Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Chemistry
versión On-line ISSN 1996-840X
versión impresa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.66 Durban ago. 2013
RESEARCH ARTICLE
Oxidized Single-Walled Carbon Nanotubes (SWCNs-COOH) as a New Catalyst for the Protection of Carbonyl Groups as Hydrazones
Maryam Kiani BorazjaniI; Hamid Reza SafaeiII; Majid PanahandehII; Ali Reza KianiI; Masoumeh KianiIII; Masoud MofarahiIV
IDepartment of Chemistry, Faculty of Basic Sciences, Bushehr Branch, Islamic Azad University, P.O. Box 7519619555, Iran
IIDepartment of Chemistry, Faculty of Basic Sciences, Shiraz Branch, Islamic Azad University, Iran
IIIDepartment of Chemistry, Payame Noor University, P.O. Box 19395-3697 Tehran, Iran
IVFaculty of Engineering, Persian Gulf University, P.O. Box 651783868, Bushehr, Iran
ABSTRACT
Nano-materials are considered as suitable heterogeneous catalysts for many organic reactions. Herein oxidized carbon nanotube (SWCNTs-COOH) has been reported as a heterogeneous catalyst, for protection of carbonyl groups as hydrazones in EtOH at 80 °C. The reactions proceed smoothly with good to excellent yields, and the SWCNTs-COOH used can be recycled.
Keywords: Carbon nanotubes, protection, catalyst, carbonyl group.
1. Introduction
The protection of carbonyl groups is an old reaction in general organic chemistry.1,2 Although several groups have reported new methods for protection of carbonyl groups as hydrazones: i) catalyzed by ZrOCl2.8H2O (10 mol%) in acetonitrile,3 ii) protection system consisting of phenyl hydrazine or 2,4-di-nitrophenylhydrazine with acidic zeolite in hexane/methanol,4 iii) Dowex polymer5 and iiii) basic alumina as heterogenic catalysts.6 In addition, protection of carbonyl groups with phenyl hydrazine or 2,4-dinitrophenylhydrazine has potential advantages. Hydrazones are useful compounds in organic chemistry; for instance, these compounds have interesting biologi-cal properties, such as anti-inflammatory, analgesic, anti-convulsant, antituberculous, antitumor, anti-HIV and anti-microbial activity.7-12 Additionally, these compounds are widely employed as ligands for metal complexes, organocatalysis and also for the syntheses of heterocyclic compounds.13-14 With this in mind, it was decided to investigate the protection of carbonyl groups with phenyl hydrazine and 2,4-dinitrophenylhydrazine using SWCNTs-COOH as catalysts.
On the other hand, materials with nano-sized tubular struc-tures including carbon and metal have been developed.15 Among the nano-structured, carbon nanotube has been a focus of extensive research due to its chemical stability, large surface area, non-toxicity, small dimensions and remarkable physical properties of these materials.16 Carbon nanotubes have shown their potential in many fields.17-19 Along with our previous research on carbon nanotubes,20-22 we now describe another application for carbon nanotubes. Although SWCNTs-COOH has been considered as a suitable heterogeneous catalyst, there are in our opinion no reports for catalytic activity of SWCNTs-COOH. Hence, in this context, we reported a mild and conve-nient procedure for protection of carbonyl groups by using SWCNTs-COOH as catalyst. The major advantages of the SWCNTs-COOH as catalyst are: (1) ease of separation from reaction mixture by filtration; and (2) catalyst recycling.
2. Experimental
The protection of carbonyl compounds schematically is shown in Scheme 1.
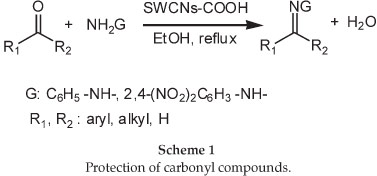
Protection of carbonyl compounds: A mixture of oxidized nano carbontube (SWCNT-COOH) (1 g),21 reagent (hydrazine derivatives) (1 mmol), carbonyl compound (1 mmol) and ethanol (5 ml) were mixed in a mortar. The reaction mixture was then poured into a round-bottomed glass equipped with a condenser and heated with stirring in an oil bath at 80 °C for a specified time (Table 3). The progress of the reaction was monitored by TLC using n-hexane/ethyl acetate. After the reaction was completed, EtOAc (2 x 20 mL) was added to the reaction mixture. Later, the resulted mixture was filtered by using a 0.45 µm polytetra-fluoroethylene filter (Millipore PTFE) to separate the SWCNT-COOH. After filtration, solvent was removed under reduced pressure to afford the respective products. NMR, IR spectra confirmed the structure of the products that compared with authentic samples obtained commercially or prepared by the reported methods. Most of the compounds are known and their analytical data were previously reported in the literature.
Recovery of SWCNTs-COOH: The SWCNTs-COOH on the filter, obtained after the filtration described in the previous section, was washed with EtOH 2-3 times to complete removal of organic residues, dried in an oven at 100-120 °C for 10-11 h and then the catalyst was re-used for the same reaction. The mass of the catalyst was checked over five cycles and it was found that the mass of SWCNT-COOH did not significantly change due to efficient filtration by using the 0.45 µm polytetrafluoroethylene filter (Millipore PTFE).
3. Results and Discussion
As shown in to Scheme 1, a systematic study of the reaction between carbonyl compounds and hydrazine derivatives was studied by using SWCNTs-COOH as catalyst in EtOH and 80 °C. The feasibility of the present protection of carbonyl compounds was first examined by using benzaldehyde as a model substrate. Hence, benzaldehyde was treated with 2,4-dinitrophenyl-hydrazine under various conditions (Table 1). As it can be seen in Table 1, the best results were achieved with SWCNTs-COOH in EtOH and 80 °C (entry 5). In entry 2, no significant product formation was observed without catalysts after 240 minutes at 80 °C.
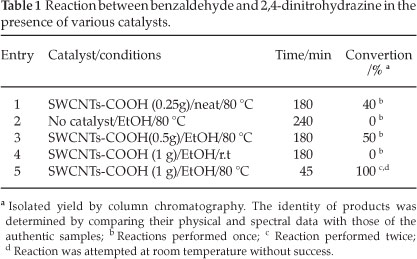
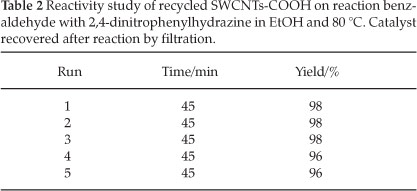
The reusability of the catalyst was checked over five cycles. As shown in Table 2, the yields of protection of carbonyl in the second, third, fourth and fifth uses of the catalyst were almost the same as that in the first use. It seems that the reduction in yield is due to a recovery error.
To establish the scope of SWCNTs-COOH as a catalyst for the protection of carbonyl compounds, structurally diverse car-bonyl compounds were treated with phenyl hydrazine and 2,4-dinitrophenyl hydrazine. The results are summarized in Table 3. As shown in Table 3, a wide range of aldehydes including either electron-donating or electron-withdrawing substituent with hydrazine derivatives were employed (entries 1-17). The protection of carbonyl compound by hydrazine derivatives should be a facile reaction due to the good electrophilic (carbonyl compound) and good nucleophilic (hydrazine derivatives) properties. The presence of electronic factors in the reactivity was observed. The presence of the methyl group in 4-methyl-benzaldehyde reduces the electrophilicity of the carbonyl carbon and the strong electron-withdrawing properties of the nitro group in 2,4-nitrophenylhydrazine decreases the nucleo-philicity of the amine group. Entry 5 is the best due to the good electrophile (aldehyde) and good nucleophile (hydrazine). In all cases, phenyl hydrazine, being more nucleophilic than 2,4-nitro-phenylhydrazine appears to require shorter reaction times and gives better yields.
Substrates such as furfural and cinnamaldehyde, being acid-sensitive substrates, were efficiently protected as the corre-sponding phenyl hydrazine and 2,4-dinitrophenylhydrazine derivatives (Table 3, entries 14-17). As is shown in Table 3, the two hydrazone derivatives were produced in very good yields.
It can also be emphasized that the reactions are very clean, the SWCNTs-COOH is recycled without a decrease in efficiency, and from the economic and environmental points of view the use of SWCNTs-COOH is an improvement.
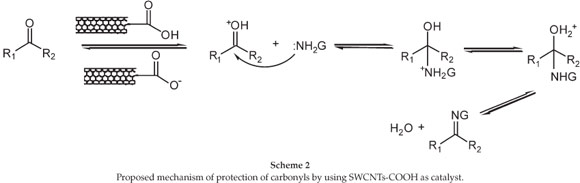
In terms of a proposed mechanism for the reaction, it is known that mildly acidic conditions (pH = 5-6) provide the best condi-tions for this reaction. Since the pH of SWCNTs-COOH is ≈5,21 it would be suitable for a carbonyl to hydrazone transformation. A tentative mechanism showing the function of the SWCNTs-COOH used in our study is presented in Scheme 2.
4. Conclusions
In summary, it is shown that SWCNTs-COOH is a highly active catalyst for the protection of carbonyl groups of aldehydes in good to excellent yields. Among the advantages of the method that can be mentioned are: (i) ease of separation catalyst from reaction mixture by filtration and (ii) catalyst recycling.
Acknowledgements
Support from the Persian Gulf University is gratefully acknowledged. The authors acknowledge the Persian Gulf University Research Council for support of this work.
References
1 T.W. Greene and Wuts, P.G.M. Protective Groups in Organic Synthesis; John Wiley and Sons, New York, 1991, pp. 188-191. [ Links ]
2 P.J. Kocienski, Protecting Groups, Thieme, Stuttgart, 1994, 156-170. [ Links ]
3 VK. Das, Das, S. and A.J. Thakur, Green Chem., 2012, 5, 577. [ Links ]
4 A. Lalitha, Pitchumani, K. and C. Srinivasan, Green Chem., 1999,173. [ Links ]
5 K. Niknam, A.R. Kiasat and S. Karimi, Synth. Commun., 2005,35,2231. [ Links ]
6 K. Niknam, A.R. Kiasat, B. Karimi and N. Heydari, Turk. J. Chem, 2007, 31, 135. [ Links ]
7 S. Rollas and S.G. Kucukguzel, Molecules, 2007,12, 1910. [ Links ]
8 F. Chimenti, E. Maccioni, D. Secci, A. Bolasco, P. Chimenti, A. Granese, O. Befani, P. Turini, S. Alcaro, F. Ortuso, M.C. Cardia and S. Distinto, J. Med. Chem, 2007, 50, 707. [ Links ]
9 J. Mao, Y. Wang, B. Wan, A.P. Kozikowski and S.G. Franzblau, Chem. Med. Chem, 2007, 2, 1624. [ Links ]
10 A. Andreani, S. Burnelli, M. Granaiola, A. Leoni, A. Locatelli, R. Morigi, M. Rambaldi, L. Varoli, N. Calonghi, C. Cappadone, G. Farruggia, M. Zini, C. Stefanelli, L. Masotti, N.S.Radin and R.H. Shoemaker, J. Med. Chem, 2008, 51, 809. [ Links ]
11 E. Noulsri, R.Richardson, S. Lerdwana, S. Fucharoen, T. Yamagishi, D.S. Kalinowski and K. Pattanapanyasat, Am. J. Hematol., 2009, 84, 170. [ Links ]
12 P. Vicini, M. Incerti, I.A. Doytchinova, P. Colla, B. Busonera and R. Loddo, Eur. J. Med. Chem., 2006,41, 624. [ Links ]
13 P. Barbazan, R. Carballo, B. Covelo, C. Lodeiro J.C. Lima and E.M. Vazquez-Lopez, Eur. J. Inorg. Chem., 2008, 2713. [ Links ]
14 S. Banerjee, S. Mondal, W. Chakraborty, S. Sen R. Gachhui, R.J. Butcher, A.M.Z. Slawin, C. Mandal and S. Mitra, Polyhedron, 2009,28, 2785. [ Links ]
15 Y. Zhu, H. Li and Y. Koltypin, Chem. Commun., 2001, 2616. [ Links ]
16 W. Zhou, X. Bai, E. Wang and S. Xie, Adv. Mater., 2009, 21, 4565. [ Links ]
17 B.A. Kakade and VK. Pillai, Appl. Surf. Sci, 2008, 254, 4936. [ Links ]
18 C.D. Doyle and J.M. Tour, Carbon, 2009, 47, 3215. [ Links ]
19 S. Banerjee, T. Hemraj-Bennyand S.S. Wong,Adv. Mater., 2005,17,17. [ Links ]
20 A. Khazaei, M.N.Soltani Rad, M. Kiani Borazjani, Sh. Saednia, M. Kiani Borazjani and D. Soudbar, Synlett., 2011, 2145. [ Links ]
21 A. Khazaei, M.N. Soltani Rad and M. Kiani Borazjani, Int. J. Nano-medicine, 2010, 5, 639. [ Links ]
22 A. Khazaei, M.Kiani Borazjani and Kh. Mansouri Moradian, Chem. Sci, 2012,124, 1127 . [ Links ]
23 B.S. Furniss, A.J. Hannaford, P.WG. Smith and A.R. Tatchell, Vogel's Textbook of Practical Organic Chemistry, 5th edn., John Wiley & Sons, New York, 1989, p. 1332. [ Links ]
24 http://www.ChemicalBook.com
 Correspondence:
Correspondence:
E-mail: maryamkiani.b@gmail.com
Received 15 February 2013
Revised 28 May 2013
Accepted 8 September 2013














