Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Chemistry
versión On-line ISSN 1996-840X
versión impresa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.66 Durban ago. 2013
RESEARCH ARTICLE
Densities and excess molar volume for the ternary systems (1-butyl-3-methylimidazolium methyl sulphate + nitromethane + methanol or ethanol or 1-propanol) at T = (303.15 and 313.15) K
Indra Bahadur; Nirmala Deenadayalu*
Department of Chemistry, Durban University of Technology, Steve Biko Campus, P.O. Box 1334, Durban 4000, South Africa
ABSTRACT
The densities of the ternary systems containing the ionic liquid 1-butyl-3-methylimidazolium methyl sulphate ([BMIM]+[MeSO4]-) were determined. The ternary systems studied were ([BMIM]+[MeSO4]- + nitromethane + methanol or ethanol or 1-propanol) at the temperatures (303.15 and 313.15) K. The ternary excess molar volumes were calculated from the experimental densities at each temperature, being negative for all mole fractions of the ionic liquid. The minimum ternary excess molar volumes increase with an increase in temperature for the systems ([BMIM]+[MeSO4]- + nitromethane + methanol or ethanol), and decrease for the system ([BMIM]+ [MeSO4]- + nitromethane + 1-propanol). The results are interpreted in terms of the alcohol chain length and the intermolecular interactions.
Keywords: Density, excess molar volume, ionic liquid, alcohol, nitromethane.
1. Introduction
Ionic liquids (ILs) are low-melting molten salts which are composed of organic cations, and inorganic anions and their properties depend on the type of cation and anion.1 ILs are studied as 'green solvents' for organic reactions, combined with a catalyst to extend its life span, and as reagents for nanomaterials.2,3 The physical properties of ILs such as density, viscosity, melting point, polarity and miscibility with water or molecular solvents, can be finely tuned by changing either the anion or the cation.2-6 ILs have other properties such as very low vapour pressure, wide electrochemical window, high recyclability, good ionic conductivities, thermal stability and nonflammability7,8 making them useful solvents for a wide range of applications.9,10 The applications of ILs are rapidly increasing in all modern scientific fields such as, separations, 11 organic synthesis, 12 homogeneous two-phase catalysis,13 extractions,14 electrochemistry,15 and polymerization processes.16
Thermophysical properties of mixtures of ILs with different solvents are useful for the design of chemical separation pro-cesses.17,18
Ternary excess molar volumes, VE123, for IL systems have been reported by various researchers.19-28 In this paper the densities were measured for the ternary systems ([BMIM]+[MeSO4]- + nitromethane + methanol or ethanol or 1-propanol) over the entire composition range at T = (303.15 and 313.15) K and at atmospheric pressure. VE123 data were calculated at T = (303.15 and 313.15) K and at atmospheric pressure for IL systems by using the experimental density values. The results are interpreted in terms of intermolecular interactions and the alcohol chain length. This work is a continuation of our research group's work on thermodynamic properties of ILs.21-26,29-35
2. Experimental
2.1. Materials
The chemicals were purchased from Aldrich or Merck or Fluka and used without further purification. The chemicals, supplier, purity and the literature and experimental density (ρ) are given in Table 1. The density of the pure chemicals was determined at T = (303.15 and 313.15) K and at atmospheric pressure. The water content in the IL was determined by using a Karl-Fischer coulometer (Metrohm797, Herisau, Switzerland) and the mass percent water content was found to be <0.0024. The purity of the pure chemicals was assessed by a comparison of the experimental density values with the literature density values where avail-able.31,36 The structure of the IL used in this work is given in Fig. 1.


2.2. Apparatus and Procedure
The ternary mixtures were prepared by transferring the pure liquids into stoppered bottles to prevent evaporation. The IL was first filled into the air-tight glass stoppered 5 cm3 glass vial and weighed. An OHAUS (Pine Brook, NJ, USA) mass balance was used to determine the mass of each component of the mixture. The mass balance has a precision of 0.0001 g. The uncertainty in the mole fraction was estimated to be 0.0006. The ternary mixtures were prepared from a spreadsheet calculation to obtain a constant z ratio (z = x2/x1) where x1 and x2 are the mole fractions of the ionic liquid and nitromethane, respectively, with varying mole fractions of methanol or ethanol or 1-propanol (x3). The densities were measured with an Anton Paar (Graz, Austria) DMA 38 vibrating U-tube densimeter. Ultra-pure water supplied by SH Calibration Service GmbH Graz, Austria and dried air were used for the calibration of the densimeter at T = 298.15 K.29 The temperature maintenance and control were regulated by a built-in thermostat controller with a temperature uncertainty of ±0.02 K. The repeatability and accuracy in experimental measurements was (±0.0002 and ±0.001) g cm-3 for density. The uncertainty in density for the ternary system was ±0.001 g cm-3. The ternary excess molar volumes were calculated from the experimental density. The experimental uncertainty in was ±0.2 cm3 mol-1. The experimental technique was validated by measuring the density for the test system (octane + toluene) at T = 298.15 K and comparing it with the literature density.37 The difference between experimental and literature density for the test system was within the experimental error.
3. Results and Discussion
Densities, ρ, and ternary excess molar volumes, VE123, for the (IL + nitromethane + methanol or ethanol or 1-propanol) systems as a function of composition at T = (303.15 and 313.15) K are given in Tables 2-4. The VE123 was calculated by using Equation (1):

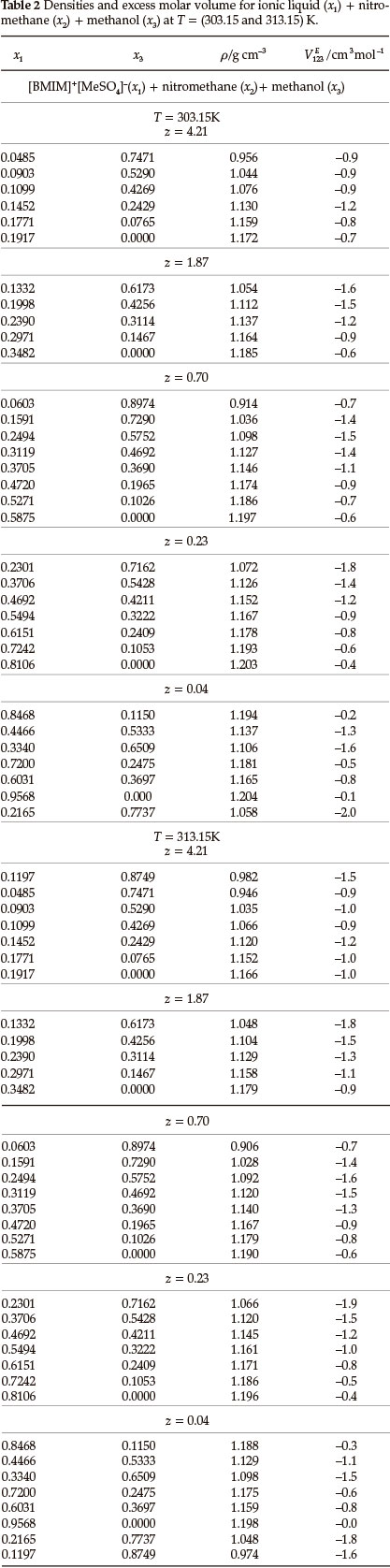
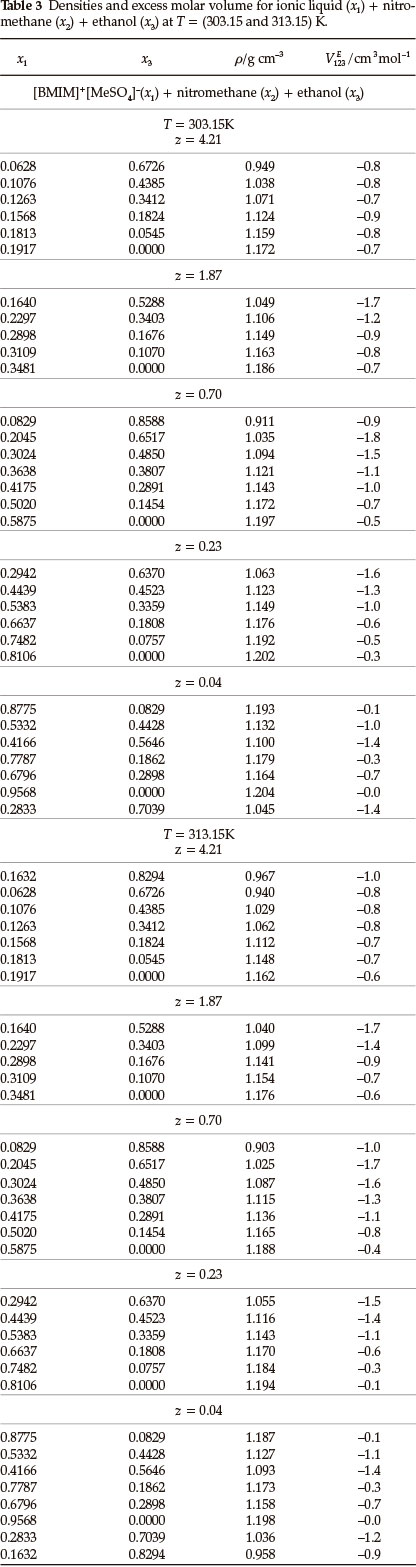
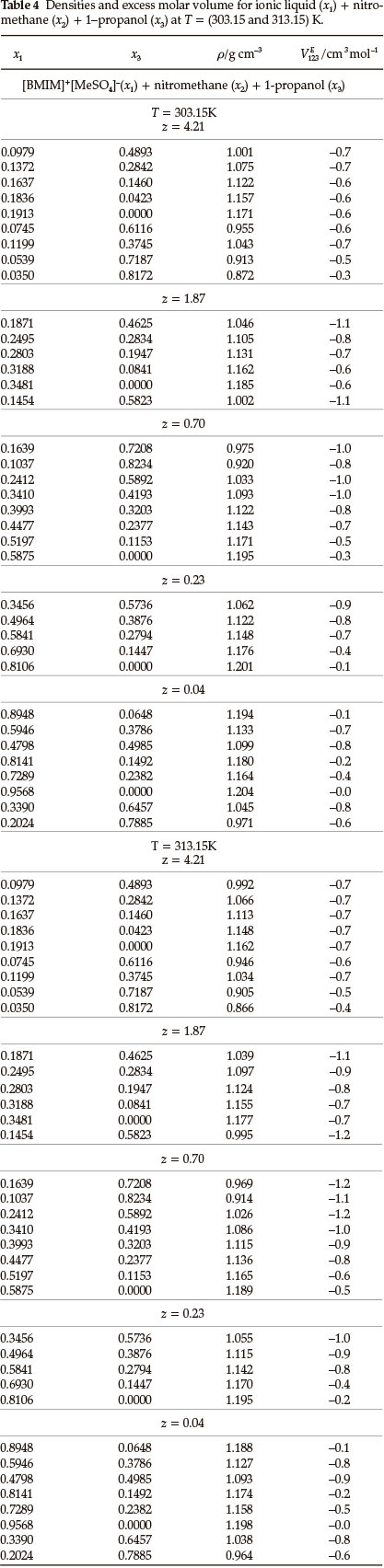
where M1, M2 and M3 are the molecular masses, ρ1, ρ2, and ρ3 are the densities of pure components 1, 2, or 3, respectively, where '1' refers to the IL, '2' to nitromethane and '3' to methanol or ethanol or 1-propanol and p is the density of the mixture.
The binary VEmdata for (IL + methanol)31, (IL + ethanol)31, (IL + 1-propanol)31, (IL + nitromethane)36, (methanol + nitromethane)38, (ethanol + nitromethane)38 and (1-propanol + nitromethane)38 were published in the literature. For each ternary system there are three sets of binary data: (i) (IL + an alcohol) (ii) (IL + nitromethane) and (iii) (an alcohol + nitromethane).
(i) (IL + nitromethane + methanol)
The binary VEm data for (IL + methanol) at the temperatures (303.15 and 313.15) K are negative for all mole fractions of the IL due to the more efficient packing and/or attractive interaction between the IL and the methanol molecules.31 The values at the temperatures (303.15 and 313.15) K are -1.444 cm3 mol-1 at x1 = 0.4386 and -1.531 cm3 mol1 at x1 = 0.5589, respectively.31
For the system (IL + nitromethane) VEm are negative at the temperatures (303.15 and 313.15) K, and at all mole fractions of the IL.25 The VEm,min values at the temperatures (303.15 and 313.15) K are -0.602 cm3 mol-1 and -0.667 cm3 mol-1, respectively, both occurring at x1 = 0.2998.36
For the binary system (methanol + nitromethane) VEm is negative at the temperature 303.15 K, and both positive and negative at the temperature 313.15 K due to the hydrogen bond rupture and dispersive interactions between unlike molecules (positive contributions) and dipole-dipole interactions and geometrical fitting of the nitromethane into the methanol structure (negative contributions).38 The VEm,minvalues at the temperatures (303.15 and 313.15) K are -0.1687 cm3 mol-1 at x1 = 0.6500 and -0.1523 cm3 mol-1 at x1 = 0.7500, respectively.38
Figures 2 and 3 are the plots of VE123 vs. mole fraction of methanol for the ternary system (IL + nitromethane + methanol). VE123 values are negative for the ternary composition curves, as can be expected, since the three binary VEm,min values are all negative. The negative values of VE123 for (IL + nitromethane + methanol) show that the (ion-dipole) interactions and packing effect between methanol, nitromethane and the IL are dominating over the dissociation of any intermolecular hydrogen bonds in methanol and nitromethane.27
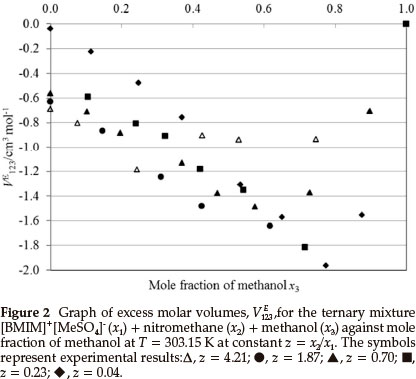
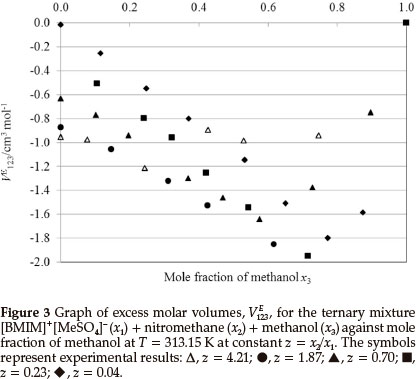
From Table 2, it can be seen that for the system ([BMIM]+ [MeSO4]- + nitromethane + methanol) VE123mindecreases with a decreasing z values (increasing mole fractions of IL) except at z = 0.70 at the temperatures (303.15 and 313.15) K and also at z = 0.04 for the temperature 313.15 K.
From Table 2, it can also be seen that the VE123,min increases with an increase in temperature for the system ([BMIM]+[MeSO4]- + nitromethane + methanol). The VE123minvalues of (-2.0 and -1.9) cm3 mol-1 at each temperature are similar in magnitude to the VEm values for the ([BMIM]+[MeSO4]- + methanol) binary system.31
(ii) (IL + nitromethane + ethanol)
The binary VmE data for (IL + ethanol) at the temperatures (303.15 and 313.15) K are negative for all mole fractions of the IL due to the same effects that occur in the binary system (IL + methanol). The VEm,minvalues at the temperatures (303.15 and 313.15) K both occur at x1 = 0.4056 and are -0.775 cm3 mol-1 and -0.869 cm3 mol-1, respectively.31 The binary excess molar volumes of (IL + ethanol) are greater than for the (IL + methanol) systems.31
For the binary system (ethanol + nitromethane) VmE are both positive and negative at the temperatures (303.15 and 313.15) K due to the same effects that occur in the binary system (methanol + nitromethane).38 The VEm,minvalues at the temperatures (303.15 and 313.15) K are -0.0292 cm3 mol-1 at x1 = 0.8500 and -0.0040 cm3 mol-1 at x1 = 0.9500, respectively.38 The binary excess molar volumes of (ethanol + nitromethane) are greater than those for the binary system (methanol + nitromethane).
Figures 4 and 5 are the plots of VE123 versus mole fraction of ethanol for the ternary system (IL + nitromethane + ethanol). VE123 values are negative for the ternary composition curves. The negative values of VE123 for the ternary system (IL + nitro-methane + ethanol) are due to the same effects that occur in the ternary (IL + nitromethane + methanol) system.


From Table 2, it is evident that for the system ([BMIM]+ [MeSO4]- + nitromethane + ethanol) VEm,mindecreases with a decreasing z value except at z = 0.23 and 0.04 for both the temperatures (303.15 and 313.15) K. Also, VEm,min increases with an increase in temperature for the system ([BMIM]+[MeSO4]- + nitromethane + ethanol). The VEm,min values of (-1.8 and-1.7) cm3 mol-1 at each temperature are similar in magnitude to the VmE values for the ([BMIM]+[MeSO4]- + ethanol) binary system.31 The VE123,minof (IL + nitromethane + ethanol) is greater than (IL+ nitromethane + methanol) for each temperature.
(iii) (IL + nitromethane + 1-propanol)
The binary VEm data for (IL + 1-propanol) at the temperatures (303.15 and 313.15) K are negative for all mole fractions of the IL due to the same effects that occur in the binary systems (IL + methanol or ethanol). The VEm,min values at the temperatures (303.15 and 313.15) K both occur at x1 = 0.4756 are -0.251 cm3 mol-1 and -0.260 cm3 mol-1, respectively.31 The binary excess molar volumes of (IL + 1-propanol) are greater than (IL + ethanol) that are greater than the (IL + methanol) system due to the increase in the alcohol chain length.31
For the binary system (1-propanol + nitromethane) VEm is positive at the temperatures (303.15 and 313.15) K due to a decrease in the intermolecular interactions. The VEm,min values at the temperatures (303.15 and 313.15) K both occur at x1 = 0.9500 and are 0.0374 cm3 mol-1 and 0.0542 cm3 mol-1, respectively.38 The excess molar volumes of (1-propanol + nitromethane) are greater than (ethanol + nitromethane) are greater than (methanol + nitromethane) system due to the increase in the alcohol chain length.38
Figures 6-7 are the plots of VE123 versus mole fraction of 1-propanol for the ternary system (IL + nitromethane + 1-propanol). VE123 values are negative for all ternary composition curves. The negative values of VE123 for (IL + nitromethane + 1-propanol) are due to similar effects that occur in the ternary (IL + nitromethane + methanol or ethanol) systems.
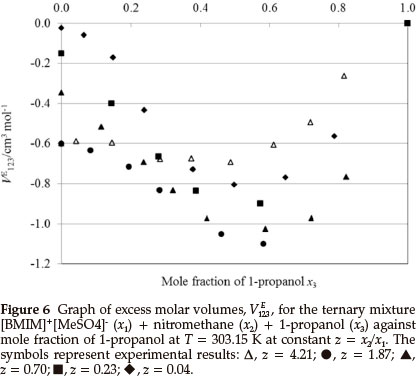

From Table 4 it can be seen that for the ternary system ([BMIM]+[MeSO4]- + nitromethane + 1-propanol) VE123,min increases with decreasing z values for both temperatures and decreases with an increase in temperature. The VE123,min of (IL + nitromethane + 1-propanol) is greater than (IL + nitromethane + ethanol) is greater than (IL + nitromethane + methanol) systems due to a decrease in intermolecular interactions among the three components and a less compact structure (a decrease in the packing effect) possibly due to the larger 1-propanol molecules. The VE123,min increases in the order (IL + nitromethane + 1-propanol) > (IL + nitromethane + ethanol) > (IL + nitromethane + methanol) at each temperature.
4. Conclusions
In this paper, the densities and VE123 have been reported for the ternary systems (IL + nitromethane + methanol or ethanol or 1-propanol) at the temperatures (303.15 and 313.15) K. The VE123 values are negative for all compositions of the IL and VE123,minincreases with an increase in temperature for the systems (IL + nitromethane + methanol or ethanol) and decreases for the system (IL + nitromethane + 1-propanol). The negative VE123 values are due to strong intermolecular interactions, as well as, a more efficient packing effect among the alcohol, nitromethane and the IL. The IL, an alcohol and/or the nitromethane are engaged in specific interactions, and the ability of the alcohol to form hydrogen bonds with the IL is increased by the presence of the nitromethane molecules. VE123,minvalues increase in the order (IL + nitromethane + 1-propanol) > (IL+ nitromethane + ethanol) > (IL+ nitromethane + methanol) at each temperature.
Acknowledgements
The authors acknowledge the National Research Foundation, South Africa, and Durban University of Technology for a postdoctoral scholarship for Dr I. Bahadur.
References
1 T. Welton, Chem. Rev.,1999, 99, 2071-2083. [ Links ]
2 S. Baj, A. Chrobok and S. Derfla, Green Chem.,2006, 8, 292-295. [ Links ]
3 K.R. Seddon, J. Chem. Technol. Biotechnol, 1997, 68, 351-356. [ Links ]
4 P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed.,2000, 39, 3772-3789. [ Links ]
5 E.R. Cooper, C.D. Andrews, P.S. Wheatley, P.B. Webb, P. Wormald and R.E. Morris, Nature, 2004, 430, 1012-1016. [ Links ]
6 H.T. Karimata and N. Sugimoto, Angew. Chem. Int. Ed., 2012,51,14161419. [ Links ]
7 T. Sato, G. Masuda and K. Takagi, Electrochim. Act., 2004, 49, 3603-3611. [ Links ]
8 M.J. Earle, P. McCormac and K.R. Seddon, Green Chem, 1999, 1, 23-25. [ Links ]
9 D. Kerle, R. Ludwig, A. Geiger and D. Paschek, J. Phys. Chem. B, 2009, 113, 12727-12735. [ Links ]
10 M. Galiński, A. Lewandowski and I. Stępniak, Electrochim. Act., 2006, 51, 5567-5580. [ Links ]
11 J.G. Huddleston, H.D. Williams, R.P. Swatloski, A.E. Visser and R.D. Rogers, Chem. Commun, 1998, 16, 1765-1766. [ Links ]
12 R. Sheldon, Chem. Commun., 2001, 23, 2399-2407. [ Links ]
13 S. Zhang, Q. Zhang and Z.C. Zhang, Ind. Eng. Chem. Res., 2004, 43, 614-622. [ Links ]
14 J. Esser, P. Wasserscheid and A. Jess, Green Chem., 2004, 6, 316-322. [ Links ]
15 M.V.B. Zanoni, E.I. Rogers, C. Hardacre and R.G. Compton, Anal. Chim. Acta, 2010, 659, 115-121. [ Links ]
16 P. Kubisa, Prog.Polym. Sci., 2009, 34, 1333-1347. [ Links ]
17 M. Freemantle, An Introduction to Ionic Liquids,RSC Publishing, Cambridge, UK, 2010. [ Links ]
18 M.T Zafarani-Mottar and H. Shekaari, J. Chem. Thermodyn., 2005, 37, 1029-1035. [ Links ]
19 E. Gómez, B. Gonzàlez, N. Calvar and Á. Domínguez, J. Chem. Thermodyn., 2008, 40, 1208-1216. [ Links ]
20 B. Gonzalez, N. Calvar, E. Gómez and Á. Domínguez, J. Chem. Thermodyn., 2008, 40, 1274-1281. [ Links ]
21 N. Deenadayalu, S. Kumar and P. Bhujrajh, J. Chem. Thermodyn., 2007, 39, 1318-1324. [ Links ]
22 N. Deenadayalu and P. Bhujrajh, J. Chem. Eng. Data, 2008, 53, 1098-1102. [ Links ]
23 N. Deenadayalu, I. Bahadur and T. Hofman, J. Chem. Eng. Data, 2010, 55, 2636-2642. [ Links ]
24 N. Deenadayalu, I. Bahadur and T. Hofman, J. Chem. Thermodyn., 2010, 42, 726-733. [ Links ]
25 N. Deenadayalu, I. Bahadur and T. Hofman, J. Chem. Eng. Data, 2011, 56, 1682-1686. [ Links ]
26 I. Bahadur, N. Deenadayalu, Z. Tywabi, S. Sen and T. Hofman, J. Chem. Thermodyn., 2012, 49, 24-38. [ Links ]
27 A.E. Andreatta, A. Arce, E. Rodil and A. Soto, J. Chem. Thermodyn., 2009, 41, 1317-1323. [ Links ]
28 A.E. Andreatta, A. Arce, E. Rodil and A.Soto, J. Solution Chem., 2010, 39, 371-383. [ Links ]
29 P.N. Sibiya and N. Deenadayalu, J. Chem. Thermodyn., 2008, 40, 1041-1045. [ Links ]
30 N. Deenadayalu, S. Sen and P.N. Sibiya, J. Chem. Thermodyn.,2009, 41, 538-548. [ Links ]
31 P.N. Sibiya and N. Deenadayalu, S. Afr. J. Chem., 2009, 62, 20-25. [ Links ]
32 P. Bhujrajh and N. Deenadayalu, J. Solution Chem., 2007, 36, 63133 N. [ Links ] Deenadayalu, K.C. Ngcongo, T.M. Letcher and D. Ramjugernath, J. Chem. Eng. Data, 2006, 51, 988-991. [ Links ]
34 N. Deenadayalu, S.H. Thango, T.M. Letcher and D. Ramjugernath, J. Chem. Thermodyn., 2006, 38, 542-546. [ Links ]
35 I. Bahadurand and N. Deenadayalu, J. Solution Chem., 2011, 40, 1528-1543 [ Links ]
36 M.A. Iglesias-Otero, J. Troncoso, E. Carballo and L. Romani, J. Chem. Eng. Data, 2008, 53, 1298-1301. [ Links ]
37 L. Moràvkovà and J. Linek, J. Chem. Thermodyn., 2008, 40, 671-676. [ Links ]
38 C-H. Tu, S-L. Lee and I-H. Peng, J. Chem. Eng. Data,2001, 46, 151-155. [ Links ]
Received 10 September 2012
Revised 16 May 2013
Accepted 7 June 2013
* To whom correspondence should be addressed. E-mail: nirmalad@dut.ac.za




![Polyaniline/SiO2 catalyzed one-pot synthesis of tetrahydrobenzo[b]pyran derivatives](/img/es/prev.gif)









