Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Chemistry
versión On-line ISSN 1996-840X
versión impresa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.66 Durban ago. 2013
RESEARCH ARTICLE
Synthesis, Characterization and Antibacterial Activity of New 1,2- and 1,4-Bis(N' -Substituted Thioureido)benzene Derivatives
Aamer SaeedI; Naeem AbbasI; Zaman AshrafII; Michael BolteIII
IDepartment of Chemistry, Quaid-I-Azam University, Islamabad 45320, Pakistan
IIDepartment of Chemistry, Allama Iqbal Open University, Islamabad, Pakistan
IIIInstitutfür Anorganische Chemie, J.W.-Goethe-Universitat, Max-von-Laue-Str. 7, D-60438 Frankfurt/Main, Germany
ABSTRACT
Synthesis of two series of 1,2- and 1,4-bis(thioureido)benzene derivatives was accomplished by the treatment of corresponding alkanoyl/aroyl chlorides with potassium thiocyanate in dry acetone to afford the respective isothiocyanates as intermediates. The latter were treated in situ with 1,2- and 1,4-diaminobenzene, respectively, to afford the title compounds in high yields. A total of sixteen new compounds are reported herein. The structures of the products were confirmed by spectroscopic techniques (IR, 1H and 13C NMR, mass spectrometry), elemental analysis and in case of 1d, by X-ray diffraction technique. All the synthesized compounds were also subjected to antibacterial bioevaluation against ten different Gram-positive and Gram-negative bacterial strains using levofloxacin as the standard drug and were shown to possess promising activities.
Keywords: Bis(thioureido)benzene, antibacterial activity, crystal structure.
1. Introduction
Thioureas are leading precursors for the synthesis of several heterocyclic systems, covering the whole field of pharmacy along with other industrial applications.1,2 Their pharmaceutical importance was demonstrated from literature as Hedgehog Inhibiting Activity3 inhibitors of trans-membrane-anchored carbonic anhydrase,4 and antifungal and antibacterial agents.5 Optically active thioureas and their oxidative cyclization benzothiazol product derivatives were shown to be antitumor agents.6 The thiourea-derived drug isoxyl is clinically used against tuberculosis. Phetsuksiri et. al. have studied the mecha-nism of action of this drug on Mycobacterium tuberculosis7 The catalytic activity of thiourea in the Morita-Baylis-Hillman reac-tion was also studied8 and further extended to bis(thiourea) catalysis.9 Tautomeric equilibrium of iminothiol/thiourea deriva-tives was studied along with their DNA interaction.10
Cyclic voltammetric data of bis(substituted thiourea)ferrocene derivatives was collected and reported.11 Metal complexes of bis- and tris-thioureas as non-linear optical material have been investigated by the Raman spectroscopy.12 Bis-urea and thiourea containing azo-moieties were shown to exhibit solution phase anion sensing and binding properties.13 Bis-(aryl)thioureas were found to be potent and selective inhibitors of te cytomegalovirus (CMV) in cultured HFF cells.14 Thiourea derivatives were used as phase change materials for thermal energy storage15 and 1,1'-(naphthalene-1,8-diyl)-3,3'-dibenzoyl-bisthiourea as an anion-binding receptor.16 In view of the aforementioned biological and synthetic significance and our previous interest17-9 in various aspects of the chemistry of thioureas, the aim of the present work was the synthesis of new bis-thiourea deriva-tives, their conversion into various heterocycles and detailed bioevaluation.
2. Results and Discussion
Freshly dried acetone was used as a solvent for the preparation of a clear solution potassium thiocyanate to which dropwise addition of a suitable alkanoyl/aroyl chloride was carried out. The corresponding alkanoyl/aroyl isothiocyantes intermediates thus obtained in situ and were treated with 1,2- or 1,4-diamino-benzene, respectively, to afford the corresponding series of 1,2-and 1,4-bis(thioureido)benzenes (Scheme 1). All the synthesized compounds were purified by recrystallization from aqueous ethanol and characterized by spectroscopic techniques and in one case by the X-ray diffraction technique.
Considering compound 1c as a typical 1,2-bis(thioureido)ben-zene derivative, the structure was supported by FTIR spectros-copy with appearance of absorption bands at 3251,3149 cm-1 for free and associated NH groups along with absorption bands at 1687 cm-1 for carbonyl carbon and stretching absorption for the thiocarbonyl carbon at 1255 cm-1. 1H NMR indicated the presence of two NH as broad singlets at 12.38 and 10.35 ppm while aromatic protons appeared in range of 7.99-7.33 ppm while alkyl protons observed in range of 2.56-0.92 ppm.
In the 13C NMR spectrum the thiocarbonyl and carbonyl group appeared at 180.5 and 174.9 ppm, respectively, the aromatic carbons at 133.4, 126.8 126.3 ppm and the alkyl carbons at 36.2, 31.0,24.3, 22.1 and 13.3 ppm. In GCMS the base peak appeared at 99 a.m.u. due to the benzoyl cation.
The structure of 1d was unequivocally confirmed by single crystal X-ray analysis (Fig. 1). Single crystals suitable for X-ray diffraction studies were obtained by slow evaporation of ethanol.
The conformation of the 1d is stabilized by two intramolecular N-H...O hydrogen bonds. Two methylene chains adopt an all-trans conformation.
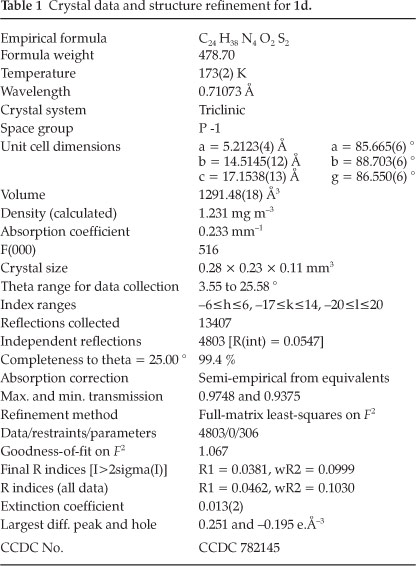
Figure 2 shows the packing diagram with a view of the bc-plane. The molecules are connected by N-H... S hydrogen bonds to zigzag chains running along [1-1 0]. The details of the structure determination are compiled in Table 1.
Considering compound 1k as a typical 1,4-bis(thioureido)ben-zene, the appearance of IR stretches at 3258 cm-1 and 3134 cm-1 for free and associated NH groups, at 1693 cm-1 for the carbonyl group and the thiocarbonyl group at 1251 cm-1 were observed. 1H NMR indicated broad singlets at 12.56 and 11.47 ppm for the two NH groups while magnetically equivalent aromatic protons appeared as a single peak at 7.67 ppm and alkyl protons observed in the range of 2.47-0.87 ppm. In 13C NMR spectrum the thiocarbonyl carbon appeared at 179.2 ppm and the carbonyl carbon at 176.0 ppm, while the aromatic carbons were observed at 135.9 and 124.7 ppm and the alkyl carbons in the range of 36.1-14.2 ppm.
2.1. Antibacterial Activity
Table 2 shows the antibacterial activity data of all synthesized compounds against ten different bacterial strains. The activity of the synthesized compounds is presented in zone of inhibition of bacterial growth in mm (Table 2). The % zone inhibition is based on the activity of the reference drug (see the experimental section for the exact formula). Among all of the tested com-pounds, 1c showed excellentactivity against Bacillussubtilis with maximum zone of inhibition of 95 % and 76 % against Staphylococ-cus aureus and Pseudomonas putida. Compound 1g exhibited max-imum inhibition against Bacillus subtilis of 90 %. Compound 2f exhibited good activityagainstanumberofbacterialstrainswith zones of inhibition: 83 %, 80 %, 73 % and 64 % against Shigella flexineri, Escherichia coli, Pasteurella multocida and Klebsiella pneumonae, respectively. Similarly compound 2g showed good activity against Escherichia coli (80 %), Shigella flexineri (70 %) and Pasteurella multocida (66 %). Compound 1 was found to be active against Staphylococcus aureus, Pasteurella multocida and Pseudomonas aeruginosa with zone inhibition of 84 %, 66 % and 53 %, respec-tively. 1,4-Bis(N'-substituted thioureido)benzene derivatives (2a, 2b, 2c and 2e) showed very poor or no zone of inhibition. The results of antibacterial bioscreening indicate that activities depend on the length of the hydrocarbon side chain (lipo/ hydrophilicity) in case of aliphatic derivatives and on the elec-tron-donating or withdrawing nature and position of substituent in case of the aromatic compounds.
3. Experimental
Melting points of all synthesized compounds were determined in an open capillary using a digital Gallenkamp (SANYO) model MPD BM 3.5 apparatus and are uncorrected. 1H NMR and 13C NMR spectra were determined with a 300 MHz Bruker AM-300 spectrophotometer. FTIR spectra were measured with a FTS 3000 MX spectrophotometer and mass spectra (EI, 70 eV) with a GC-MS instrument (Agilent Technologies 1200 series USA).
3.1. Crystal Structure Determination of 1d
X-ray data were collected on a STOE IPDS-II diffractometer with graphite-monochromated MoKG radiation. An empiri-cal absorption correction with the PLATON program20 was performed. The structure was solved by direct methods and refined with full-matrix least-squares on F2 using the program SHELXL97.21 H-atoms bonded to carbon atoms were placed on ideal positions and refined with fixed isotropic displacement parameters using a riding model. H-atoms bonded to N were freely refined.
3.2. General Procedure for Synthesis of Bis(AT-substituted Thioureido)benzenes
To a solution of KSCN (1.0 mmol) in dry acetone (50 mL) was dropwiseaddedalkanoyl/aroylchloride(1.0mmol)withvigorous stirring during 30 min. A solution of 1,2-phenylene diamine/ 1,4-phenylene diamine (0.5 mmol) in dry acetone (60 mL) was dropwise added and the reaction mixture was refluxed for 2-4 h and then poured onto ice-cold water. Filtration of solids obtained followed by washing with cold water and crystalliza tion from ethanol afforded the title compounds. A total of 16 new compounds are reported.
1,2-Bis(N'-butanoylthioureido)benzene (1a)
Yield: 68 %, Rf: 0.67 (Solvent for Rf petroleum ether: Ethyl acetate, 1:1), m.p. 209-210 °C, IR: 3256 (N-H), 3139 (N-H), 2956 (CH), 1697 (C = O), 1269 (C=S) cm-1.1H NMR (300 MHz, CDCl3): d 12.26 (s, 2H, CO-NH-CS), 11.51 (s, 2H, CS-NH-C), 7.84-7.81 (m, 2H, ArH), 7.35-7.32 (m, 2H, ArH), 2.42 (t, 4H, J = 7.1 Hz, 2CH2), 1.60-1.53 (m, 4H, 2CH2), 0.91-0.87 (t, 6H, J = 6.9 Hz, 2CH3), 13C NMR (75 MHz, CDCl3): d 180.6,175.5,133.6,127.4,127.0,37.9, 18.2,13.8. EIMS m/z (%): 366 [M+] (14), 71 (100 %), 87 (50). Anal. calcd. for C16H22N4O2S2: C, 52.43, H, 6.05, N, 15.29, S, 17.50 %, found: C, 52.31, H, 6.19, N, 15.41, S, 17.62 %.
1,2-Bis(N'-pentanoyl thioureido)benzene (1b)
Yield: 72 %, Rf: 0.74 (Solvent for Rf petroleum ether: Ethyl acetate, 1:1), m.p. 178-180 °C, IR: 3244 (N-H), 3140 (N-H), 2958 (CH), 1690 (C=O), 1257 (C=S) cm-1.1H NMR (300 MHz, CDCl3): d 12.02 (s, 2H, CO-NH-CS),11.49 (s, 2H, CS-NH-C), 7.85-7.51 (m, 2H, ArH), 7.32-7.16 (m, 2H, ArH), 2.42 (t, 4H, J = 7.0 Hz, 2CH2), 1.66-1.42 (m, 8H, 4CH2), 0.92-0.87 (t, 6H, J = 6.9 Hz, 2CH3), 13C NMR (75 MHz, CDCl3): d 180.5,175.6,130.6,128.3,126.1,37.4, 18.2,15.6,13.8. EIMS m/z (%): 394 [M+] (17), 85 (100 %), 101 (49). Anal. calcd. for C18H26N4O2S2: C, 54.79, H, 6.64, N, 14.20, S, 16.25 %. found: C, 54.12, H, 6.87, N, 14.42, S, 16.10 %.
1,2-Bis(N'-hexanoyl thioureido)benzene (1c)
Yield: 64 %, Rf: 0.76 (Solvent for Rf petroleum ether: Ethyl acetate, 1:1), m.p. 138-140 °C, IR: 3251 (N-H), 3149 (N-H), 2955 (CH), 1687 (C=O), 1255 (C=S) cm-1.1H NMR (300 MHz, CDCl3): d 12.38 (s, 2H, CO-NH-CS), 10.35 (s, 2H, CS-NH-C), 7.99-7.96 (m, 2H, ArH), 7.37-7.33 (m, 2H, ArH), 2.56 (t, 4H, J = 7.2,2CH2), 1.751.65 (m, 4H, 2CH2), 1.42-1.34 (m, 8H, 4CH2), 0.92 (t, J = 6.8, 6H, 2CH3), 13C NMR (75 MHz, CDCl3): d 180.5, 174.9, 133.4, 126.8, 126.3, 36.2, 31.0, 24.38, 22.1,13.3. EIMS m/z (%): 422 [M+] (16), 99 (100 %), 115 (32). Anal. Calcd. for C20H30N4O2S2: C, 56.84, H, 7.16, N, 13.26,S, 15.17 %. found: C, 56.42, H, 7.01, N, 13.47,S, 15.32 %.
1,2-Bis(N'-octanoyl thioureido)benzene (1d)
Yield: 61 %, Rf: 0.79 (Solvent for Rf petroleum ether: Ethyl acetate, 1:1), m.p. 137-138 °C, IR: 3268 (N-H), 3167 (N-H), 2959 (CH), 1695 (C=O), 1262 (C=S) cm-1.1H NMR (300 MHz, CDCl3): d 12.26 (s, 2H, CO-NH-CS),11.50 (s, 2H, CS-NH-C), 7.86-7.83 (m, 2H, ArH), 7.36-7.31 (m, 2H, ArH), 2.51 (t, 4H, J = 7.5 Hz, 2CH2), 1.56-1.51 (m, 4H, 2CH2), 1.39-1.20 (m, 16H, 8CH2), 0.86 (t, J = 6.5 Hz, 6H, 2CH3), 13C NMR (75 MHz, CDCl3): d 180.6,175.6, 133.5,127.3, 126.9, 36.1, 31.3, 30.1, 28.9, 24.8, 22.5, 14.4. EIMS m/z (%): 478 [M+] (10), 127 (100 %), 143 (35). Anal. Calcd. for C24H38N4O2S2: C, 60.21, H, 8.00, N, 11.70, S, 13.40 %. found: C, 60.31, H, 8.36, N, 11.88, S, 13.66 %.
1,2-Bis(N'-benzoyl thioureido)benzene (1e)
Yield: 69 %, Rf: 0.68 (Solvent for Rf petroleum ether: Ethyl acetate, 1:1), m.p. 156-158 °C, IR: 3234 (N-H), 3142 (N-H), 1672 (C=O), 1244 (C=S) cm-1.1H NMR (300 MHz, CDCl3): d 12.63 (s, 2H, CO-NH-CS), 10.48 (s, 2H, CS-NH-C), 8.06-8.00 (m, 6H, ArH), 7.70-7.65 (m, 2H, ArH), 7.56-7.51 (m, 4H, ArH), 7.44-7.41 (m, 2H, ArH),13C NMR (75 MHz, CDCl3): d 180.5,167.9,133.6,133.3,132.2, 128.6,128.3,127.2,126.5. EIMS m/z (%): 434 [M+] (15), 105 (100 %), 121 (40). Anal. Calcd. for C22H18N4O2S2: C, 60.81, H, 4.18, N, 12.89, S, 14.76 %. found: C, 60.45, H, 4.26, N, 12.77, S, 14.69 %.
1,2-Bis(N'-(2-chlorobenzoyl) thioureido)benzene (1f)
Yield: 72 %, Rf: 0.58 (Solvent for Rf petroleum ether: Ethyl acetate, 1:1), m.p. 200-202 °C, IR: 3250 (N-H), 3142 (N-H), 1670 (C=O), 1240 (C=S) cm-1.1H NMR (300 MHz, CDCl3): d 12.22 (s, 2H, CO-NH-CS), 12.14 (s, 2H, CS-NH-C), 7.95-7.92 (m, 2H, ArH), 7.56-7.49 (m, 6H, ArH), 7.42-7.37 (m, 4H, ArH), 13C NMR (75 MHz, CDCl3): d 180.4, 168.1, 134.7, 133.7, 132.6, 130.5, 130.0, 129.6,127.6,127.4,127.1.EIMS m/z(%): 503 [M+] (10), 138 (100 %), 155 (27). Anal. Calcd. for C22H16Cl2N4O2S2: C, 52.49, H, 3.20, N, 11.13, S, 12.74 %. found: C, 52.20, H, 3.11, N, 11.09, S, 12.88 %.
1,2-Bis(N'-(4-chlorobenzoyl)thioureido)benzene (1g)
Yield: 66 %, Rf: 0.71 (Solvent for Rf petroleum ether: Ethyl acetate, 1:1), m.p. 210-212 °C, IR: 3230 (N-H), 3152 (N-H), 1670 (C=O), 1251 (C=S) cm-1.1H NMR (300 MHz, CDCl3): d 12.32 (s, 2H, CO-NH-CS), 12.11 (s, 2H, CS-NH-C), 7.87-7.80 (m, 2H, ArH), 7.62-7.54 (m, 6H, ArH), 7.42-7.36 (m, 4H, ArH), 13C NMR (75 MHz, CDCl3): d 178.5, 168.1, 134.8, 133.7, 131.5, 130.9, 130.2, 128.6, 127.1. EIMS m/z (%): 503 [M+] (11), 138 (100 %), 155 (29). Anal. Calcd. for C22H16Cl2N4O2S2: C, 52.49, H, 3.20, N, 11.13, S, 12.74 %. found: C, 52.67, H, 3.29, N, 11.25, S, 12.45 %.
1,2-Bis(N'-(4-nitrobenzoyl)thioureido)benzene (1h)
Yield: 58 %, Rf: 0.54 (Solvent for Rf petroleum ether: Ethyl acetate, 1:1), m.p. 179-181 °C, IR: 3260 (N-H), 3148 (N-H), 1669 (C=O), 1254 (C=S) cm-1.1H NMR (300 MHz, CDCl3): d 12.30 (s, 2H, CO-NH-CS),11.24 (s,2H, CS-NH-C), 7.94-7.83 (m,4H,ArH), 7.55-7.49 (m, 4H, ArH), 7.37-7.31 (m, 4H, ArH), 13C NMR (75 MHz, CDCl3): d 180.6, 168.3, 139.7, 138.8, 133.9, 130.5, 129.7, 124.6, 123.4. EIMS m/z (%): 524 [M+] (11), 150 (100 %), 166 (24). Anal. Calcd. for C22H16N6O6S2: C, 50.38, H, 3.07, N, 16.02, S, 12.23 %. found: C, 50.48, H, 3.19, N, 16.29, S, 12.34 %.
1,4-Bis(N'-butanoyl thioureido)benzene (2a)
Yield: 75 %, Rf: 0.52 (Solvent for Rf petroleum ether: Ethyl acetate, 1:1), m.p. 180-181 °C, IR: 3261 (N-H), 3151 (N-H), 2959 (CH), 1691 (c=O), 1250 (C=S) cm-1.1H NMR (300 MHz, CDCl3): d 12.39 (s, 2H, CO-NH-CS), 11.01 (s, 2H, CS-NH-C), 7.59 (s, 4H, ArH), 2.36 (t, 4H, J= 6.99 Hz, 2CH2), 1.52-1.50 (m, 4H, 2CH2), 0.90-0.79 (t, 6H, J = 6.9 Hz, 2CH3), 13C NMR (75 MHz, CDCl3): d 179.8,176.6,134.4,124.4,38.6,18.8,13.1. EIMS m/z (%): 366 [M+] (15), 71 (100 %), 87(55). Anal. calcd. for C16H22N4O2S2: C, 52.43, H, 6.05, N, 15.29, S, 17.50 %. found: C, 52.29, H, 6.21, N, 15.01, S, 17.41 %.
1,4-Bis(N'-pentanoyl thioureido)benzene (2b)
Yield: 71 %, Rf: 0.77 (Solvent for Rf petroleum ether: Ethyl acetate, 1:1), m.p. 215-217 °C, IR: 3254 (N-H), 3164 (N-H), 2953 (CH), 1677 (C=O), 1261 (C=S) cm-1.1H NMR (300 MHz, CDCl3): d 11.98 (s, 2H, CO-NH-CS), 11.36 (s, 2H, CS-NH-C), 7.92 (s, 4H, ArH), 2.56 (t, 4H, J = 7.0 Hz, 2CH2), 1.74-1.39 (m, 8H, 4CH2), 0.93-0.87 (t, 6H, J = 6.9 Hz, 2CH3), 13C NMR (75 MHz, CDCl3): d 180.4, 175.4, 134.6,123.1, 37.5, 18.4, 15.9, 12.2. EIMS m/z (%): 394 [M+] (20), 85 (100 %), 101 (41). Anal. calcd. for C18H26N4O2S2:C, 54.79, H, 6.64, N, 14.20, S, 16.25 %. found: C, 54.42, H, 6.96, N, 14.31, S, 16.36 %.
1,4-Bis(N'-hexanoyl thioureido)benzene (2c)
Yield: 74 %, Rf: 0.79 (Solvent for Rf petroleum ether: Ethyl acetate, 1:1), m.p. 168-169 °C, IR: 3258 (N-H), 3134 (N-H), 2938 (CH), 1693 (C=O), 1251 (C=S) cm-1.1H NMR (300 MHz, CDCl3): d 12.56 (s, 2H, CO-NH-CS), 11.47 (s, 2H, CS-NH-C), 7.67 (s, 4H, ArH), 2.47 (t, 4H, J = 7.5, 2CH2), 1.59-1.54 (m, 4H, 2CH2), 1.29-1.27 (m, 8H, 4CH2), 0.87 (t, 6H, J= 6.3, 2CH3), 13C NMR (75 MHz, CDCl3): d 179.2,176.0,135.9,124.7,36.1,31.1,24.4,22.3, 14.2. EIMS m/z (%): 422 [M+] (15), 99 (100 %), 115 (35). Anal.Calcd. for C20H30N4O2S2: C, 56.84, H, 7.16, N, 13.26, S, 15.17 %. found: C, 56.57, H, 7.09, N, 13.11, S, 15.14 %.
1,4-Bis(N'-octanoyl thioureido)benzene (2d)
Yield: 69 %, Rf: 0.80 (Solvent for Rf petroleum ether: Ethyl acetate, 1:1), m.p. 170-172 °C, IR: 3242 (N-H), 3155 (N-H), 2959 (CH), 1686 (C=O), 1261 (C=S) cm-1.1H NMR (300 MHz, CDCl3): d 12.56 (s, 2H, CO-NH-CS), 11.46 (s, 2H, CS-NH-C), 7.66 (s, 4H, ArH), 2.45 (t, 4H, J = 7.5, 2CH2), 1.58-1.53 (m, 4H, 2CH2), 1.31-1.25 (m, 16H, 8CH2), 0.86 (t, J = 6.4, 6H, 2CH3), 13C NMR (75 MHz, CDCl3): d 179.1,176.0,135.9,124.7,36.2,31.5,28.8,24.7, 22.5,21.1,14.4. EIMS m/z (%): 478 [M+] (11), 127 (100 %), 143 (37). Anal. Calcd. for C24H38N4O2S2: C, 60.21, H, 8.00, N, 11.70, S, 13.40 %. found: C, 60.33, H, 8.24, N, 11.69, S, 13.12 %.
1,4-Bis(N'-benzoyl thioureido)benzene (2e)
Yield: 70 %, Rf: 0.57 (Solvent for Rf petroleum ether: Ethyl acetate, 1:1), m.p. 223-225 °C, IR: 3235 (N-H), 3167 (N-H), 1655 (C=O), 1261 (C=S) cm-1.1H NMR (300 MHz, CDCl3): d 12.59 (s, 2H, CO-NH-CS), 10.88 (s, 2H, CS-NH-C), 7.99-7.78 (m, 6H, ArH), 7.67 (s, 4H, ArH), 7.38-7.21 (m, 4H, ArH), 13C NMR (75 MHz, CDCl3): d 178.3,166.1,134.5,131.2,129.8,127.2,125.6,123.7. EIMS m/z (%): 434 [M+] (14), 105 (100 %), 121 (46). Anal. Calcd. for C22H18N4O2S2: C, 60.81, H, 4.18, N, 12.89, S, 14.76 %. found: C, 60.69, H, 4.37, N, 12.50, S, 14.91 %.
1,4-Bis(N'-(2-chloro benzoyl) thioureido)benzene (2f)
Yield: 64 %, Rf: 0.69 (Solvent for Rf petroleum ether: Ethyl acetate, 1:1), m.p. 175-177 °C, IR: 3230 (N-H), 3136 (N-H), 1667 (C=O), 1257 (C=S) cm-1.1H NMR (300 MHz, CDCl3): d 12.22 (s, 2H, CO-NH-CS), 12.14 (s, 2H, CS-NH-C), 7.84-7.79 (m, 2H, ArH), 7.74 (m, 4H, ArH), 7.67 (s, 4H, ArH), 7.33-7.28 (m, 2H, ArH), 13CNMR (75 MHz, CDCl3): d 181.6,166.4,134.5,133.4,131.8,130.2, 128.6,126.6,125.9,124.0. EIMS m/z (%): 503 [M+] (14), 138 (100 %), 155 (20). Anal. Calcd. for C22H16Cl2N4O2S2: C, 52.49, H, 3.20, N, 11.13, S, 12.74 %. found: C, 52.25, H, 3.40, N, 11.21, S, 12.91 %. 1,4-Bis(N'-(4-chloro benzoyl) thioureido)benzene (2g)
Yield: 67 %, Rf: 0.71 (Solvent for Rf petroleum ether: Ethyl acetate, 1:1), m.p. 230-232 °C, IR: 3257 (N-H), 3142 (N-H), 1665 (C=O), 1249 (C=S) cm-1.1H NMR (300 MHz, CDCl3): d 12.02 (s, 2H, CO-NH-CS), 11.31 (s, 2H, CS-NH-C), 7.84 (d, 4H, J = 7.5 Hz, ArH), 7.61 (s, 4H, ArH), 7.50 (d, 4H, J=7.5 Hz, ArH), 13C NMR (75 MHz, CDCl3): d 179.6, 168.1, 133.7, 131.7, 130.5, 132.9, 127.0, 124.1. EIMS m/z (%): 503 [M+] (13), 138 (100 %), 155 (21). Anal. Calcd. for C22H16Cl2N4O2S2: C, 52.49, H, 3.20, N, 11.13, S, 12.74 %. found: C, 52.39, H, 3.07, N, 11.00, S, 12.51 %.
1,4-Bis(N'-(4-nitro benzoyl) thioureido)benzene (2h)
Yield: 61 %, Rf: 0.68 (Solvent for Rf petroleum ether: Ethyl acetate, 1:1), m.p. 234-236 °C, IR: 3242 (N-H), 3149 (N-H), 1658 (C = O), 1241 (C=S) cm-1.1H NMR (300 MHz, CDCl3): d 12.25 (s, 2H, CO-NH-CS), 11.45 (s, 2H, CS-NH-C), 7.94 (d, 4H, J = 7.6 Hz, ArH), 7.65 (s, 4H, ArH), 7.56 (d, 4H, J = 7.5 Hz, ArH), 13C NMR (75 MHz, CDCl3): d 179.9, 166.4, 139.8, 138.0, 133.5, 131.5, 128.9, 124.6. EIMS m/z (%): 524 [M+] (14), 150 (100 %), 166 (17) Anal. Calcd. for C22H16N6O6S2: C, 50.38, H, 3.07, N, 16.02, S, 12.23 %. found: C, 50.59, H, 3.21, N, 16.21, S, 12.12 %.
3.3. Pharmacological Assays
In vitro evaluation of antibacterial activity of the compounds was carried out by agar well diffusion assay20 against ten differ-ent Gram-positive and Gram-negative bacteria (Pasteurella multocida (P.m.), Bacillus subtilis (B.s.), Escherichia coli (E.c.), Staph-ylococcus aureus (S.a.), Pseudomonas putida (P.p.), Pseudomonas aeruginosa (P.a.), Salmonella typhi (S.t.), Micrococcus luteus (M.l.),
Shigella flexineri (S.f.) and Klebsiella pneumoniae (K.p.)). Antibacterial activity was determined by using the Mueller Hinton Agar (MHA).n The fresh inoculums of these bacteria were prepared and diluted by sterilized normal saline. The turbidity of these cultures was adjusted by using 0.5 Mc-Farland. A homogeneous bacterial lawn was developed by sterile cotton swabs. The inoculated plates were bored by 6 mm sized borer to make the wells. The sample dilutions were prepared by dissolving each sample (1.0 mg) in 1.0 mL of DMSO used as negative control in this bioassay. The equimolar concentration of Levofloxacin (1.0 mg mL-1), a broad spectrum antibiotic (positive control) was prepared. These plates were incubated at 37 °C for 24 hours. Antibacterial activity of the compounds was determined by measuring the diameter of zone of inhibition (mm, ± standard deviation) and presented by subtracting the activity of the negative control in Table 2. The % zone inhibition is therefore defined as:

4. Conclusion
Two novel series of 1,2- and 1,4-bis(N'-substituted thioureido) benzenes (a total of 16 new compounds) were synthesized by treatment with 1,2- and 1,4-diaminobenzene with respective isothiocyanates produced in situ. The X-ray single crystal diffrac-tion analysis of compound 1d shows that the conformation is stabilized by two intramolecular N-H...O hydrogen bonds and the two methylene chains adopt an all-trans conformation. The results of antibacterial screening indicated that compound 1c from 1,2-bis(N'-substituted thioureido)benzene series showed excellent activity against Bacillus subtilis, Staphylococcus aureus and Pseudomonas putida, respectively. Whereas amongst 1,4-bis(N'-substituted thioureido)benzene series, compound 2f exhibited good activity against Shigella flexineri, Escherichia coli, Pasteurella multocida and Klebsiella pneumonae compared with levofloxacin, the standard drug.
Acknowledgement
N.A. gratefully acknowledges a research scholarship from HEC Islamabad under the HEC Indigenous PhD Scholarship 5000 Scheme.
References
1 V.V Kachhadia, M.R. Patel and H.S. Joshi, J. Serb. Chem. Soc, 2005, 70, 153-161. [ Links ]
2 D. Wilson, M.A. Arada, S. Alegret and M. del Valle, J. Hazard. Mater., 2010,181, 140-146. [ Links ]
3 A. Solinas, H. Faure, H. Roudaut, E. Traiffort, A. Schoenfelder, A. Mann, F. Manetti, M. Taddei and M. Ruat, J. Med. Chem., 2012, 55, 1559-1571. [ Links ]
4 J. Moeker, K. Teruya, S. Rossit, B.L. Wilkinson, M. Lopez, L.F. Bornaghi, A. Innocenti, C.T. Supuran and S.-A.Poulsen, Bioorg. Med. Chem., 2012, 20, 2392-2404. [ Links ]
5 N.A. Mohamed and N.A.A. El-Ghany, Int. J. Biol. Macromol, 2012,50, 1280-1285. [ Links ]
6 S.N. Manjyula, N.M. Noolvi, K.V. Parihar, S.A.M. Reddy, V. Ramani, A.K. Gada, G. Singh, N.G. Kutty and C.M. Rao, Eur. J. Med. Chem., 2009,44, 2923-2929. [ Links ]
7 B. Phetsuksiri, M. Jackson, H. Scherman, M. McNeil, G.S. Besra, A.R. Baulard, R.A. Slayden, A.E. DeBarber, C.E. Barry, M.S. Baird, D.C. Crick and P.J. Brennan, J. Biol. Chem., 2003, 278, 53123-53130. [ Links ]
8 Y. Sohtome, N.Takemura, R. Takagi, Y. Hashimoto and K. Nagasawa, Tetrahedron, 2008, 64, 9423-9429. [ Links ]
9 Y. Nakayama, T. Gotanda and K. Ito, Tetrahedron Lett., 2011, 52, 6234-6237. [ Links ]
10 M. Breton, M. Bessodes, S. Bouaziz, J. Herscovici, D. Scherman and D. Mignet, Biophys. Chem, 2009,145, 7-16. [ Links ]
11 Y.-F. Yuan, S.-M. Ye, L.-Y. Zhang, B. Wang and J.-T. Wang, Polyhedron, 1997,16, 1713-1718. [ Links ]
12 R.G. Kumari, V. Ramakrishnan, M.L. Carolin, J. Kumar, A. Sarua and M. Kuball, Spectrochim. Acta, Part A, 2009, 73, 263-267. [ Links ]
13 B. Garg, T. Bisht and S.M.S. Chauhan, Sens. Actuators, B, 2012, 168, 318-328. [ Links ]
14 C. Alkan, Y. Tek and D. Kahraman, Turk. J. Chem. 2011, 35, 769-777. [ Links ]
15 F. Aydin, N. Tunoglu, D. Aykac, N.B. Arslan and C. Kazak, Turk. J. Chem., 2012, 36, 764-777. [ Links ]
16 A. Saeed, U. Shaheen, A. Hameed and S.Z. Haider Naqvi, J. Fluorine Chem., 2009, 130, 1028-1034. [ Links ]
17 A. Saeed, M.F. Erben and U. Flörke, J. Mol. Struc., 2010, 982, 91-99. [ Links ]
18 A. Saeed, A. Mumtaz and H. Ishida, J. Sulfur Chem., 2011, 32, [ Links ]
19 A. Saeed, M.F. Erben and M. Bolte, Spectrochimica Acta A, 2013, 102, 408-413. [ Links ]
20 M.I. Okeke, C.U. Iroegbu, E.N. Eze, A.S. Okoli and C.O. Esimone, J. Ethnopharm, 2001, 78, 119. [ Links ]
21 A.L. Spek, Acta Cryst., 2009, D65, 148-155 [ Links ]
22 G.M. Sheldrick, Acta Cryst., 2008, A64, 112-122). [ Links ]
 Correspondence:
Correspondence:
E-mail: aamersaeed@yahoo.com
Received 31 December 2012
Revised 20 May 2013
Accepted 19 August 2013














