Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Chemistry
versão On-line ISSN 1996-840X
versão impressa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.67 Durban Jan. 2014
RESEARCH ARTICLE
Students' conceptions about the sub-microscopic approach to explanations in chemistry throughout their BSc degree course
Boingotlo M. Serobatse; Mailoo Selvaratnam; Helen P. Drummond*
Department of Chemistry, North West University (Mafikeng Campus), Private BagX2046, Mmabatho, 2735, South Africa
ABSTRACT
The main objective of this study was to test chemistry students' competence, throughout the entire BSc course at North-West University (Mafikeng Campus), in the use of the important and widely applicable strategy of explaining the facts, principles and laws of chemistry in terms of the properties of the constituent sub-microscopic particles. Seventeen questions were used for systematically testing all basic aspects of chemistry. The aspects tested included the following: the types of particles present and their properties in various types of matter (e.g. metals, non-metals, ionic compounds, covalent compounds, mixtures, gases, liquids, solids, solutions); the changes in the nature, arrangement and properties of the particles during both physical and chemical changes; the explanation of physical and chemical properties of substances in terms of the properties of the constituent particles. Student performance was consistently bad and it did not improve as they progressed from year to year in their BSc course. More than half of them (average performance in all questions) had difficulty in answering the questions. Analysis of students' answers suggested that most students' difficulties were due to their not identifying clearly the problem that had to be solved and their not using the relevant principles and reasoning to solve the problems. Instead, most students tried to solve problems by recalling knowledge, procedures and solutions they had learnt. The lack of improvement from year to year indicates the need for continuously training students in the sub-microscopic approach to interpretations and explanations throughout the BSc course, and not just in the first year as is done presently.
Keywords: Sub-microscopic particles, sub-microscopic approach, properties of matter, explanations in chemistry.
1. Introduction
A very important approach to interpreting, understanding and explaining the facts, principles and laws of chemistry, and maybe all the sciences, is in terms of the properties of the sub-microscopic particles (molecules, atoms, ions, electrons, protons, neutrons) present in the system being studied. This approach, the sub-microscopic approach to explanations, is very widely applicable and one of the main objectives of chemistry courses should be to train students to consistently use this approach. This approach should become a mental habit and should be used automatically whenever explanations have to be given.1
A large number of research studies have been reported in this field, over a long period of time2-9. Studies have reported that many students fail to explain phenomena or chemical reactions because they do not represent matter at the particulate level. Most of these studies, however, have involved students in schools. Not much work has been done in this field on university students and little work has been published of studies involving students in South Africa. This paper reports the results of a study done on chemistry students at North-West University (Mafikeng Campus) throughout their university course (BSc first, second and third years). The main aim of the study was to test students' competence in the use of this approach.
2. Objectives and Method of Study
The main objective of the study was to find out and compare the competence of BSc first, second and third year chemistry students in using the sub-microscopic approach.
The method of study was mainly the analysis of students' answers to carefully designed questions, not only to obtain information about their competence but also to find out the possible reasons for their difficulties. Discussions were also held with the different groups of students. The first year students answered the questionnaire during a chemistry lecture period, while the second and third year students who wrote volunteered to do so during a free period. The questionnaire was administered by the authors under test conditions, and all students finished in under an hour.
The questions used for testing were all designed by us to systematically test all basic aspects of chemistry that need, for their understanding, a consideration of the particles present and their properties. The aspects tested included knowledge and understanding of the following:
•types of particles present and their arrangement and properties in various types of matter (metals, non-metals, ionic compounds, covalent compounds, mixtures, gases, liquids, solids and solutions);
•the changes in the properties (e.g. motion, bonding, arrangement) of the particles during physical and phase changes (liquid → vapour, solid → liquid);
•the changes in the arrangement of the particles and bonding during chemical changes;
• explanation of the physical properties of substances in terms of the properties (e.g. mass, motion, charge, size, structure, shape, polarity) of the particles;
• explanation of the chemical properties of substances in terms of the properties of the particles.
Different types of questions were used as test items. These included: true/false type; filling-in blanks type; short-answers; multiple choice. The simplest possible questions for testing the objectives of the study were designed.
3. The Question Paper
The Question paper used for testing is given below. What each of the 17 questions in the paper is intended to test will be stated in the results and discussion section along with discussion of students' performance.
3.1. The Questions used for Testing
Question 1
Indicate whether each property stated below is a macroscopic property ('bulk' property) or 'sub-microscopic' property. Mark the correct answer with the symbol /X/
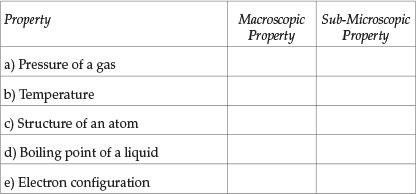
Question 2
Indicate whether each statement given below is a description or explanation. Mark the correct answer with the symbol /X/
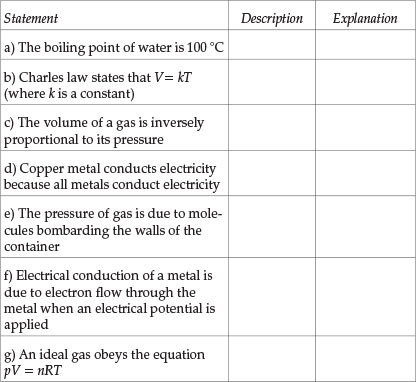
Question 3
Fill-in the blanks in the following statements (To illustrate the type of answer required, the answer to part (a) of the Question is given.)
The particles (molecules, atoms, ions, electrons, protons) that exist as stable units in:
(a) Hydrogen gas are.......H2 molecules.................
(b) A mixture of nitrogen and ammonia gases are...........
(c) Liquid water are.....................................
(d) Copper metal are....................................
(e) Solid sodium chloride are.............................
(f) An aqueous solution of sodium chloride is ..............
(g) An aqueous solution of glucose is ......................
Question 4
Liquid water (H2O) is boiled to form vapour. Which one of the following represents the particles present in the vapour?
(a) H2 molecules and O2 molecules
(b) H2O molecules
(c) H2 molecules, O2 molecules and H2O molecules
(d) H2O molecules, H+ ions and OH- ions
(e) H atoms and O atoms
Question 5
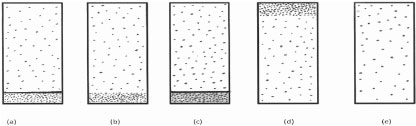
Which one of the following diagrams best represents, from a molecular viewpoint, a closed vessel containing a sample of liquid water in equilibrium with its vapour?
Question 6
The questions below concern two samples of hydrogen gas, one at 25 °C and the other at 50 °C. Both samples are at the same pressure and volume.
(a) In 1 cm3 of the gas sample, which sample (at 25 °C or 50 °C) will have the larger number of molecules?
(b) In which sample will the molecules move faster?
(c) Which one of the following will approximately represent the number of the H2 molecules present in 1 cm3 of the gas at 25 °C? (Number of molecules in one mole = 6 x 1023)
(i) one million (106) (ii) one billion (109)
(iii) billion x billion (1018) (iv) greater than 1018
(v) less than 106
Question 7
The following questions concern ice, in which the particles present are H2O molecules.
Indicate whether each of the following statements is True or False.
True (T)/False (F)
(a) The H2O molecules are in fixed positions and they do not show any type of motion.
(b) The H2O molecules show vibrational motion.
(c) The amplitude of the vibrational motion of the molecules increases when temperature is increased.
(d) The O-H bonds in the H2O molecules show vibrational motion.
(e) An H2O molecule in ice is bonded to other H2O molecules by covalent bonds.
Question 8
Consider the outermost electron in an atom of Na and in an atom of K. In which atom will
(a) the distance between this electron and the atomic nucleus be larger?
(b) the force of attraction between this electron and the atomic nucleus be larger?
(c) this electron be more easily removed from the atom?
Question 9
The H2O molecule has an angular structure and the bond angle is 108 °. The molecule is polar with the O atom having the negative charge and the H atoms having positive charges. Draw a diagram that shows the structure obtained when three H2O molecules bond together by intermolecular forces.
Question 10
The volume of a fixed amount of a gas increases when it is heated at constant pressure. Which one of the following is the main reason for this increase?
(a) Increase in the size of the molecules
(b) Increase in the number of molecules
(c) Increase in the speed of the molecules
(d) Increase in the volume of the spaces between the molecules
(e) Increase in the temperature of the gas
Question 11
Which one of the following represents the type of bonds that break when ice melts to form water?
(a) O-H bonds
(b) O=O bonds
(c) H-H bonds
(d) Intermolecular bonds between H2O molecules
(e) Intramolecular bonds within the H2O molecules
Question 12
Give balanced chemical equations, using only the particles (i.e. atoms, ions, electrons, molecules) that are involved, for the following processes:
(a) The removal of 2 electrons from a copper atom (Cu)
(b) The reaction that occurs when zinc (Zn) metal is added to an aqueous solution of copper sulphate (CuSO4)
(c) The formation of a precipitate of silver chloride (AgCl) when a silver nitrate (AgNO3) solution is added to a sodium chloride (NaCl) solution
(d) The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH)
Question 13
State the number of bonds broken, and the number of bonds formed, (Hint: when a hydrogen molecule dissociates to form H atoms, H-H → H + H, one H-H bond is broken)
(a) when two molecules of H2 react with O2 according to the equation 2H2 + O2 → 2H2O
(b) when three molecules of H2 react with N2 to form NH3 according to the equation
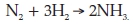
(c) when one molecule of silver chloride (AgCl), which is insoluble, is formed when solutions of silver nitrate (AgNO3) and sodium chloride (NaCl) are mixed.
Question 14
This Question concerns sodium chloride (NaCl). Indicate whether the following statements are true or false.
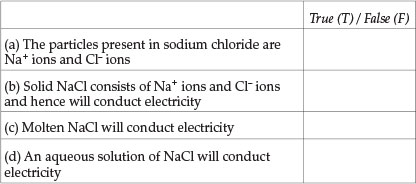
Question 15
Consider the following molecules:
O2 (Mr = 32), CO2 (Mr = 44), NH3 (Mr = 17), CH4 (Mr = 16). (Mr = relative molecular mass)
Arrange them in the order of increasing
(a) molecular speed
(b) density (for gases containing these molecules, at the same temperature and pressure)
Question 16
Two solid objects A and B are formed by the packing of large hydrocarbon molecules. The molecules in A and B have the same number of atoms but have different shapes. The molecules in object A are linear and in object B they are branched (see fig.)
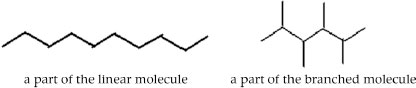
Deduce which object (A or B) will (briefly indicate your reasoning)
(a) have the higher density
(b) have the higher melting point
Question 17
Consider the addition, in the gas phase, of Cl2 to CH2=CH2 and to CH3CH2CH=CHCH2CH3. The products formed respectively will be CH2ClCH2Cl and CH3CH2CHClCHClCH2CH3
Which reaction will you expect to be faster? Indicate briefly your reasoning.
4. Results and Discussion
Students' performance in each of the 17 questions used for testing is shown in Table 1. Columns 2,3 and 4 show respectively the percentages of BSc first year, second year and third year students who correctly answered the questions given in column 1. Column 5 shows the average performance of all the students tested in each question.
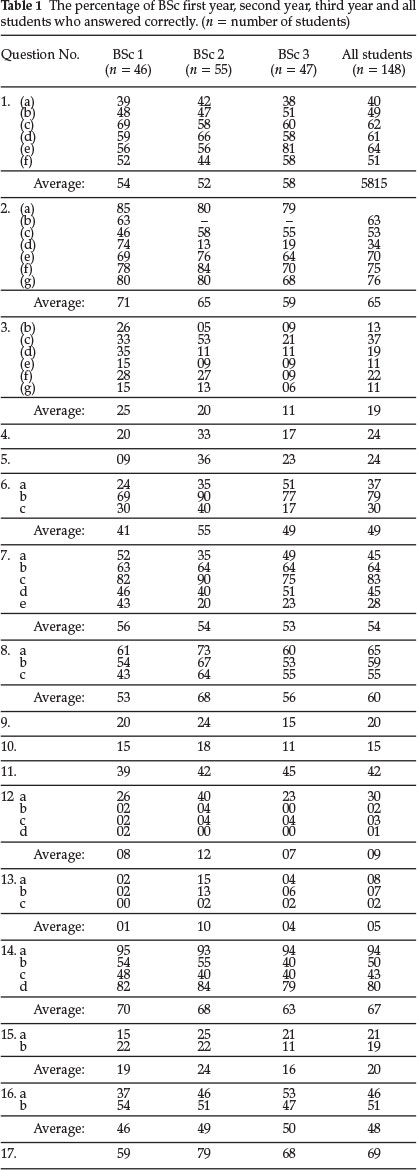
It can be seen that students' performance is poor in all the questions, and also that in many questions there was no improvement in performance as students progressed from year to year. In some questions performance actually decreased in successive years. This was surprising but may be due to the fact that the sub-microscopic approach to explanations is explicitly taught at our university only during the first year. The implicit assumption made that students will continue to use this important approach in later courses is therefore not valid. It is therefore necessary to ensure that students use this important approach throughout their BSc degree course.
Students' performance in each Question (see Table 1) will now be analysed. Question 1 tests the understanding of the distinction between macroscopic properties and sub-microscopic properties and Question 2 checks whether students recognize that an explanation of macroscopic properties has to be given in terms of the properties of the constituent sub-microscopic particles. About a half of the students tested had difficulty in answering Question 1 correctly (average performance in all parts). Students' performance in Question 2 was better: nearly two-thirds of students answered correctly. Those students who have difficulty in distinguishing between descriptive statements and explanatory statements will be seriously handicapped in their learning because descriptive and explanatory aspects of knowledge need to be learnt in different ways. Also, many students' failure in some examination questions is due to their giving a descriptive answer when what was required was an explanatory answer. The part in Question 2 that was most poorly answered was (d): two-thirds of the students thought erroneously that 'copper metal conducts electricity because all metals conduct electricity' was an explanatory statement. This error was probably due to the word 'because' in the statement.
Although Questions 3 and 4 essentially test recall of very basic knowledge about the type of particles present in systems, they were very poorly answered by all the groups of students tested. From the data in Table 1 it can be seen that less than a quarter of the students answered the questions correctly (average performance of the three groups of students in both the questions). It was surprising, for example, that more than 60 % of the students tested did not know that the particles that exist in liquid water (Question 3(c)) and in water vapour (Question 4) are H2O molecules. Concerning Questions 3 (f) and (g), the main source of error was due to students not recognizing that aqueous solutions always contain H2O molecules: they considered only the solute particles. The main error in Question 4 was due to students thinking that H2O molecules in liquid water decompose into H2 and O2 molecules when it is boiled.
Questions 5-7 essentially test the arrangement and properties (e.g. motion) of molecules in the gaseous, liquid and solid phases. Though Question 5 merely tests awareness of the different 'density' of the molecules in the two phases in a liquid vapour equilibrium system, student performance was poor. Only about a quarter of all the students tested recognized that response (b) is correct. The majority thought, surprisingly, that the molecules are distributed uniformly in the liquid and vapour phases (response (e)). Student performance in Questions 6 and 7, which test respectively some properties (e.g. motion) of the molecules in the gaseous and solid states, was much better. About a half of all the students who were tested answered correctly.
Question 8 tests some basic principles needed for understanding chemical reactions of the atoms of metals. Student performance was good: about 60 % of all students tested answered correctly.
Question 9 was very poorly answered. Only about 20 % of students answered it correctly. This Question is more difficult than most of other questions used in the Question paper because its answering needs more conceptual knowledge (e.g. of polar molecules and intermolecular attraction) and also some reasoning.
Questions 10 and 11 test ability to interpret/explain physical changes in terms of the constituent particles and their properties. Student performance in Question 10 was very poor with only about 15 % of them answering correctly. The majority of students thought erroneously that response (e) was correct. Increase in temperature cannot, however, be considered to be the reason for the increase in gas volume when it is heated: it is an experimental fact. Similarly, increase in the speed of the molecules (response (c)) is not the main reason for the increase in volume. The correct response, which interprets increase in gas volume, when heated, in terms of the increase in the volume of the space between the molecules, is (d). Question 11 tests an important principle that distinguishes physical and chemical changes. In physical changes the bonds that break are bonds between different molecules, while in chemical changes the bonds that break are covalent bonds between atoms within molecules. The correct response to this Question is therefore (d). More than half of the students tested had difficulty and they thought that the bonds that break when ice melts are covalent O-H bonds within the molecules.
Questions 12 and 13 are mainly concerned with the breaking and forming of covalent bonds during chemical changes. Student performance was very poor. Though part (a) of Question 12 is easy, about 30 % of the students could not answer it: it was surprising that they could not write the simple equation for the removal of two electrons from a copper atom (Cu → Cu2+ + 2e-). The very poor performance in parts (b), (c) and (d) of Question 12 was mainly due to students not answering the questions asked, which was to write the equations using the particles involved in the reactions (which are ions). Most students wrote the equations correctly in terms of molecules. For example, about 70 % of them wrote correctly the equation for the reaction between hydrochloric acid and sodium hydroxide using molecules (HCl + NaOH → NaCl + H2O). It seems that the question did not emphasize sufficiently strongly that they should only use the particles involved. This suggests that most students do not focus sharply on the questions asked and try to use reasoning to obtain the solutions. Instead, the strategy they often use to answer questions is recall of knowledge that they have learnt. Question 13 was also very poorly answered: less than 10 % of students answered it correctly. The very poor performance was due to students not writing the given balanced equations in terms of the bonds in the reactant and product molecules. In terms of bonds, the reaction 2H2 + O2 → 2H2O could be rewritten as 2 (H-H) + (O=O) → 2 (H-O-H) which would show that two H-H bonds and one O = O bond are broken and four O-H bonds are formed during the reaction 2H2 + O2 → 2H2O.
Questions 14-16 test competence in the explanation of macroscopic physical properties using the properties of the particles present in the system. Student performance in Question 14, which tests knowledge of the necessity for the presence of mobile ions in a compound for it to conduct electricity, was good. In contrast, student performance in the next question, which tests explanation of molecular speed and gas density in terms of the mass of the molecules, was very poor. Only about 20 % of students answered correctly. This poor performance was surprising because 'common sense' can be used to infer that molecular speed will decrease when molecule mass increases. Though Question 16 appears to be more difficult than Question 15, because its solution needs the correlation of density/melting point with some properties of molecules and intermolecular forces, student performance was much better. About a half of all the students tested answered correctly.
The final question, Question 17, tests competence in explaining chemical properties in terms of the structures of molecules. Student performance was good, about 70 % answered correctly. The second year students answered particularly well: nearly 80 % of them gave the correct answer. This could be because orientation of reactant molecules is taught in kinetics in the second year physical chemistry module.
5. Conclusions and Recommendations
The results of the study show that more than a half of all the students tested had difficulty in answering the questions. One of the reasons for poor student performance in some questions (e.g. Questions 10 and 12) may be due to the questions not emphasizing sufficiently strongly that they should only use the particles involved for explanations. It may have been better, for example, to give Question 12(d) as a multiple choice question that included the following as possible alternatives: The particles actually taking part in the reaction between NaOH and HCl solutions are (i) NaOH molecules and HCl molecules, (ii) H+ ions and OH- ions, etc.
Analysis of students' answers and subsequent discussions with them revealed that the main reason for most students' difficulties was due to their trying to answer questions by recall of knowledge and procedures they had learnt. They do not identify the problem clearly and do not deduce the solution logically using a consistent approach6,10,11. For example, about a third of the students gave 'opposite' answers to the questions involving temperature and pressure (parts (a) and (b) of Question 1) and involving structure of an atom and electron configuration (parts (c) and (e) of Question 1), though they are similar types of quantities. If a consistent approach had been used, students who thought, for example, that pressure is a macroscopic property should have thought that temperature is also a macroscopic property.
From the results it can also be concluded that more than half of the students tested had difficulty with the use of the sub-microscopic approach for interpretations and explanations. This will seriously handicap learning and problem solving since 'the study of atomic and molecular structure is considered a sine qua non in chemical education.'12 The sub-microscopic approach is well recognized4,7,13 to be 'the most useful and most powerful way of thinking in chemistry.... by adopting this approach [students] will find learning, remembering and understanding chemistry much easier.'14 Since the use of the sub-microscopic approach is central to learning and understanding chemistry, students should constantly be reminded and taught to use this approach throughout their chemistry courses.
References
1 M. Selvaratnam, S. Afr. J. Chem., 1998, 51, 2-6. [ Links ]
2 S. Novick and J. Nussbaum, Sci. Educ, 1981, 65, 187-196. [ Links ]
3 D.L. Shepherd and J.W. Renner, Sch. Sci. Math., 1982, 82, 650-665. [ Links ]
4 D.L. Gabel, K.V Samuel and D. Hunn. J. Chem. Educ., 1987, 64, 695-697. [ Links ]
5 S.C. Nurrenbern and M. Pickering, J. Chem Educ, 1987, 64, 508-510. [ Links ]
6 R. Ben-Zvi, B. Eylon and J.Silberstein. Educ. Chem, 1988, 25, 89-92. [ Links ]
7 J. Snir, C.L Smith and G. Raz. Sci. Educ, 2003, 87, 794-830. [ Links ]
8 M.J. Sanger and A.J. Phelps. J. Chem. Educ, 2007, 84, 870-874. [ Links ]
9 H. Ozmen. Inter. J. Enviro. Sci. Educ, 2011, 6, 99-121. [ Links ]
10 H.P. Drummond and M. Selvaratnam. S. Afr. J. Chem, 2008,61,56-62. [ Links ]
11 H.PDrummondandM.Selvaratnam. S.Afr.J. Chem.,2009,62,179-184. [ Links ]
12 G. Tsaparlis. J. Chem. Educ, 1997, 74, 922-925. [ Links ]
13 A.H. Johnstone, J. Comput. Assist. Learn, 1991, 7, 75-83. [ Links ]
14 M. Selvaratnam and H.P. Drummond, A Guided Approach to Learning Chemistry, 2nd edn., North-West University, Mafikeng, South Africa, 2013. [ Links ]
Received 4 April 2013
Revised 10 March 2014
Accepted 10 March 2014
* To whom correspondence should be addressed. E-mail: helen.drummond@nwu.ac.za














