Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.66 Durban Aug. 2013
RESEARCH ARTICLE
Cesium salts of phosphotungstic acid: comparison of surface acidity, leaching stability and catalytic activity for the synthesis of β-ketoenol ethers
Ezzat Rafiee*; Masoud Kahrizi
Inorganic Chemistry Department, Faculty of Chemistry, Razi University, Kermanshah, 67149, Iran
ABSTRACT
Catalytic activity of CsxH3-xPW12O40 catalysts were investigated in the synthesis of β-ketoenol ethers. It was found that activity; acidity, solubility and consequently, recoverability of these catalysts are related to cesium content. A series of β-ketoenol ether derivatives were synthesized by using Cs25H0.5PW12O40 catalyst in high to excellent yields. This catalyst showed highest surface acidity and lowest solubility in reaction media in comparison with the other cesium content salts.
Keywords: Polyoxometalates, cesium salts, surface acidity, β-ketoenol ethers, heterogeneous catalysis.
1. Introduction
Heteropoly acids (HPAs) and their acidic salts with the Keggin structure are well-known as environmentally friendly and economically viable solid acids have gained increasing attention owing to their ease of handling and high catalytic activities.1-3 Although HPAs are useful solid catalysts, the number of acid sites on the surface are small because of the low surface area (about 5 m2/g). However, their catalytic activity can be improved by increasing the HPA dispersion, which can be achieved by the partial neutralization of the HPA with different cations (e.g. K+, Cs+,NH4+) to form porous insoluble salts.4 The most-studied insoluble salt of HPA is cesium salt of tungstophosphoric acid, CsxH3-xPW12O40 (CsxPW), a well-known acidic catalyst in which the residual protons are more acidic than the homogeneous acid catalysts (e.g. H2SO4 and p-toluenesulfonic acid). Properties of HPAs such as solubility, crystalline structure, porosity, surface area, amount of water of crystallization and thermal stability are sensitive to the amount of Cs substituted.4-6 Considering the economic and environmental advantages of CsxPW catalysts, several researchers have focused their attention to the characterization and catalytic application of these catalysts in several organic processes.7-10 Taking into account the increasing demand for clean chemical processes catalyzed by CsxPW salts, herein we report their catalytic application in the synthesis of β-ketoenol ethers. Our goal in choosing β-ketoenol ethers lies in the fact that they have been widely used as important intermediates in various organic transformations, they are valuable in the preparation of enantiomerically pure bioactive compounds, and they act as dienophiles in Diels-Alder reactions.11-17 The activity of Csx PW (X = 0,1, 2, 2.5 and 3) catalysts used in the same operating conditions, has been compared together. Also, surface acidity and solubility of these catalysts in reaction media have been investigated.
2. Experimental
2.1. General
All reagents and solvents used in this work were obtained from Fluka, Aldrich or Merck and were used without further purification. UV-vis spectra were obtained with an Agilent (8453) UV-vis diode-array spectrometer using quartz cells of 1 cm optical path. NMR spectra were recorded on a BrukerAvance 200 MHz NMR spectrometer with CDCl3 as the solvent and TMS as the internal standard.
2.2. Preparation of the Catalysts
The CsxPW catalysts were prepared by adding the required amount of aqueous cesium carbonate to aqueous solution of H3PW12O40 (PW) with stirring. An appropriate amount of the aqueous solution of Cs2CO3 (0.10 M) was added dropwise to the aqueous solution of PW (0.08 M) at room temperature with vigorous stirring. The Cs content was adjusted by the amount of Cs2CO3 solution added. From the beginning of the addition of Cs2CO3, very fine particles (precipitates) were formed to make the solution milky. The precipitate obtained was dried in a rotary evaporator.
2.3. General Procedure for the Synthesis of β-Ketoenol Ethers
A mixture of dimedone (1 mmol) and alcohol (3 mL) was stirred well in the presence of the catalyst (0.2 g) at 80 °C. After completion of the reaction as indicate by TLC, the catalyst was removed by filtration, and washed with acetonitrile and dried at 100 °C for reuse. The filtrate was concentrated and the product was purified by column chromatography on silica gel using ethylacetate/hexane as eluent. All products were identified by comparing their spectral data with those of the authentic samples.18-21
1HNMR data for products are presented below.
Table 2, entry 1 (a): δh (200 MHz, CDCl3): 1.29 (6H, s, Me), 2.19 (2H, s, CH2), 2.47 (2H, s, CH2), 3.64 (3H, s, OMe), 5.31 (1H, brs, CH).
Table 2, entry 2(b): δh (200 MHz, CDCl3): 1.27 (6H, s, Me), 1.41 (3H, m, OCH2CH3), 2.21 (2H, s, CH2), 2.46 (2H, s, CH2),4.12 (2H, m, OCH2CH3), 5.30 (1H, brs, CH).
Table 2, entry 5 (c): δh (200 MHz, CDCl3): 0.96 (3H, m, OCH2CH2CH2), 1.27 (6H, s, Me), 1.63 (2H, m, OCH2CH2CH3), 2.23 (2H,s,CH), 2.44 (2H, s, CH2), 3.41 (2H, m, OCH2CH2CH3), 5.31 (1H, brs, CH).
Table 2, entry 6(d): δh (200 MHz, CDCh): 0.93 (3H, m, OCH2CH2CH2CH3). 1.25 (6H, s, Me), 1.38 (2H, m, OCH2CH2CH2CH3),1.52 (2H, m, OCH2CH2CH2CH3), 2.21 (2H, s, CH2), 2.45 (2H, s, CH2), 3.42 (2H, m, OCH2CH2CH2CH3), 5.31 (1H, brs, CH).
Table 2, entry 7(e): δh (200 MHz, CDCh): 0.91 (3H, m, OCH2CH2(CH2)3CH3), 1.23 (6H, s, Me), 1.34 (6H, m, OCH2CH2(CH2)3CH3),1.56 (2H, m, OCH2CH2(CH2)3CH3), 2.24 (2H, s, CH2), 2.47 (2H, s, CH2), 3.36 (2H, m, OCH2CH2(CH2)CH3), 5.30 (1H, brs, CH).
Table 2, entry 8(f): δΗ (200 MHz, CDQ3): 1.19 (6H, s, Me), 1.31 (6H, d, J 7.0 Hz, CH(CH3)2), 2.21 (2H, s, CH2),2.35 (2H, s, CH2),4.46 (1H, m, OCH(CH3)2), 5.29 (1H, brs, CH).
Table2, entry 10(g): δΗ (200 MHz, CDQ3): 1.15 (6H, s,Me),1.321.49 (6H, m, cyclohexyl), 1.61 1.98 (4H, m, cyclohexyl), 2.19 (2H, s, CH2), 2.30(2H, s, CH2), 4.08 4.13 (1H, m, OCH), 5.36 (1H, brs, CH).
Table 2, entry 11(h): 1.26 (6H, s, Me), 2.28 (2H, s, CH2), 2.49 (2H, s, CH2),4.68 (2H, s, OCH2), 5.30 (1H, brs, CH), 7.28-7.41 (5H, m, phenyl).
Table 2, entry 12(i): 1.25 (6H, s, Me), 2.26 (2H, s, CH2), 2.48 (2H, s,CH2), 3.76 (3H, s,OMe), 4.71 (2H, s, OCH2), 5.30 (1H, brs, CH), 6.88-7.12 (4H, m, phenyl).
Table 2, entry 13(j): 1.25 (6H, s, Me), 2.22 (2H, s, CH2), 2.45 (2H, s, CH2), 4.88 (2H, s, OCH2), 5.30 (1H, brs, CH), 7.63-8.19 (4H, m, phenyl).
Table 2, entry 14(k): 1.27 (6H, s, Me), 2.21 (2H, s, CH2), 2.48 (2H, s, CH2), 4.91 (2H, s, OCH2), 5.32 (1H, brs, CH), 7.51-8.22 (4H, m, phenyl).
Table 2, entry 15(l): 1.26 (6H, s, Me), 2.28 (2H, s, CH2), 2.50 (2H, s, CH2), 3.78 (3H, s. OMe), 4.75 (2H, s, OCH2), 5.31 (1H, brs, CH), 6.79 7.08 (4H, m, phenyl).
Table 2, entry 16(m): 1.25 (6H, s, Me), 2.21 (2H, s, CH2), 2.44 (2H, s, CH2), 4.66 (2H, m, OCH2), 5.31 (1H, brs, CH), 6.36 (1H, m, HC=CH), 6.57 (1H, m, HC=CH), 7.18 7.58 (5H, m, phenyl).
3. Results and Discussion
Initially, etherification of dimedone with ethanol as model substrates was performed in the presence of 0.2 g of PW as catalyst at various temperatures. With increasing the temperature from room temperature to 80 °C, the yield increased from 10 % to 61 %. The results showed that the reaction temperature had a significant effect on the reaction, and further experiments were carried out at 80 °C. Also it is remarkable to note that in the absence of the catalyst, reaction did not proceed at 80 °C even after long reaction times.
Various types of CsxPW were examined to find the best catalyst (Table 1). Cs25PW was the best catalyst among the others (Table 1, entry 4). The acidity measurement of the catalysts by means of potentiometric titration with n-butylamine was carried out to find if there is any relationship between acidic properties and catalytic reactivity. The n-butylamine is considered a strong base, so its adsorption could be expected on sites of different acid strength. The total solid acidity without distinguishing the type of acidity is titrated. In this method, the initial electrode potential (Ei) indicates the maximum strength of the acid sites and the value from which the plateau is reached indicates the total number of acid sites that are present in the titrated solid.22 As can be seen, the measured values (Ei) are in good agreement with the theoretical bulk Brönsted acidity of these catalysts. As expected, strength of the acid sites decreases with increasing cesium content (Table 1). In CsxPW, surface acidic sites decreased (mmol amine g-1 catalyst) slightly from x = 0tox = 2, but these amounts increased when the Cs content changed from x = 2tox = 2.5 (Table 1, entries 1-4). Consequently, the dependence of surface acidity and catalytic activity of CsxPW to Cs content is remarkable. For Cs25PW surface area is large and catalytic activity of Cs2.5PW was ascribed to its high surface acidity.

One of the most important factors in the evaluation of a heterogeneous catalytic reaction is the catalyst stability. Stability tests on CsxPW with different cesium contents were conducted to find the most stable catalyst for our study. For this purpose, the materials were treated in ethanol at 80 °C for 100 min followed by filtering of the solid catalysts. Catalyst leaching in the supernatant was determined by measuring the concentration of PW via UV-vis spectroscopy. The UV-vis spectra for the catalysts are shown in Fig. 1a. The spectroscopic measurements revealed that different Cs salts have different leaching tendencies. The amount of released PW was significant for materials with low cesium content (x < 2). In contrast, Cs25PW exhibited the highest

leaching stability in ethanol (Fig. 1b), suggesting that it is able to work as a heterogeneous catalyst in alcohols. As a result, Cs25PW was selected as the best catalyst in terms of surface acidity, catalytic activity and stability.
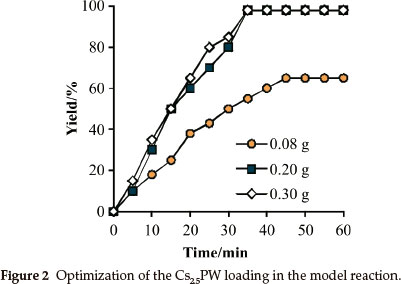
In the next step of our investigation, the reaction between dimedone (1 mmol) and ethanol (3 mL) was carried out using different amounts of Cs25PW. As seen in Fig. 2, 0.2 g of Cs25PW showed excellent yield in 35 min. Also, it should be noted that, no improvement in yield or reaction time was observed when catalyst loading increased from 0.2 to 0.3 g. In the presence of 0.2gofCs25PW, the etherification reaction involving dimedone and ethanol successively proceeded at 80 °C to afford the corresponding β-ketoenol ethers in 95 % yield.
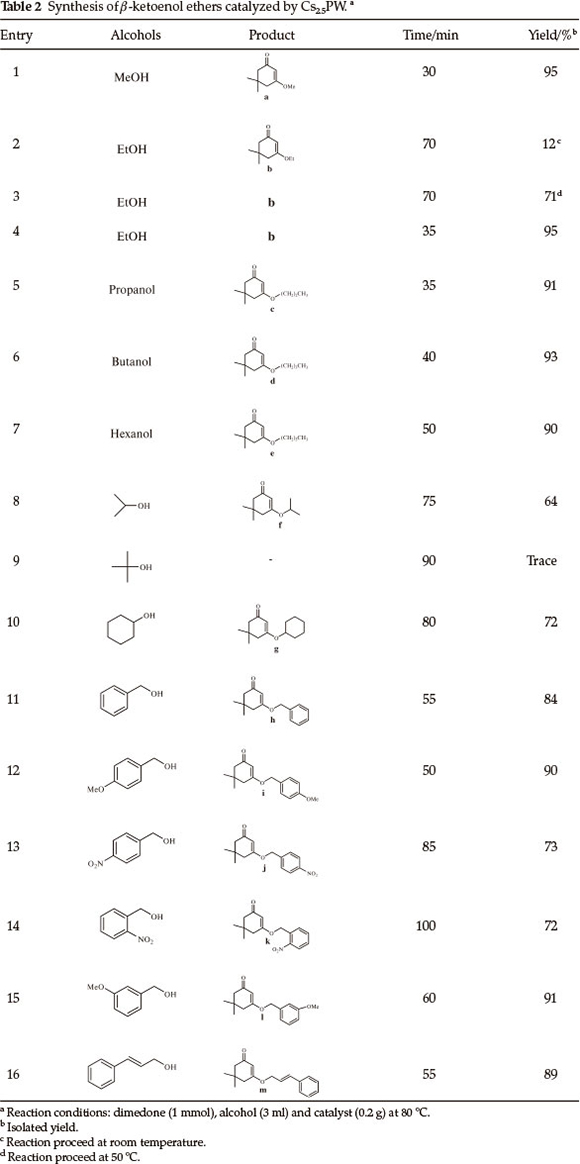
This new methodology allowed us to prepare the β-ketoenol ether derivatives (Table 2). Reactions of different alcohols including secondary, vinyl and benzyl alcohols were examined in the presence of 0.2 g of Cs2.5PW and at 80 °C. All the reactions proceeded expeditiously, delivered good to excellent product yields and accommodated a wide range of aliphatic and aromatic alcohols bearing both electron-donating and electron-withdrawing substituent (Table 2). Aromatic alcohols with electron-withdrawing groups (Table 2, entries 13 and 14) gave relatively lower yields. We believe that this is mainly due to the laborious formation of carbocation produced from the precursor bearing an electron-withdrawing group. An important feature of this procedure is that the secondary alcohols, which normally produce low yields due to their steric effects (Table 2, entries 8, 10) were also used with similar success to provide the corresponding β-ketoenol ethers. But, with tert-butyl alcohol trace amount of product was obtained even after 90 min.
Many of the catalysts which can catalyze the synthesis of β-ketoenol ethers suffer from the drawback of green chemistry such as recovery and reusability of the catalyst. Therefore the possibility of recycling the catalyst is of concern. For this purpose, 2 g of the catalyst was used in the reaction of dimedone and ethanol as a model reaction, and the experiments were properly scaled up. When the reaction completed, the catalyst was recovered and reused in the next run. This process was carried out over three runs without appreciable reduction in the catalytic activity of the catalyst (Fig. 3).

A suggested mechanism for the reaction of dimedone with alcohols in the present of Cs2.5PW as a Brönsted acid catalyst is shown in Scheme 1. The reaction between dimedone and different alcohols proceeds through the formation of benzyl or alkyl cations. The solid acid catalyst protonates hydroxylic oxygen, and generates a cationic centre on the initial alcohol after dehydration. Under the present conditions, due to the resonance of oxygen with adjacent é-bonding electrons, dimedone as a nucleophile combined with the stable carbocation to produce β-ketoenol ether after the releasing H+.

The reaction data, along with some literature data for comparison, are given in Table 3. These catalysts showed good reactivity. However, the use of solvent, microwave irradiation conditions and non-recyclable or reusable catalysts in these reported systems are not beneficial to industrial and synthetic applications.

4. Conclusions
In conclusion, we have demonstrated that Cs25PW is an efficient and green catalyst for the synthesis of β-ketoenol ethers. This catalyst showed highest surface acidity and lowest solubility in reaction media in comparison with the others (Cs1PW, Cs2PW, and Cs3PW). This green procedure offer some advantages such as short reaction time, high yields, simple work-up, cost-effective recovery and the reusability of catalyst without a significant change in its activity.
Acknowledgement
The authors thank the Razi University Research Council for supporting this work.
References
1 J.K. Rajput and G. Kaur, Tetrahedron, 2010, 53, 646-649. [ Links ]
2 K.A. Rocha, N.V Rodrigues, I.V Kozhevnikov and E.V Gusevskaya, Appl. Catal. A: Gen., 2010, 374, 87-94. [ Links ]
3 R. Wang, G. Zhang and H. Zhao, Catal. Today, 2010, 149, 117-121. [ Links ]
4 T. Okuhara and T. Nakato, Catal. Surv. JPN, 1998, 2, 31-44. [ Links ]
5 T. Okuhara, H. Watanabe, T. Nishimura, K. Inumaru and M. Misono, Chem. Mater., 2000, 12, 2230-2238. [ Links ]
6 D.A. Friesen, D.B. Gibson and C.H. Langford, Chem. Commun., 1998, 543-544. [ Links ]
7 L.R. Pizzio and M.N. Blanco, Appl. Catal. A: Gen., 2003, 255, 265-277. [ Links ]
8 L. Matachowski, A. Zieba, M. Zembala and A. Drelinkiewicz, Catal. Lett., 2009, 133, 49-62. [ Links ]
9 K.M. Parida, S. Rana, S. Mallick and D. Rath, J. Colloid Interface Sci., 2010, 350, 132-139. [ Links ]
10 A.S. Dias, S. Lima, M. Pillinger and A.A. Valente, Carbohyd. Res., 2006, 341, 2946-2953. [ Links ]
11 D.J. H.Hart and C.S. Lai, Synlett, 1989, 49-51. [ Links ]
12 H.O. House and G.H. Rasmusson, J. Org. Chem, 1963, 28, 27-30. [ Links ]
13 H.E. Zimmerman and E.E. Nesterov, J. Am. Chem. Soc. , 2003, 125, 5422-5430. [ Links ]
14 J.O. Bunte, S. Rinne, C. Schafer, B. Newmann, H.G. Stammler and J. Mattaya, Tetrahedron Lett., 2003 ,44, 45-48. [ Links ]
15 G. Tanyeli and D. Ozdemirhan, Tetrahedron Lett., 2003, 44, 7311-7313. [ Links ]
16 A.S. Demir and O. Sesenoglu, Org. Lett, 2002, 4, 2021-2023. [ Links ]
17 M.S. Furness, T.P. Robison, D.J. Goldsmith and J.P. Bowen, Tetrahedron Lett, 1999, 40, 459-462. [ Links ]
18 H. Mansilla and M.M. Afonso, Synth. Commun., 2008, 38, 2607-2618. [ Links ]
19 R.S. Bhosale, S.V Bhosale, S.V Bhosale, T. Wang and P.K. Zubaidha, Tetrahedron Lett. , 2004, 45, 7187-7188. [ Links ]
20 Z.S. Cui, Z.H. Zhang and S.F. Liu, J. Chem. Res., 2006, 390 393. [ Links ]
21 B. Banerjee, S.K. Mandal and S.C. Roy, Chem. Lett., 2006, 35, 16 17. [ Links ]
22 E. Rafiee, Z. Zolfagharifar, M. Joshaghani and S. Eavani, Appl. Catal. A: Gen., 2009, 365, 287-291. [ Links ]
23 B.P.V. Lingaiah, T. Yakaiah, A.C. Shekhar, A.R. Kumar, G. Sathaiah, G.V Reddy, B. Narsaiah and P.S. Rao, Indian J. Chem., 2011, 50B, 1667-1670. [ Links ]
24 B. Das, K. Laxminarayana and B. Ravikanth, J. Mol. Catal. A: Chem. , 2007, 271, 131-133. [ Links ]
Received 16 January 2013
Revised 3 May 2013
Accepted 14 May 2013
* To whom correspondence should be addressed. E-mail: ezzat_rafiee@yahoo.com / e.rafiei@razi.ac.irtion














