Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Chemistry
versión On-line ISSN 1996-840X
versión impresa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.66 Durban ago. 2013
RESEARCH ARTICLE
One-Pot, Simple and Efficient Synthesis of Triaryl-1H-imidazoles by KMnO4/CuSO4
Ahmad Khorramabadi-zad; Mohammad Azadmanesh; Somaye Mohammadi
Faculty of Chemistry, Bu-Ali Sina University, Hamedan 65174, Iran
ABSTRACT
2,4,5-Triarylimidazoles have been obtained in excellent yields by the one-pot three-component condensation of bis-aryl a-hydroxyketones, aromatic aldehydes and ammonium acetate by the action of inexpensive, readily available and nontoxic KMnO4/CuSO4 under mild reaction conditions. The present method is simple, efficient, cost-effective and eco-friendly.
Keywords: Triarylimidazoles, Three-component reaction, One-pot synthesis, Oxidation, bis-aryl a-hydroxyketone, KMnO4, CuSO4.
1. Introduction
The development of simple, efficient and general synthetic methods for widely used organic compounds from readily available reagents is one of the major challenges in organic synthesis. In 1858 Debus1 reported the reaction of glyoxal and ammonia, a pioneering novel synthetic route to imidazole. The imidazole nucleus is a fertile source of biologically important molecules. They are well known as inhibitors of P38MAP kinase,2 herbi-cides,3 anti-inflammatory and antithrombotic agents,4-5 plant growth regulators6 and therapeutic agents.7
Redziszewski and Japp proposed the first synthesis of the imidazole core in 1882, starting from 1,2-dicarbonyl compounds, aldehydes and ammonia, to obtain 2,4,5-triarylimidazoles.8 Trisubstituted imidazole derivatives are widely used as organic materials and photographic materials,9-11 as well as in textile industry, e.g. to increase composition resistance and fluorescent whiteners on textile. It is also known that these compounds play key roles in many kinds of biological and pharmacological activities such as anti-HIV, anti-convulsant,12 calcium antagonist and as inhibitors of thromboxane A2 synthase,13 anthihistaminic,14 tranquillizing,15 anti-parkinsonism16 and MAO inhibiting activi-ties.17
Since their discovery, imidazoles have attracted attention because of their chemical and biochemical properties. Even today, after more than 150 years, research in imidazole chemistry continues unabated.
Several procedures reported for the synthesis of triaryl-1H-imidazoles through condensation reactions of a-hydroxyketones with ammonium acetate and various aromatic aldehydes in the presence of H2SO4,18 HOAc,19 DMSO,20 organocatalysts in HOAc,21 a variety of solid catalysts,22 ionic liquids23 and different metal complexes.24 Ceric ammonium nitrate,25 iodine,26 NaHSO3,27 zeolites HY/silica gel,28 ZrCl4,29 NiCl4.6H2O,30 TBAB (tetrabutyl ammonium bromide)31 and microwave irradiation using Al2O3 or acetic acid32 have also been reported for the preparation of imidazoles. However, some of the synthesis protocols reported above suffer from one or more disadvantages, such harsh reaction conditions, poor yields, prolonged reaction times, and the use of expensive equipments (microwave or ultrasonic), corrosive reagents, difficult work-up and often extensive acid catalysts. On the other hand, a literature survey showed that a KMnO4/CuSO4 mixture is a good choice for the oxidation of alcohols, oxidative coupling of primary aromatic amines as well as conversion of aryl and alkyl sulfides into their corresponding sulfones,33 and the recently reported synthesis of quinoxalines.34 Therefore, it was decided to use this mixture for the preparation of 2,4,5-triaryl imidazoles (Fig. 1).
2. Results and Discussion
As was mentioned above, the KMnO4/CuSO4 system could be used for the oxidation of bis-aryl a-hydroxyketones to bis-aryl diketones. In fact, control tests confirmed that the co-presence of KMnO4 and CuSO4 is necessary for the reaction to proceed. When use was made of this mixture in hot EtOH media, MnO2 was generated in situ by the action of KMnO4 on hot EtOH during the reaction. This freshly prepared active MnO2 is a mild oxidant which can easily oxidize benzylic hydroxyl group into the corresponding carbonyl group, for which three routes have been proposed.35
In all previously reported procedures for the synthesis of 2,4,5-triarylimidazoles, starting from either bis-aryl α-hydroxy-ketones or bis-aryl diketones, the reaction time is higher when the starting material are bis-aryl a-hydroxyketones. However, in the case of KMnO4/CuSO4 in EtOH, the reverse is true, i.e. the reaction time for bis-aryl a-hydroxyketones is much less than that of bis-aryl diketones. In addition, the yields with bis-aryl a-hydroxyketones as starting materials are higher than those of bis-aryl diketones (see Table 1).
It seems that bis-aryl α-hydroxyketones are first oxidized to bis-aryl diketones, with the subsequent formation of Mn2+ which may act as an effective Lewis acid catalyst. In the case of bis-aryl diketones, practically no Mn2+ is formed, and Cu2+ is the only existing Lewis acid catalyzing the reaction. As a control test, running the reaction of 1,2-diphenyl-1,2-ethanedione in the presence of Mn2+ and Cu2+ reveals that the co-presence of Mn+2 may be the key point for the higher reaction rate of bis-aryl 1,2-diketones.
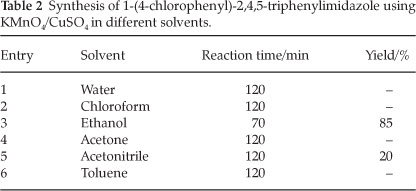
We have also studied the effect of different solvents on the synthesis of 2-(4-chlorophenyl)-4,5-diphenyl-1H-midazole, the results of which are summarized in Table 2. Among those examined, ethanol was found to be the most efficient solvent, regarding both the shorter reaction times and higher yields. This is due to the role of ethanol acting both as a solvent and as a reducing agent for the in situ formation of active MnO2.
In conclusion, this is the first report using a KMnO4/CuSO4 mixture for the synthesis of 2,4,5-triaryl-1H-imidazoles. The novelty of this procedure is that the reaction times for the synthesis of 2,4,5-triaryl-1H-imidazoles from bis-aryl α-hydroxy-ketones are less than those of reactions using bis-aryl diketones. Hence, it allows direct, one-pot synthesis of triarylimidazoles from bis-aryl a-hydroxyketones. Other advantages of this procedure are the use of ethanol as a green solvent, a cheap and readily available oxidizing agent, and high product yields when starting from bis-aryl α-hydroxyketones.
3. Experimental
Melting point apparatus, Stuart model SMP3, was used for measuring melting points. IR spectra were recorded on a PerkinElmer series II spectrum. 1H NMR and 13C NMR spectra were recorded in DMSO-d6 using Bruker DRX-400 spectrometer at 400 and 100 MHz respectively. All chemicals and solvents were purchased from Aldrich, Merck and Fluka, and were used as received. Melting points and spectral data of all products are fully consistent with those of the reported ones.
3.1. Preparation of KMnO4/CuSO4 Mixture
The optimized amounts of KMnO4 and CuSO4, i.e. 5 mmol and 1 mmol, respectively, were ground together in a mortar to obtain a purple powder.
3.2. General Procedure for the Synthesis of 2,4,5-Triaryl Imidazoles
α-Hydroxy ketone (1.0 mmol), aromatic aldehyde (1.0 mmol), ammonium acetate (2.5 mmol) and the mixture of KMnO4/ CuSO4 (0.4 mmol) were stirred and refluxed in ethanol. The progress of the reaction was monitored by TLC. After completion of the reaction, the mixture was poured in cold water (50 mL), the precipitated solid was filtered, washed several times with water, dried and recrystallized from EtOH or acetone:water (9:1) to get the corresponding 2,4,5-triaryl-1H-imidazoles. The experimental results are summarized in Table 3.
3.3. Spectral Data of Imidazole Derivatives
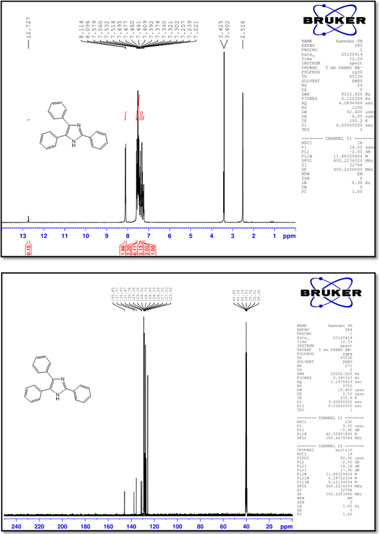
2,4,5-Triphenyl-1 H-imidazole (1)
M.p.: 275-276 °C; 1H NMR (400 MHz, DMSO-d6): d = 7.24 (t, 1H, J = 7.2 Hz), 7.32 (t, 2H, J = 7.2 Hz), 7.39 (t, 2H, J = 7.2 Hz), 7.44-7.53 (m, 6H), 7.57 (d, 2H, J = 7.6 Hz), 8.10 (d, 2H, J = 7.2 Hz), 12.73 (1H, br) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 125.7, 127.0, 127.5, 128.3, 128.6, 128.7, 128.8, 128.9, 129.2, 129.2, 130.7, 131.5, 135.6, 137.6, 145.8 ppm; IR (KBr, cm-1): 3434, 3031, 1601, 1503, 1455, 1202, 1125, 966, 765, 733, 695.
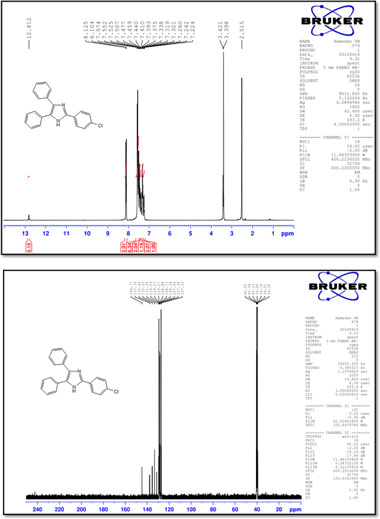
2-(2-Chlorophenyl)-4,5-diphenyl-1 H-imidazole (2)
M.p.: 198-200 °C; 1H NMR (400 MHz, DMSO-d6): δ = 7.24 (t, 1H, J = 7.1 Hz), 7.31-7.39 (m, 3H), 7.42-7.53 (m, 6H), 7.57 (d, 2H, J = 7.2 Hz) 7.61-7.64 (m, 1H), 7.81-7.83 (m, 1H), 12.69 (br, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 127.1,127.6,127.7, 128.2, 128.4, 128.7, 129.2, 130.5, 130.6, 130.7, 131.3, 132.0, 132.1, 135.5, 137.4, 143.8 ppm; IR (KBr, cm-1): 3448, 3059, 1601, 1503, 1478, 1320, 1201, 1070, 761, 693, 605.
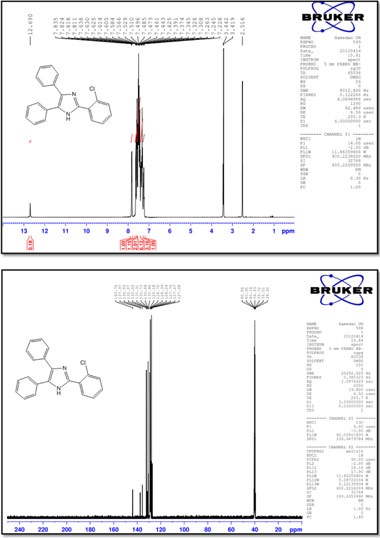
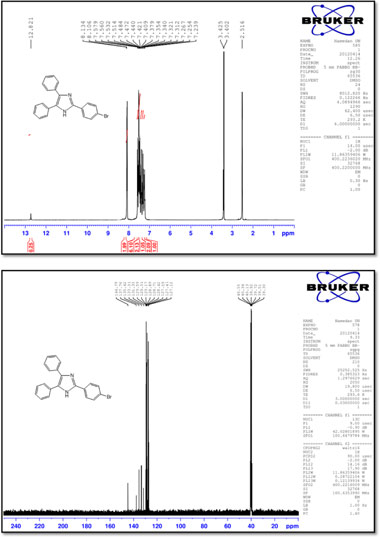
2-(4-Chlorophenyl)-4,5-diphenyl-1 H-imidazole (3)
M.p.: 262-264 °C; 1H NMR(400 MHz, DMSO-d6): δ = 7.22-7.57 (m, 12H), 8.11 (d, 2H, J = 8.4 Hz), 12.81 (br, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 127.1,127.3,127.6,128.4,128.7,128.9, 129.2, 129.3, 129.6, 131.3 133.2, 135.4, 137.8, 144.8 ppm; IR (KBr, cm-): 3440,3059,1602,1503,1486,1448,1324,1128,1091,969,832, 766, 732, 697, 605.
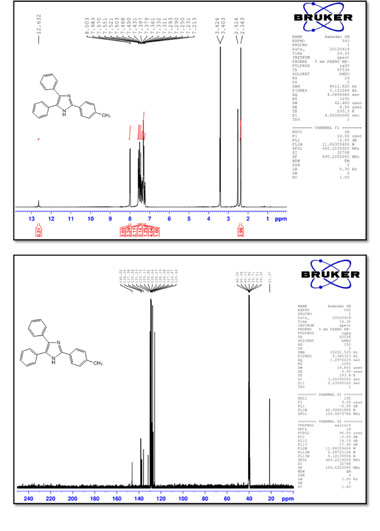
2-p-tolyl-4,5-diphenyl-1 H-imidazole (4)
M.p.: 236-237 °C; 1H NMR (400 MHz, DMSO-d6): δ = 2.36 (s, 3H), 7.23 (t, 1H, J = 7.4 Hz), 7.31 (t, 4H, J = 7.2 Hz), 7.38 (t, 1H, J = 7.2 Hz),7.45 (t, 2H, J = 7.4 Hz), 7.51 (d, 2H, J = 7.2 Hz), 7.56 (d, 2H, J = 7.6 Hz), 7.99 (d, 2H, J = 8.0 Hz) 12.63 (br, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 21.3, 125.6, 127.0, 127.5, 128.1, 128.2, 128.3, 128.7, 128.9, 129.1, 129.7, 131.5, 135.7, 137.4, 138.2, 146.0 ppm; IR (KBr, cm-1): 3420,3030,1600,1494,1450,1128,1070, 969, 823, 764, 731, 695, 672.
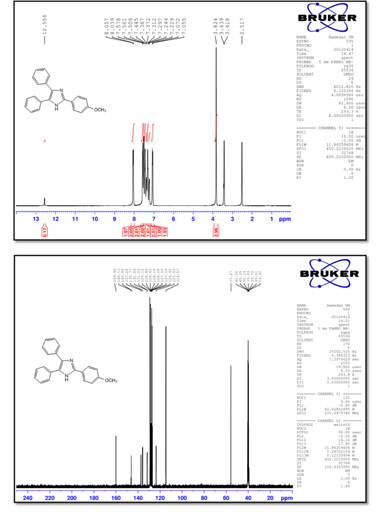
2-(4-Methoxyphenyl)-4,5-diphenyl-1 H-imidazole (5)
M.p.: 232-235 °C; 1H NMR (400 MHz, DMSO-d6): δ = 3.82 (s, 3H), 7.06 (d, 2H, J = 6.8 Hz), 7.23-7.58 (m, 10H), 8.05 (d, 2H, J = 7.2 Hz), 12.56 (br, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 55.7, 114.6, 123.5, 126.9, 127.2, 127.5, 128.0, 128.1, 128.6, 128.8, 129.1, 131.6, 131.7, 135.8, 137.2, 146.0, 146.1, 159.9 ppm; IR (KBr, cm-1): 3431,3060,1614,1493,1293,1249,1177,1030,968,829,765,695.
2-(4-Bromophenyl)-4,5-diphenyl-1 H-imidazole (6)
M.p.: 264-266 °C; 1H NMR (400 MHz, DMSO-d6): δ = 7.25 (t, 1H, J = 7.4 Hz), 7.33 (t, 2H, J = 7.0 Hz), 7.40 (t, 1H, J = 7.8 Hz), 7.46 (t, 2H, J = 7.2 Hz), 7.51-7.58 (m, 6H), 8.12 (d, 2H, J = 7.2 Hz), 12.82 (br, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): d = 127.1, 127.4, 127.6, 128.4, 128.7, 128.9, 129.2, 129.3, 129.6, 131.3, 133.3, 135.4, 137.8,144.8 ppm; IR (KBr, cm-1): 3445,3028,1602,1501,1483,1449, 1432, 1129, 1069, 826, 765, 729, 695, 500.
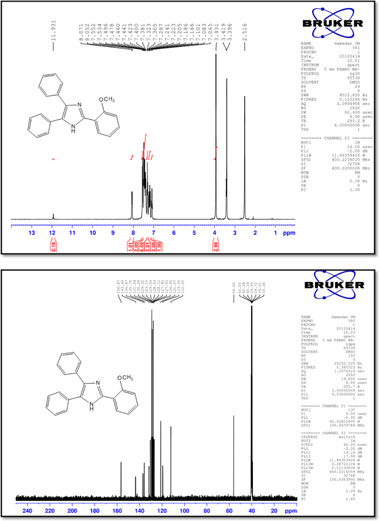
2-(2-Methoxyphenyl)-4,5-diphenyl-1 H-imidazole (7)
M.p.: 208-210 °C; 1H NMR (400 MHz, DMSO-d6): δ = 3.93 (s, 3H), 7.08 (t, 1H, J = 7.2 Hz), 7.17-7.24 (m, 2H), 7.30 (t, 2H, J = 7.2 Hz), 7.38-7.50 (m, 6H), 7.54 (d, 2H, J = 7.2 Hz), 8.06 (d, 1H, J = 7.6 Hz), 11.93 (br, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 112.0, 119.3, 121.1, 126.9, 127.5, 127.8, 128.1, 128.6, 129.0, 129.1, 129.3, 130.3, 131.6, 135.7, 136.9, 143.5, 156.5 ppm; IR (KBr, cm-1): 3450,3063,1601,1585,1481,1470,1446,1250,1099,1018,764,747, 694, 613.
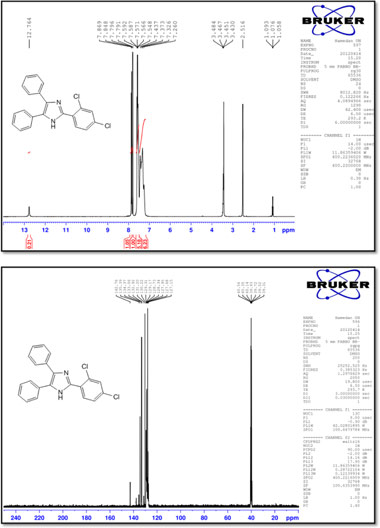
2-(2,4-Dichlorophenyl)-4,5-diphenyl-1 H-imidazole (8)
M.p.: 178-180 °C; 1H NMR (400 MHz, DMSO-d6): δ = 7.26-7.59 (m, 11H), 7.79 (d, 1H, J = 7.6 Hz), 7.86 (d, 1H, J = 7.6 Hz), 12.76 (br, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 127.1,127.7,127.9, 128.3, 128.7, 129.2, 129.3, 130.2, 131.2, 132.9, 133.1, 134.4, 135.4, 142.8 ppm; IR (KBr, cm-1): 3446,3069,1594,1552,1501,1476,1426, 1322, 1135, 1101, 879, 768, 700, 497.
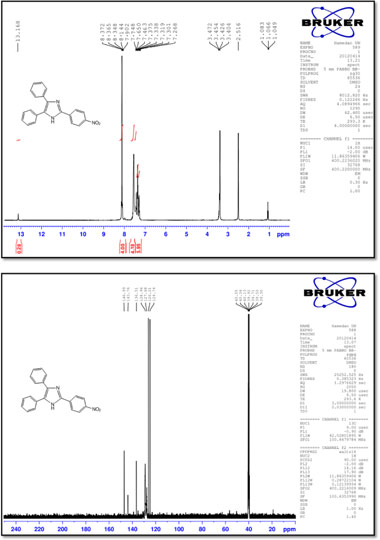
2-(4-Nitrophenyl)-4,5-diphenyl-1 H-imidazole (9)
M.p.: 240-242 °C; 1H NMR (400 MHz, DMSO-d6): δ = 7.27-7.75 (m, 10H), 7.90-8.37 (m, 4H), 13.17 (br, 1H), ppm; 13C NMR (100 MHz, DMSO-d6): δ = 124.7,126.2,127.7,129.0,136.5,143.8, 147.0 ppm; IR (KBr, cm-1): 3393,3081,1600,1582,1512,1442,1336, 1245, 1109, 968, 853, 765, 695.
2-Phenyl-4,5-di-p-tolyl-1 H-imidazole (10)
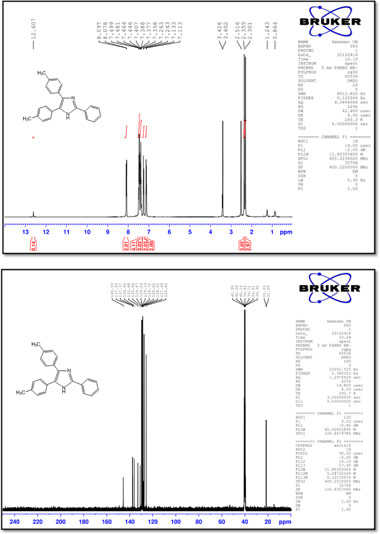
M.p.: 269-271 °C; 1H NMR (400 MHz, DMSO-d6): δ = 2.30 (s, 3H), 2.36 (s, 3H), 7.12 (d, 2H, J = 8.0 Hz), 7.25 (d, 2H, J = 8.0 Hz), 7.38-7.50 (m, 7H), 8.09 (d, 2H, J = 7.6 Hz), 12.61 (br, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 21.2,21.3,125.6,127.5,128.2, 128.6, 128.7, 129.1, 129.2, 129.7, 130.8, 132.9, 136.0, 137.4, 137.5, 145.5 ppm; IR (KBr, cm-1): 3435,3032,1520,1497,1460,1411,1322, 1126, 816, 721, 689.
2,4,5-Tri-p-tolyl-1 H-imidazole (11) M.p.: 238-240 °C; 1H NMR (400 MHz, DMSO-d6): δ = 2.30 (3H, s), 2.36 (s, 6H), 7.12 (d, 2H, J = 8.0 Hz), 7.24 (d, 2H, J = 8.0 Hz), 7.29 (d, 2H, J = 8.0 Hz), 7.39 (d, 2H, J = 8.0 Hz), 7.45 (d, 2H, J = 8.0 Hz), 7.97 (d, 2H, J = 8.4 Hz), 12.51 (br, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 21.2, 21.3, 21.4, 125.6, 127.5, 127.9, 128.2, 128.6, 128.7, 129.2, 129.6, 129.7, 132.9, 135.9, 137.2, 137.4, 138.0, 145.7ppm; IR (KBr, cm-1): 3442,3021,1520,1500,1431,1319,1125, 969, 818, 727, 503.
Acknowledgements
We gratefully acknowledge the support of this work by Research Council of Bu-Ali Sina University.
References
1 H. Debus, Liebigs Ann. Chem, 1858,107, 199-208. [ Links ]
2 J.C. Lee, J.T. Laydon, P.C. McDonnell, T.F. Gallagher, S. Kumar, D. Green and D. McNulty, Nature 1994, 372, 739-746. [ Links ]
3 W. Töpfl, U.S. Patent 4921955, 1990. [ Links ]
4 P. Arthur Page, W. Helen Lyng and R. Solomon, Eur. Pat. Appl. EP 58890, 1982. [ Links ]
5 J.G. Lombardino and E.H. Wiseman, J. Med. Chem., 1974, 17, 1182-1188. [ Links ]
6 R. Schmierer, T. Maier, K. Bauer, H. Bieringer, H. Bürstell and B. Sachse, U.S. Patent 4820335 1987. [ Links ]
7 J. Heeres, L.J.J. Backx,J.H. Mostmans and J. Vancustem, J.Med. Chem., 1979, 22, 1003-1005. [ Links ]
8 B. Radziszewski, Chem. Ber., 1882,15, 1493-1496. [ Links ]
9 K. Etou, M. Nanhei and S. Tanaka, U.S. Patent 4405705, 1983. [ Links ]
10 T. Ogawa, U.S. Patent 4786588, 1988. [ Links ]
11 H. Oldenwalder, H. Lange and U. Dahlhaus, U.S. Patent 6451520 B1, 2002. [ Links ]
12 P. Renukadevi, J.S. Biradar, S.P. Hiremath and S.Y. Manjunath, Indian J. Heterocycl. Chem, 1997, 6, 277-280. [ Links ]
13 P. Cozzi, G. Carganico, D. Fusar, M. Grossoni, M. Menichincheri, V. Pinciroli, R. Tonani, F. Vaghi and P. Salvati, J. Med. Chem., 1993, 36, 2964-2972. [ Links ]
14 J. Mohan and A. Kumar, Indian J. Heterocycl. Chem., 2002,12, 41-44. [ Links ]
15 Y.E. Shealy, J.A. Montgomery and W.R. Laster, Biochem. Pharmacol., 1962,11, 674-676. [ Links ]
16 S. Meenakshi, R. Reenakalsi, K.S. Dixit, C. Nath and J.P. Barthwal, Indian J. Chem., Sect B, 1990, 29B, 85-87. [ Links ]
17 M. Harfenist, F.E. Soroko and G.M. McKenzie, J. Med. Chem, 1978,21, 405-409. [ Links ]
18 H. Weinmann, M. Harre, K. Koeing, E. Merten and U. Tilestam, Tetrahedron Lett, 2002, 43, 593-595. [ Links ]
19 S. Sarshar, D. Siev and A.M.M. Mjalli, Tetrahedron Lett. , 1996, 37, 835-838. [ Links ]
20 N.G. Clark and E. Cawkill, Tetrahedron Lett., 1975,16, 2717-2720. [ Links ]
21 D.E. Frantz, L. Morency, A. Soheili, J.A. Murry, E.J.J. Grabowski and R.D. Tillyer, Org. Lett., 2004, 6, 843-846. [ Links ]
22 R.B. Sparks and A.P. Combs, Org. Lett., 2004, 6, 2473-2475. [ Links ]
23 S.A. Siddiqui, U.C. Narkhede, S.S. Palimkar, T. Daniel, R.J. Lahoti and K.V. Srinivasan, Tetrahedron, 2005, 61, 3539-3546. [ Links ]
24 L.M. Wang, Y.H. Wang, H. Tian, Y.F. Yao, J.H. Shao and B. Liu, J. Fluorine Chem, 2006,127, 1570-1573. [ Links ]
25 A. Shaabani, A. Maleki and M. Behnam, Synth. Commun., 2009, 39, 102-110. [ Links ]
26 M. Kidwai, P. Mothsra, V. Bansal and R. Goyal, Monatsh. Chem, 2006, 137, 1189-1194. [ Links ]
27 J.N. Sangshetti, N.D. Kokare, S.A. Kothakar and D.B. Shinde, Monatsh. Chem., 2008,139, 125-127. [ Links ]
28 S. Balalaie, A. Arabanian and M. Hashtroudi, Monatsh. Chem., 2000, 131, 945-948. [ Links ]
29 G.V.M. Sharma, Y. Jyothiand P.S. Lakshmi, Synth. Commun.,2006,36, 2991-3000. [ Links ]
30 M.M. Heravi, K. Bakhtiari and H.A. Oskooie, J. Mol. Catal. A Chem., 2007, 263, 279-281. [ Links ]
31 M.V. Chary, N.C. Keerthysri, S.V.N. Vupallapati, N. Lingaiah and S. Kantevari, Catal. Commun, 2008, 9, 2013-2017. [ Links ]
32 A.Y. Usyatinsky and Y.L. Khmelnitsky, Tetrahedron Lett., 2000, 41, 5031-5034. [ Links ]
33 A. Shaabani and D.G. Lee, Tetrahedron Lett, 2001,42, 5833-5836. [ Links ]
34 A. Khorramabadi-zad, M. Azadmanesh and S. Mohammadi, S. Afr. J. Chem., 2013, 66, 113-116. [ Links ]
35 G. Tojo, and M.I. Fernandez, Oxidation of Alcohols to Aldehydes and Ketones: A Guide to Current Common Practice (Basic Reactions in Organic Synthesis), Springer, New York, 2006. [ Links ]
 Correspondence:
Correspondence:
E-mail: khoram@gmail.com
Received 16 April 2013
Revised 6 May 2013
Accepted 16 August 2013




![Synthesis and characterization of ethyl 2-methyl-7-oxo-7H-isoxazolo[2,3-α ]pyrimidine-6-carboxylate derivatives](/img/es/prev.gif)