZnO has attracted most interest due to its unique properties.31-37 The use of conventional powder catalyst reveals disadvantages with stirring during the reaction and in separation of powder after the reaction. The preparation of thin-film catalysts makes it possible to overcome these disadvantages and potentially explore industrial applications.38 ZnO nanoparticles thin-films are prepared by different techniques such as metal organic chemical vapour deposition, sol-gel, hydrothermal, thermal evaporation, oxidation and anodization.39-45 Although the use of ZnO nanoparticles (NPs) thin-film as a catalyst is proposed herein for the first time, there are other applications of ZnO NPs thin-film such as optical and electrical devices and in solar cells.46-48
In spite of the fact that ZnO NPs have emerged previously as a useful catalyst in water or ethanol for the synthesis of 2-amino-4H-chromenes,49,50 in this paper we introduce ZnO nanoparticles on a thin-film glass substrate by the mild hydrothermal method and then used it for the first time as an efficient heterogeneous catalyst for the preparation of racemic 3-amino-1aryl-1H-benzo[f]chromene-2-carbonitriles (4) via a three-component condensation of aromatic aldehydes, malononitrile and b-naphthol in aqueous media at 60 °C (Scheme 1).
2. Results and Discussion
Intially, the ZnO NPs thin-film was prepared by a facile hydrothermal solution method51 using zinc acetate dihydrate. In this process, the ZnO film synthesis includes three principal steps: (i) solution preparation; (ii) coating; and (iii) heat treatment. For the first step, the particle formation is discussed including nucleation and growth, particle size, morphology and colloidal stability. These three steps involve several parameters such as: (i) nature and concentration of precursor; solvent and additive and solution aging time of the chemical system; (ii) coating method, thickness and substrate for the coating step; and (iii) pre- and post-heat treatment for the last step.
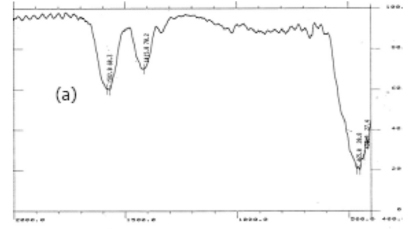
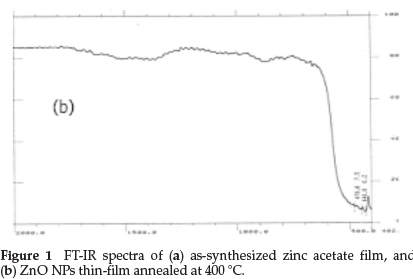
FT-IR analysis of as-synthesized zinc acetate film is presented in Fig. 1a; the absorption peak at 1582 cm-1 is due to C=O arising from the bridging type metal-acetate bonding (M-OCOO-M), those at 1415 cm-1 are the C-O stretching frequencies and 1340 cm-1 is a weakly C-O mode from an acetic acid molecule (CH3-COOH). Figure 1b shows the FT-IR spectrum of ZnO NPs thin-film annealed at 400 °C which shows the absence of absorption bands corresponding to organics and hydroxyl groups, indicating complete removal of organics.
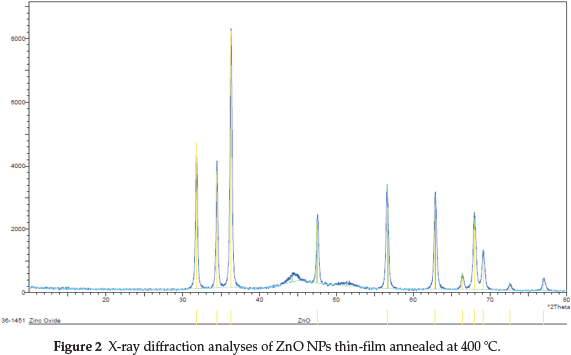
The XRD patterns of annealed thin-films ZnO NPs are crystalline with hexagonal wurtzite structure, and their XRD peaks at 2Θ angle 31.9, 34.6 and 36.2 ° are corresponding to diffraction from planes (002), (101) and (110), respectively (JCPDS:36-1451) (Fig. 2).
The optical absorption spectra of the thin-film in the UV-visible wavelength range are presented in Fig. 3. It can be seen that the film has high transparency in the visible range. The absorption at higher wavelengths in the visible region is low at wavelength around 484 nm. Further, a sharp increase of absorption occurs at around 361 nm, indicating the ZnO absorption.

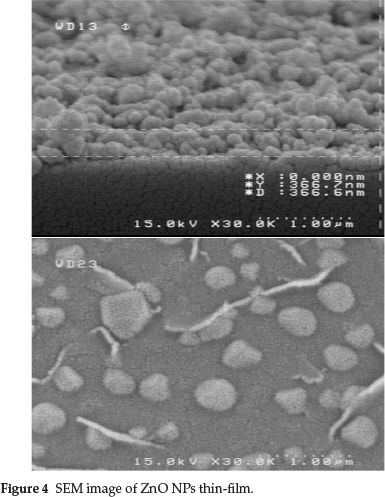
Figure 4 shows the surface morphology of ZnO NPs within the thin-film. In general, the film is homogeneous and continuous. Separate coating layers are not visible for the sintered film. The random distribution of grains suggests a random nucleation mechanism. Film thickness from the SEM image was found to be 366 nm.
For optimization of the reaction conditions, ZnO NPs thin-film was investigated as a catalyst in the model reaction of 3-chloro-benzaldehyde, malononitrile and b-naphthol in various solvents which were chosen for comparison. The best result was obtained in aqueous media (Table 1, entries 2 and 3-5). It is noticeable that in aqueous media and in the absence of catalyst the reaction was very slow and obtained in lower yields (Table 1, entry 1).
An increase of the temperature from 50 °C to 90 °C, showed that further increase of the temperature above 60 °C shortened the reaction time while having no measurable effects on the yield (Table 1, entries 2 and 6-9).
The scalability of the reaction protocol is presented in Table 2. The results showed that the present method is effective for large-scale synthesis (Table 2, entries 2 and 3) as well as routine scale (Table 2, entry 1). It is interesting that the higher yields of products were obtained for large-scale synthesis. All data of Table 2 are provided for optimized conditions.
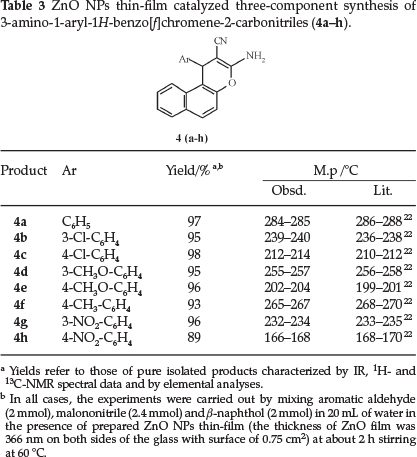
To expand the efficiency and applicability of our protocol, we used various aromatic aldehydes to produce the corresponding products in high to excellent yields. The results are summarized in Table 3. The structures of products (4a-h) were confirmed by IR, 1H- and 13C-NMR spectral data and elemental analysis.
A comparison of the efficiency of presented new protocol with several pervious methods in the reaction of aromatic aldehydes, malononitrile and b-naphthol for the synthesis of corresponding products is given in Table 4. The results show that this method has some merits in comparison with the known methods on terms of yields, thermal conditions, and reaction times.
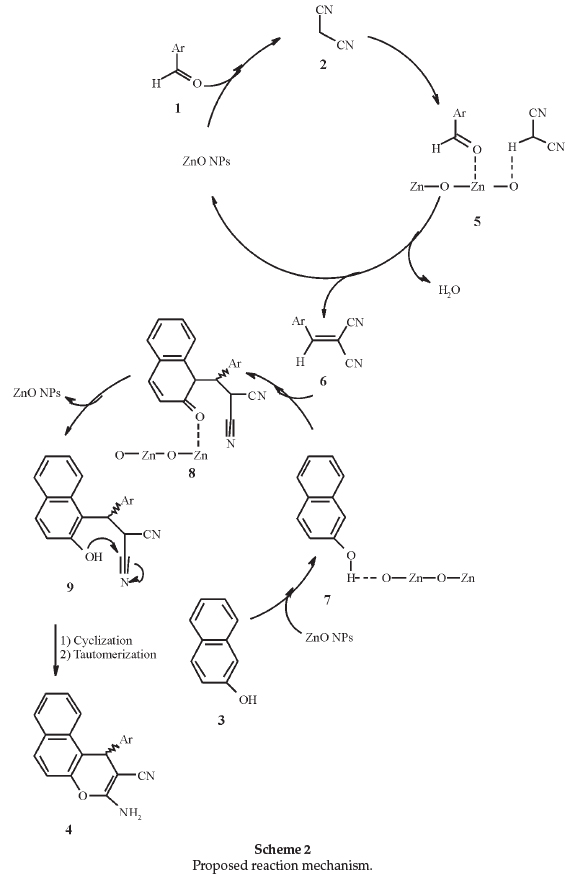
A plausible mechanism for the formation of the product is suggested in Scheme 2: the ZnO facilate Knoevenagel condensation of aromatic aldehyde 1 and malononitrile 2 for the formation of alkene 6, through Lewis acid sites (Zn2+) which are coordinated to the oxygen of the aldehyde and active it for nucleophilic attack of malononitrile. ZnO can also active methylene group of b-naphthol 3 so that deprotonation occurs in the presence of Lewis basic sites (O2-) to generate intermediate 7 which then ads to alkene 6 by Michael type addition to produce the Michael adduct 8. Intramolecular cyclization of intermediate 9, which is obtained from the enolization of 8, gives product 4 after tautomerization.
The recyclability of the catalyst is an important factor from economical and environmental points of view. For this reason, the reusability of the ZnO nanoparticle thin-film was examined in model reaction under the optimized reaction conditions by separating film from the reaction mixture washing with ethanol, and drying in vacuo. It was found that the ZnO film could be recycled for at least 15 cycles without significant change in activity.
3. Experimental
3.1. Materials and Methods
All chemicals used in this work purchased from Merck and Fluka in high purity. Melting points were determined with Electrothermal 9100 melting point apparatus and were uncorrected. FT-IR spectra were obtained using a Bruker, Equinox 55, Golden Gate Micro-ATR spectrometer. 1H-NMR and 13C-NMR spectra were run on a Bruker DRX-500 AVANCE at 300 and 75 MHz, respectively using TMS as internal standard and, Acetone-d6 as solvent. Elemental analyses were carried out using a Foss Heraeus CHN-O-Rapid elemental analyser (Foss Heraeus, GmbH, Hanau, Germany). Microscopic morphology of catalyst was visualized by scanning electron microcopy SEM: Philips XL30. Powder X-ray diffraction (XRD) patterns were collected from a diffractometer with X'PertPro monochromatized Cu Ka radiation (λ =1.54 Ä) operated on 35 mA and 40 kV current, in the range 2θ = 580 °, from Philips Company. The optical transmittance and reflectance of the films were recorded on an AvaSpec-2048 UV-VIS-NIR spectrometer with drift accessory in the wavelength range from 200 to 500 nm. The TLC plates (silica gel 60 F-254) used in this work were purchased from Merck Inc.
3.2. General Procedure
3.2.1. Preparation of ZnO NPs Thin-film Catalyst
Corresponding preparation of the ZnO film according to conditions of entry 1 in Tables 2 and 3: 1.6 g of zinc acetate dihydrate (99.98 %) was dissolved into 50 mL of methanol, (>99.9 %) and stirred on a magnetic stirrer for 10 min. Then a small amount (0.2 mL) of glacial acetic acid (>99.9 %) was added to the mixture and stirred at 40 °C for 10 min, the obtained transparent solution was transferred to an autoclave. The glass substrate (15 x 5 mm) was pre-cleaned with water and detergent, and then cleaned in methanol and acetone for 10 min each by using an ultrasonic cleaner and then cleaned with deionized water and dried. The hydrothermal technique51 was used for the deposition of the sample on the surface of glass substrate. The cleaned glass substrate was transferred to autoclave and aged at 60 °C for 48 h, and then the coated film was dried at 100 °C in air for 30 min. The process was repeated for a second time to prepare film with desired thickness. Finally, the ZnO NPs thin-film was heat-treated at 400 °C in a furnace. The film thickness was found to be 366 nm from the SEM image of ZnO NPs thin-film.
Corresponding preparation of the ZnO film according to conditions of entry 2 in Table 2:3.2 g of zinc acetate dihydrate (99.98 %) was dissolved into 75 mL of methanol, (>99.9 %) and stirred on a magnetic stirrer for 15 min. Then, a small amount (0.3 mL) of glacial acetic acid (>99.9 %) was added to the mixture and stirred at 40 °C for 15 min, the obtained transparent solution was transferred to an autoclave. The glass substrate (15 x 7 mm) was pre-cleaned with water and detergent, and then cleaned in methanol and acetone for 15 min each by using an ultrasonic cleaner and then cleaned with deionized water and dried. The hydrothermal technique51 was used for the deposition of the sample on the surface of glass substrate. The cleaned glass substrate was transferred to autoclave and aged at 60 °C for 48 h, and then the coated film was dried at 100 °C in air for 30 min. The process was repeated for a second time to prepare film with desired thickness. Finally, the ZnO NPs thin-film was heat treated at 400 °C in a furnace. The film thickness was found to be 366 nm from the SEM image of ZnO NPs thin-film.
Corresponding preparation of the ZnO film according to conditions of entry 3 in Table 2: 6.4 g of zinc acetate dihydrate (99.98 %) was dissolved into 100 mL of methanol, (>99.9 %) and stirred on a magnetic stirrer for 20 min. Then, a small amount (0.6 mL) of glacial acetic acid (>99.9 %) was added to the mixture and stirred at 40 °C for 20 min, the obtained transparent solution was transferred to an autoclave. The glass substrate (15 x 15 mm) was pre-cleaned with water and detergent, and then cleaned in methanol and acetone for 15 min each by using an ultrasonic cleaner and then cleaned with deionized water and dried. The hydrothermal technique51 was used for the deposition of the sample on the surface of glass substrate. The cleaned glass substrate was transferred to autoclave and aged at 60 °C for 48 h, and then the coated film was dried at 100 °C in air for 30 min. The process was repeated for a second time to prepare film with desired thickness. Finally, the ZnO NPs thin-film was heat-treated at 400 °C in a furnace. The film thickness was found to be 366 nm from the SEM image of ZnO NPs thin-film.
3.2.2. Synthesis of 3-Amino-1-aryl-1H-benzo[f]chromene-2-carbonitriles
Corresponding synthesis of compounds (4a-h) according to conditions of entry 1 in Tables 2 and 3: A mixture of aromatic aldehyde (2 mmol), malononitrile (2.4 mmol), b-naphthol (2 mmol) and water (20 mL) were taken in 50 mL round-bottom flask. The ZnO NPs thin-film (15 x 5 mm) was then dipped into the sample and the mixture was stirred at 60 °C for 2 h. The progress of the reaction was monitored with TLC (n-hexane: ethyl acetate, 5: 2). Upon completion of the reaction, the ZnO NPs thin-film was removed from the reaction mixture, for reuse by washing with EtOH, and drying in vacuo at 80 °C for several hours. The reaction mixture was then filtered. The residue was recrystallized from EtOH 95 % to give the pure product.
Corresponding synthesis of compound (4b) according to conditions of entry 2 in Table 2: A mixture of 3-chlorobenzaldehyde (5 mmol), malononitrile (6 mmol), b-naphthol (5 mmol) and water (30 mL) were taken in 100 mL round-bottom flask. The ZnO NPs thin-film (15 x 7 mm) was then dipped into the sample and the mixture was stirred at 60 °C for 2 h. The progress of the reaction was monitored with TLC (n-hexane: ethyl acetate, 5: 2, Rf = 0.48). Upon completion of the reaction, the ZnO NPs thin-film was removed from the reaction mixture, for reuse by washing with EtOH, and drying in vacuo at 80 °C for several hours. The reaction mixture was then filtered. The residue was recrystallized from EtOH 95 % to give the pure product.
Corresponding synthesis of compound (4b) according to conditions of entry 3 in Table 2: A mixture of 3-chlorobenzaldehyde (10 mmol), malononitrile (12 mmol), b-naphthol (10 mmol) and water (60 mL) were taken in 150 mL round-bottom flask. The ZnO NPs thin-film (15 x 15 mm) was then dipped into the sample and the mixture was stirred at 60 °C for 2 h. The progress of the reaction was monitored with TLC (n-hexane: ethyl acetate, 5: 2, Rf = 0.48). Upon completion of the reaction, the ZnO NPs thin-film was removed from the reaction mixture, for reuse by washing with EtOH, and drying in vacuo at 80 °C for several hours. The reaction mixture was then filtered. The residue was recrystallized from EtOH 95 % to give the pure product.
3.2.3. Spectroscopic Data of the Products
3-Amino-1-phenyl-1 H-benzo[f]chromene-2-carbonitrile (4a) White solid, yield: 0.289 g (97 %), m.p. 284-285 °C (lit: 286-288 °C22) (n-hexane: ethyl acetate, 5:2, Rf = 0.43). IR (KBr) (nmax/cm-1): 3465, 3346,2181,1665,1600,1500,1210,1076. Ή-NMR: <5 = 5.20 (s, 1H, H-1), 6.60 (bs, 2 H, NH2), 7.20 (m, 3 H, H-Ar), 7.38 (m, 2 H, H-Ar), 7.75 (d, J = 7.8 Hz, 2 H, H-Ar), 7.82 (d, J = 8.1 Hz, 3 H, H-Ar) ppm. 13C-NMR: <5 = 39.53, 58.37,116.12,117.21,120.95,124.06,125.34, 127.03, 127.46, 128.90, 129.12, 129.93, 131.26, 146.15, 147.27, 130.62,160.14 ppm. Anal. calcd. for C20H14N2O (298.34): C 80.51, H 4.73, N 9.39; found: C 80.43, H 4.61, N 9.52.
3-Amino-1-(3-chlorophenyl)-1H-benzo[f]chromene-2-carbonitrile (4b): White solid, yield: 0.316 g (95 %), m.p. 239-240 °C (lit: 236-238 °C22) (n-hexane: ethyl acetate, 5: 2, Rf = 0.48). IR (KBr) (vmax/crn-1): 3431, 3328, 2181, 1646, 1588, 1515, 1220, 1080. Ή-NMR: d = 5.39 (s, 1 H, H-1), 6.34 (bs, 2 H, NH), 7.29 (m, 5 H, H-Ar), 7.46 (m, 3 H, H-Ar), 7. 92 (m, 2 H, H-Ar), ppm. 13C-NMR: d = 41.27, 55.40, 59.10, 115.08, 118.33, 119.49, 120.66, 120.86, 123.38, 123.93, 126.47, 126.59, 127.67, 133.27, 137.20, 143.06, 145.77,147.60,159.89 ppm. Anal. calcd. for CioHuClNiO (332.79): C 72.18, H 3.94, N 8.42; found: C 72.13, H 4.15, N 8.52.
3-Amino-1-(4-chlorophenyl)-1H-benzo[f]chromene-2-carbonitrile (4c): White solid, yield: 0.326 g (98 %), m.p. 212-214 °C (lit: 210-212 °C22) (n-hexane: ethyl acetate, 5: 2, Rf = 0.50). IR (KBr) (vmax/crn-1): 3420, 3330, 2191, 1643, 1591, 1460, 1259, 1112. Ή-NMR: d = 4.94 (s, 1 H, H-1), 7.10 (bs, 2 H, NH2), 7.19 (s, 2 H, H-Ar), 7.28 (d, J = 8.5 Hz, 2 H, H-Ar), 7.39 (d, J = 8.5 Hz, 2 H, H-Ar), 7.59 (m, 2 H, H-Ar), 7.85 (d, J = 8.5 Hz, 1 H, H-Ar), 8.25 (d, J = 8.5 Hz, 1 H, H-Ar) ppm. 13C-NMR: d = 40.73, 57.88, 115.60, 117.24, 120.78, 122.98, 125.44, 127.61, 128.95, 129.21, 130.14, 130.49, 131.26, 131.62, 145.11, 147.25,160.16. Anal. calcd. for C20H13ClN2O (332.79): C 72.18, H 3.94, N 8.42; found: C 72.03, H 3.75, N 8.33.
3-Amino-1-(3-methoxyphenyl)-1H-benzo[f]chromene-2-carbonitrile (4d): White solid, yield: 0.312 g (95 %), m.p. 255-257 °C (lit: 256-258 °C22) (n-hexane: ethyl acetate, 5: 2, Rf = 0.41). IR (KBr) (vmax/crn-1): 3460, 3350, 2983, 2180, 1665, 1607, 1500, 1234, 1103, 1080. Ή-NMR: d = 3.73 (s, 3 H, OCH3), 5.16 (s, 1 H, H-1), 6.05 (m, 2 H, H-Ar), 6.69 (bs, 2 H, NH2), 6.74 (d, J = 7.7 Hz, 1 H, H-Ar), 7.16 (m, 1H, H-Ar), 7.27 (d, J = 8.8 Hz, 1 H, H-Ar), 7.40 (m, 1 H, H-Ar), 7.72 (m, 2 H, H-Ar), 7.81 (d, J = 8.8 Hz, 2 H, H-Ar) ppm. 13C-NMR: d = 41.05, 54.96, 58.23, 111.62, 114.87, 116.03, 117.18, 119.61, 121.92, 124.08, 125.38, 127.53, 128.88, 129.93, 131.29, 131.66, 132.23, 147.16, 147.68, 159.78, 160.18. Anal. calcd. for C21H16N2O2 (328.37): C 76.81, H 4.91, N 8.53; found: C 76.93, H 4.76, N 8.59.
3-Amino-1-(4-methoxyphenyl)-1 H-benzo[f]chromene-2-carbonitrile (4e): White solid, yield: 0.315 g (96 %), m.p. 202-204 °C (lit: 199-201 °C22) (n-hexane: ethyl acetate, 5: 2, Rf = 0.42). IR (KBr) (nmax/cm-1): 3450, 3337, 2980, 2180, 1665, 1600, 1500, 1247, 1190, 1075. Ή-NMR: d = 3.70 (s, 3 H, OCH3), 5.16 (s, 1 H, H-1), 5.63 (m, 2 H, H-Ar), 6.76 (bs, 2 H, NH2), 7.09 (d, J = 8.5 Hz, 2 H, H-Ar), 7.25 (d, J = 8.5 Hz, 2 H, H-Ar), 7.38 (m, 2 H, H-Ar), 7.70 (t, J = 7.3 Hz, 1 H, H-Ar), 7.78 (d, J = 8.7 Hz, 1 H, H-Ar) ppm.13C-NMR: d = 42.49, 60.16, 63.41, 119.23, 121.15, 121.97, 125.76, 128.88, 130.05, 132.19, 133.23, 133.62, 134.54, 135.37, 136.01, 143.10, 151.88, 163.02,164.74. Anal. calcd. for C21H16N2O2 (328.37): 76.81, H 4.91, N 8.53; found: C 76.99, H 5.04, N 8.65.
3-Amino-1-(4-methylphenyl)-1H-benzo[f]chromene-2-carbonitrile (4f): White solid, yield: 0.291 g (93 %), m.p. 265-267 °C (lit: 268-270 °C22) (n-hexane: ethyl acetate, 5: 2, Rf = 0.47). IR (KBr) (Vmax/cm-1): 3463, 3355, 2960, 2170, 1665, 1605, 1500, 1270, 1190, 1080. Ή-NMR: d = 2.20 (s, 3 H, CH3), 5.16 (s, 1H, H-1), 6.32 (bs, 2 H, NH2), 7.05 (m, 4 H, H-Ar), 7.26 (d, J = 8.7Hz, 1 H, H-Ar), 7.34 (d, J = 5.2 Hz, 2 H, H-Ar), 7.70 (t, J = 5.7 Hz, 1 H, H-Ar), 7.82 (d, J = 8.7 Hz, 2 H, H-Ar) ppm. 13C-NMR: d = 20.99, 39.20, 58.51, 116.23, 117.21, 120.98, 124.09, 125.31, 127.39, 128.87, 129.67, 129.83, 130.63, 131.25, 136.11, 143.25, 147.20, 160.03. Anal. calcd. for C21H16N2O (312.37): C 80.75, H 5.16, N 8.97; found: C 80.63, H 5.05, N 9.12.
3-Amino-1-(3-nitrophenyl)-1H-benzo[f]chromene-2-carbonitrile (4g): White solid, yield: 0.330 g (96 %), m.p. 232-234 °C (lit: 233-235 °C22) (n-hexane: ethyl acetate, 5: 2, Rf = 0.41). IR (KBr) (Vma/cm-1): 3466, 3340, 2185, 1660, 1610, 1580, 1500, 1334, 1270, 1080, 760. Ή-NMR: d = 5.36 (s, 1H, H-1), 6.00 (bs, 2H, NH2), 7.31 (d, J = 9.0 Hz, 1H, ArH), 7.39-7.51 (m, 3H, ArH), 7.61 (d, J = 8.28 Hz, 2H, ArH), 7.82-7.87 (m, 2H, ArH), 7.97 (s, 1H, ArH), 8.02(d, J = 8.16 Hz, 1H, ArH) ppm. 13C-NMR: d = 42.54, 62.20, 119.77,122.05, 125.36, 126.51, 127.01, 128.66, 130.33, 132.58, 133.78,135.18,135.62,136.05,138.89,152.17,153.12,165.17. Anal. calcd. for C20H13N3O3 (343.34): C 69.97, H 3.82, N 12.24; found: C 69.88, H 3.75, N 12.12.
3-Amino-1-(4-nitrophenyl)-1H-benzo[f]chromene-2-carbonitrile (4h): Pale lemon solid, yield: 0.306 g (89 %), m.p. 166-168 °C (lit: 168-170 °C22) (n-hexane: ethyl acetate, 5: 2, Rf = 0.53). IR (KBr) (Vmax/cm-1): 3460, 3320, 2185, 1665, 1600, 1560, 1500, 1325, 1270, 1190,1080. Ή-NMR: d = 5.35 (s, 1H, H-1), 6.05 (bs, 2H, NH2), 7.30 (d, J = 9.0 Hz, 1H, ArH), 7.35-7.42 (m,4H, ArH), 7.54-7.58 (m,1H, ArH), 7.82-7.86 (m, 2H, ArH), 8.10 (dd, J = 6.96 & 6.92 Hz, 2H, ArH) ppm. 13C-NMR: d = 42.58, 117.45, 120.62, 127.01, 128.17, 129.19, 131.39, 131.90, 132.61, 134.18, 135.29, 151.13, 155.64, 163.77. Anal. calcd. for C20H13N3O3 (343.34): C 69.97, H 3.82, N 12.24; found: C 69.83, H 4.04, N 12.16.
Conclusion
In conclusion, ZnO nanoparticles thin-film was prepared with a mild hydrothermal method and well characterized by various techniques such as XRD, FT-IR and SEM; it was found to catalyze the three-component reaction of aromatic aldehydes, malononitrile and b-naphthol efficiently, affording 3-amino-1-aryl-1H-benzo[f]chromene-2-carbonitrile derivatives (4) in high to excellent yields. Compared to known methods, satisfactory results were obtained with high yields, mild reaction conditions, short reaction time, reusability of the catalyst, easy work-up, inexpensive reagents and environmentally friendly procedures.
Acknowledgement
Financial support from the East Tehran Branch, Islamic Azad University, is gratefully acknowledged.
References
1 H.V. Rensburg, P.S.V Heerden, B.B. Bezuidenhoudt and D. Ferreira, Tetrahedron Lett., 1997, 38, 3089-3092. [ Links ]
2 P.O. 'Kennedy and R.D. Thornes, eds., Coumarins: Biology, Applications and Mode of Action, Wiley & Sons, Chichester, U.K, 1997. [ Links ]
3 L. Alvey, S. Prado, V Huteau, B. Saint-Joanis, S. Michel, M. Koch, T. Cole, F. Tillequin and L. Janin, Bioorg. Med. Chem., 2008, 16, 8264-8272. [ Links ]
4 T. Symeonidis, M. Chamilos, J. Hadjipavlou-Litina, M. Kallitsakis and E. Litinas, Bioorg. Med. Chem. Lett., 2009,19, 1139-1142. [ Links ]
5 J.L. Wang, D. Liu, Z.J. Zhang, S. Shan, X. Han, S.M. Srinivasula, C.M. Croce, E.S. Alnemri and Z. Huang, Proc. Natl. Acad. Sci. USA., 2000, 97, 7124-7129. [ Links ]
6 F. Cheng, A. Ishikawa, Y. Ono, T. Arrheniusa and A. Nadzana, Bioorg. Med. Chem. Lett., 2003,13, 3647-3650. [ Links ]
7 D. Grée, S. Vorin, L. Manthati, F. Caijo, G. Viault, F. Manero, P. Juin and R. Grée, Tetrahedron Lett., 2008, 49, 3276-3278. [ Links ]
8 W. Kemnitzer, S. Jiang, H. Zhang, S. Kasibhatla, C. Crogan-Grundy, C. Blais, G. Attardo, R. Denis, S. Lamoth, H. Gourdeau, B. Tseng, J. Drewe and X. Cai, Bioorg. Med. Chem. Lett., 2008,18, 5571-5575. [ Links ]
9 M.M. Khafagy, A.H.F.A. El-Wahas, F.A. Eid and A.M. El-Agrody, Farmaco, 2002, 57, 715-722. [ Links ]
10 K., Mazaahir, S. Shilpi, R. Khalilur and S.T. Sharanjit, Bioorg. Med. Chem. Lett., 2005, 15, 4295-4298. [ Links ]
11 B.S. Kumar, N. Srinivasulu, R.H. Udupi, B. Rajitha, Y.T. Reddy, P.N. Reddy and P.S. Kumar, Russ. J. Org. Chem., 2006,42, 1813-1815. [ Links ]
12 R.R. Kumar, S. Perumal, P. Senthilkumar, P. Yogeeswari and D. Sriram, Bioorg. Med. Chem. Lett., 2007,17, 6459-6462. [ Links ]
13 V.K. Tandon, M. Vaish, S. Jain, D.S. Bhakuni and R.C. Srimal, Indian J. Pharm. Sci., 1991, 53, 22-23. [ Links ]
14 M. Longobardi, A. Bargagna, E. Mariani, P. Schenone and E. Marmo, Farmaco, 1990,45, 399-404. [ Links ]
15 H. Bedair, A. El-Hady, S. Abd El-Latif, H. Fakery and M. El-Agrody, Farmaco, 2000, 55, 708-714. [ Links ]
16 M. Heravi, K. Bakhtiari, V Zadsirjan and F. Bamoharram, Bioorg. Med. Chem. Lett., 2007, 17, 4262-4265. [ Links ]
17 C. Bruhlmann, F. Ooms, P. Carrupt, B. Testa, M. Catto, F. Leonetti, C. Altomare and A. Cartti, J. Med. Chem., 2001, 44, 3195-3198. [ Links ]
18 S.R. Kesten, T.G. Heffner, S.J. Johnson, T.A. Pugsley, J.L. Wright and L.D. Wise, J. Med. Chem., 1999, 42, 3718-3725. [ Links ]
19 M. Kidwai, S. Saxena, M.K. Rahman Khan and S.S. Thukral, Bioorg. Med. Chem. Lett., 2005, 15, 4295-4298. [ Links ]
20 S. Balalaie, S. Ramezanpour, M. Bararjanian and J.H. Gross, Synth. Commun., 2008, 38, 1078-1089. [ Links ]
21 K. Gong, H.L. Wang, D. Fang and Z.L. Liu, Catal. Commun., 2008, 9, 650-653. [ Links ]
22 M.S. Rao, B.S. Chhikara, R. Tiwari, A. Nasrolahi Shirazi, K. Parang and A. Kumara, Chem. Biol. Interface, 2012, 2, 362-372. [ Links ]
23 J. Albadi, A. Mansournezhad and Z. Azarian, Iranian J. Org. Chem., 2013, 5, 963-966. [ Links ]
24 A.M. El-Maghraby, Org. Chem. Int., 2014, Article ID 715091, 6 pp. [ Links ]
25 V.P. Reddy, A.V. Kumar, K. Swapna and K.R. Rao, Org. Lett., 2009,11, 951-953. [ Links ]
26 N. Mittapelly, B.R. Reguri and K. Mukkanti, Der Pharma Chemica., 2011, 3, 180-189. [ Links ]
27 S. Abdolmohammadi and M. Afsharpour, Chin. Chem. Lett., 2012, 23, 257-260. [ Links ]
28 S. Abdolmohammadi and S. Balalaie, Comb. Chem. High T. Scr., 2012, 15, 395-399. [ Links ]
29 S. Abdolmohammadi, M. Mohammadnejad and F. Shafaei, Z. Naturforsch. B., 2013, 68b, 362-366. [ Links ]
30 M. Tajbakhsh, E. Alaee, H. Alinezhad, M. Khanian, F. Jahani, S. Khaksar, P. Rezaee and M. Tajbakhsh, Chin. J. Catal., 2012, 33, 1517-1522. [ Links ]
31 K.M. Parida, S.S. Dash and D.P. Das, J. Colloid Interface Sci., 2006, 298, 787-793. [ Links ]
32 Z. Mirjafary, H. Saedian, A. Sadeghi and F.M Atloubi Moghaddam, Catal. Commun., 2008, 9, 299-306. [ Links ]
33 M.Z. Kassaee, F. Movahedi and H. Masrouri, Synlett, 2009,8,1326-1330. [ Links ]
34 I. Yavari and S. Beheshti, J. Iran. Chem. Soc., 2011, 8, 1030-1035. [ Links ]
35 B.V Kumar, H.S.Naik,D. Girija and B.V Kumar, J. Chem. Sci.,2011,123, 615-621. [ Links ]
36 S. Abdolmohammadi, Comb. Chem. High T. Scr., 2013,16, 32-36. [ Links ]
37 J. Safaei-Ghomi, M.A. Ghasemzadeh and S. Zahedi, J. Mex. Chem. Soc., 2013, 57, 1-38. [ Links ]
38 N.V Kaneva, G.G. Yordanov and C.D. Dushikin, Bull. Mater. Sci., 2010, 33, 111-117. [ Links ]
39 Y. Zhang, F. Zhu, J. Zhang and L. Xia, Nanoscale Res. Lett., 2008, 3, 201-204. [ Links ]
40 F.E. Ghodsi and H. Absalan, Acta Phys. Pol. A., 2010,118, 659-664. [ Links ]
41 S. Ilican, Y. Caglar and M. Caglar, J. Optoelectron. Adv. Mater., 2008,10, 2578-2583. [ Links ]
42 K. Sun, W. Wei, Y. Ding, Y. Jing, Z.L. Wang and D. Wang, Chem. Commun., 2011,47, 7776-7778. [ Links ]
43 M.N. Kamalasanan and S. Chandra, Thin Solid Films, 1996, 288, 112-115. [ Links ]
44 G. Adamopoulos, A. Bashir, W.P. Gillin, S. Georgakopoulos, M. Shkunov, M.A. Baklar, N. Stingelin, D.D.C. Bradley and T.D. Anthopoulos, Adv. Funct. Mater., 2011,11, 525-531. [ Links ]
45. L. Znaidi, Mater. Sci. Eng. B., 2010, 174, 18-30. [ Links ]
46 E. Fortunato, A. Goncalves, A. Marques, A. Viana, H. Aguas, L. Pereira, I. Ferreira, P. Vilarinho and R. Martins, Surf. Coat. Tech., 2004,180, 20-25. [ Links ]
47 A. Tsukazaki, M. Kubota, A. Ohtomo, T. Onuma, K. Ohtani, H. Ohno, S.F. Chichibu and M. Kawasaki, Jpn. J. App. Phys., 2005, 44, 643-645. [ Links ]
48 N.K. Hassan and M.R. Hashim, Sains Malays., 2013, 42, 193-196. [ Links ]
49 M. Hosseini-Sarvari and S. Shafiee-Haghighi, Collect. Czech. Chem. Commun., 2011, 76, 1285-1298. [ Links ]
50 S. Zavar, Arabian J. Chem., In Press. [ Links ]
51 Y.H. Ni, X.W. Wei, J.M. Hong and Y. Ye, Mater. Sci. Eng., B, Solid State Mater. Adv. Technol., 2005, 121, 42-47. [ Links ]
Received 30 June 2014
revised 9 September 2014
*To whom correspondence should be addressed.