Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.67 Durban Jan. 2014
RESEARCH ARTICLE
Reaction of hydrazine hydrate with oxalic acid: synthesis and crystal structure of dihydrazinium oxalate
Rajendran SelvakumarI; Thathan PremkumarI, II, *; Vadivelu ManivannanIII Kaliannan SaravananI; Subbiah GovindarajanI, *
IDepartment of Chemistry, Bharathiar University, Coimbatore - 641 046, India
IIThe University College/Department of Chemistry, Sungkyunkwan University, Suwon 440-746, South Korea
IIIDepartment of Materials Science, Ponnaiyah Ramajeyam Institute of Science and Technology, Prist University, Thanjavur- 613 403, India
ABSTRACT
The reaction of oxalic acid with hydrazine hydrate (in appropriate mole ratio) forms the dihydrazinium oxalate under specific experimental condition. The title compound is a molecular salt containing two discrete hydrazinium cations and an oxalate anion. The oxalate anion is perfectly planar and there is a crystallographic centre of symmetry in the middle of the C-C bond. The C-O bond distances are almost equal indicating the presence of resonance in the oxalate ion. The crystal packing is stabilized by intermolecular N-H · O and N-H · N hydrogen bonds. The oxalate ions are linked together end to end through hydrogen bonds (via N2H5+ ions) and run parallel to the [101] direction. It is interesting to note that each oxalate group in the structure is surrounded by six hydrazinium ions through hydrogen bonding. Similarly, each hydrazinium ion is surrounded by three oxalate and one hydrazinium ion.
Key WORDS: : Hydrazine, oxalic acid, dihydrazinium oxalate, crystal structure.
1. Introduction
Hydrazine, a weaker base than ammonia, is a diacidic base that forms N2H5+ [hydrazinium (+1)] and N2H62+ [hydrazinium (+2)] ionic salts with mineral as well as carboxylic acids. Hydrazinium (+1) salts are more common and they can be obtained with a wide variety of acids whereas hydrazinium (+2) salts are formed only with strong acids. Dibasic acids are known to form N2H5HA, (N2H5)2A and N2H5HA.H2A type of salts (H2A = dibasic acid) with hydrazine. Though a number of hydazinium salts of the former two types with different dibasic acids have been reported, it appears that no report is available in literature on the hydrazinium salts of the third type, viz. N2H5HA.H2A, except that of oxydiacetic acid.1
Hydrazine forms two types of salts with oxalic acid, viz. N2H4∙H2C2O4 and 2N2H4∙H2C2O4. Pratt and Richards2 studied these compounds with proton magnetic resonance and satisfactorily explained that these compounds could be formulated as N2H5∙HC2O4 and (N2H5)2C2O4, respectively. To prepare (N2H5)2C2O4, Pratt and Richards first prepared N2H6C2O4 salt which was then treated with an aqueous solution of hydrazine hydrate.2 The structure of N2H5∙HC2O4 was extensively and thoroughly studied by X-ray3,4 and neutron5 diffraction methods and found to consist of chains of N2H5+ and HC2O4- ions. The chains are cross-linked by N-H∙∙∙O bonds from the N2H5+ ions, thus forming a three-dimensional network. Though much has been reported on the N2H5HC2O4 salt, the crystal structure of (N2H5)2C2O4 has not been determined. The structures of oxalic acid and its salt are shown below.
The spectral and thermal properties of this salt have been well studied,6 but the crystal structure has not been reported so far.
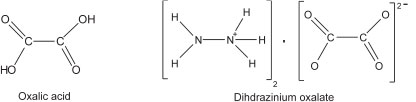
The preparation of the single crystals (suitable for single crystal X-ray analysis) of dihydrazinium oxalate is difficult (the crystals obtained only in ice-cold conditions). When the reaction was carried out above 25 °C, the title compound was not obtained; instead only monohydrazinium oxalate was realized showing the thermal transformation of the dihydrazinium oxalate to the monohydrazinium salt. This may be the main reason that the crystal structure of dihydrazinium oxalate [(N2H5)2C2O4] salt has not been reported so far, though the crystal structure of mono-hydrazinium salt of oxalic acid has been realized around four decades ago (~1973).3,4 Interestingly, a successful attempt has been made to determine the crystal structure of the (N2H5)2C2O4 and the results are presented in this paper.
2. Experimental
2.1. Preparation of Dihydrazinium Oxalate, (N2H5)2C2O4
Dihydrazinium oxalate was prepared by dissolving hydrazine hydrate and oxalic acid (in the mole ratio 2:1) in equal volume of water-alcohol mixture, in ice-cold conditions (yield: 85 %; mp 147 °C). When the reaction was carried out above 25 °C the title compound was not obtained; instead only monohydrazinium oxalate was realized showing the thermal transformation of the dihydrazinium oxalate to the mono salt. Elemental analysis: C (%): 15.35 (Cald. 15.58); H (%): 6.28 (6.54); N (%): 36.40 (36.36); and % hydrazine: 41.0 (Cald. 41.58). The hydrazine content of the salt was determined volumetrically using a standard KIO3 (0.025 M) solution under Andrews' conditions.7
2.2. Single Crystal X-ray Diffraction Analysis
Preliminary examination and data collection were performed using a Bruker SMART Charge Coupled Device (CCD) Detector system single crystal X-ray diffractometer at 218 K using graphite monochromated Mo Kα radiation (λ = 0.71073 Å) equipped with a sealed tube X-ray source. The double-pass method of scanning was used to exclude any noise. The collected frames were integrated using an orientation matrix determined from the narrow frame scans. SMART and SAINT software packages8 were used for data collection and data integration. Analysis of the integrated data did not show any decay. Final cell constants were determined by a global refinement of xyz centroids. Collected data were corrected for systematic errors using SADABS9 based on the Laue symmetry using equivalent reflections. Structure solution and refinements were carried out using the SHELXTL software package.10
3. Results and Discussion
The dihydrazinium oxalate crystals are colourless and soluble in water. The detailed experimental procedure and reaction involved for the preparation of titled salt are explained in Scheme 1. The analytical data (elemental and volumetric analyses) agree well with the proposed formula for the (N2H5)2C2O4 salt. The hydrazine content (41.0 %) of the salt was determined volumetrically using a standard KIO3 (0.025 M) solution under Andrews' conditions,7 which was consistent (calculated value 41.58 %) with the formula of the salt, (N2H5)2C2O4, determined by single crystal X-ray diffraction method.
It is interesting to note that oxalic6 and succinic acids11 form both hydrazinium and dihydrazinium salts, whereas malonic acid forms only mono-hydrazinium salt, viz. N2H5(HOOC-CH2-COO). The reasons are very difficult to generalize.
As reported,6 oxalic acid forms both salts. The hydrogenoxalate anion which is represented as 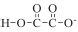 has a strong -I effect due to the
has a strong -I effect due to the 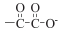 group that makese the O-H bond more labile. This leads to the formation of a dihydrazinium salt. In the case of hydrogenmalonate anion, the O-H bond is stabilized by the formation of a six-membered ring anion due to strong intramolecular hydrogen bonding.
group that makese the O-H bond more labile. This leads to the formation of a dihydrazinium salt. In the case of hydrogenmalonate anion, the O-H bond is stabilized by the formation of a six-membered ring anion due to strong intramolecular hydrogen bonding.
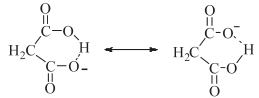
Hence a proton cannot be abstracted by a weak base like N2H4, which prevents the formation of dihydrazinium salts. In hydrogensuccinate anion, the intramolecular hydrogen bonding is not preferred due to an increase in size of the ring. Also the -I effect of the carboxylate group makes the O-H bond more labile. This helps to form the dihydrazinium salt.
Single crystals obtained for (N2H5)2C2O4 salt has been studied using single crystal X-ray diffraction methods. The crystal data including experimental details, bond length, bond angle and hydrogen bonding parameters are given in Tables 1-3.
The ORTEP (Oak Ridge Thermal Ellipsoid Plot) diagram of the title compound is shown in Fig. 1. The oxalate ions are linked together into infinite chains by O · Ή-N bonds and the bond distances vary from 2.8095 (9) to 3.6974 (12) Å. The C2O42- skeleton is perfectly planar and there is a crystallographic centre of symmetry in the middle of C-C bond. The covalent bond distances withintheC2O42-ionareC-C = 1.5597(15),C-O1 = 1.2613(9)and C-O2 = 1.2477 (9) Å. The C-O bond distances are almost equal indicating the presence of resonance in the oxalate ion. The N-N distance of the N2H5+ ion is 1.4478 (10) Å which is in agreement with the value reported for N2H5HC2O4 (N-N bond length is 1.443 (5) Å) and CH3COON2H5 (N-N bond length is 1.462 (12) Å). The bond angles within the N2H5+ ion are all fairly close to tetrahedral and there seems to be a significant difference between the two ends. In the NH2 end, the bond angles are equal and nearer to 106°, whereas in the NH3 end the angles are around 110°.
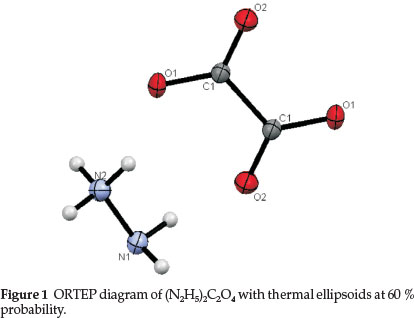
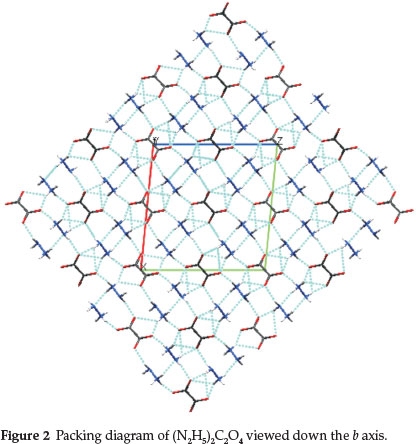
The structure is built from N2H5+ and C2O42- ions, which are joined by hydrogen bonds (Fig. 2). The oxalate ions are linked together end to end through hydrogen bond (via N2H5+ ions) and run parallel to [101] direction. The chains are cross-linked by N-H∙∙∙O bonds from the donor N2H5+ ions, thus forming a three-dimensional network. In the lattice, the adjacent N2H5+ ions are also linked to each other through N-H∙∙∙N bond, with the NH2 part of one hydrazinium ion to the NH3 end of another. It is interesting to note that each oxalate group in the structure is surrounded by six hydrazinium ions through hydrogen bonding. Similarly, each hydrazinium ion is surrounded by three oxalate and one hydrazinium ion.
4. Conclusions
The reaction of oxalic acid with hydrazine hydrate (in appropriate mole ratio) forms the dihydrazinium oxalate at specific experimental conditions. Note that when the reaction is carried out above 25 °C the title compound was not obtained; instead only monohydrazinium oxalate was realized showing the thermal transformation of the dihydrazinium oxalate to the mono salt. The crystal structure of dihydrazinium oxalate was determined. In the salt, the cations and anions form a rigid three-dimensional network through hydrogen bonding. It is interesting to note that each oxalate group in the structure is surrounded by six hydrazinium ions through hydrogen bonding. Similarly, each hydrazinium ion is surrounded by three oxalate and one hydrazinium ion.
References
1 S. Yasodhai and S. Govindarajan, J. Therm. Anal., 2000, 62, 737-745. [ Links ]
2 I.L. Pratt and R.E. Richards, Trans. Faraday Soc, 1953, 49, 744-.751. [ Links ]
3 N.B.K. Ahmed, R. Liminga and I. Olovsson, Acta Chem. Scand., 1968, 22, 88-96. [ Links ]
4 J.O. Thomas, Acta Cryst. B, 1973, 29, 1767-1776. [ Links ]
5 A. Nilsson, R. Liminga and I. Olovsson, Acta Chem. Scand., 1968, 22, 719-731. [ Links ]
6 D. Gajapathy, S. Govindarajan and K.C. Patil, Thermochim. Acta, 1983, 60, 87-92. [ Links ]
7 A.I. Vogel, A Text Book of Quantitative Inorganic Analysis, 4th edn., Longman, London, 1986. [ Links ]
8 Bruker Analytical X-ray; APEX2 and SAINT; BrukerAXS Inc.,Madison, Wisconsin, USA, 2004. [ Links ]
9 R.H. Blessing, Acta Cryst. A, 1995, 51, 33-38. [ Links ]
10 G.M. Sheldrick, Acta Cryst. A, 2008, 64, 112-122. [ Links ]
11 K. Kuppusamy, B.N. Sivasankar and S. Govindarajan, Thermochim. Acta, 1995, 259, 251-262. [ Links ]
Received 15 July 2013
Revised 28 November 2013
Accepted 10 December 2013
* To whom correspondence should be addressed. E-mail: T.P.: thathanpremkumar@gmail.com; S.G.: drsgovind@yahoo.co.in














