Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Chemistry
versión On-line ISSN 1996-840X
versión impresa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.66 Durban ago. 2013
RESEARCH ARTICLE
Adsorption of dyes using different types of sand: A review
Olugbenga Solomon Bello*; Isah Adewale Bello; Kayode Adesina Adegoke
Department of Pure and Applied Chemistry, Ladoke Akintola University of Technology P.M.B. 4000, Ogbomoso, Oyo State, Nigeria
ABSTRACT
The threat posed by increasing amount of dyes on daily basis, especially on our ecosystem, has brought a serious search for more efficient low-cost adsorbents. Sand is mixed with cement and water to make concrete, used in the manufacture of brick, glass and other materials, and it can also be used as a medium for the filtration of water. Sand, which is ubiquitous, has been used as an adsorbent because of its enormous availability in the local environment. This review reveals that further research must be conducted to bring to the fore the expansive laboratory, industrial and environmental usage of sand materials as dye adsorbents. Consequently, the usage of different types of sand in the field of adsorption science represents a viable and powerful tool, resulting into the superior improvement in pollution control and environmental preservation.
Keywords: Adsorption, dyes, low-cost adsorbents, sand.
Table of Contents
1. Introduction 117
2. Different types of sand materials and their uses 119
3. Sand materials used as adsorbents for dyes treatment 124
4. Challenges and future prospects 127
5. Conclusion 127
Acknowledgement 127
References 127
1. Introduction
Several dyes and their break-down products are toxic for living organisms because dyes are not easily degradable and are generally not removed from wastewater by conventional wastewater treatment systems; this makes it difficult to remove dyes from effluent.1,2 Organic dyes are an integral part of many industrial effluents which demand an appropriate method to dispose of them. Commonly suggested methods include biodegradation, photo-catalytic, photolytic and advanced oxidative degradation of these solutions.3-7 Considerable interest has recently been focused on using the adsorption technique for the removal of some dyes from solutions on various adsorbent surfaces such as clays,8-9 fly ash,10-11 peat,12 activated carbon,13-14 polymers15-16 and alumina.17-18 The process is known to be simple and efficiently treat dyes in concentrated form.
In practice, adsorption techniques are versatile and easy to adopt but adsorbent materials are costly and some cannot be regenerated for large-scale applications. There is therefore a clear need to use low-cost, renewable and easily available adsorbent material for such purposes, otherwise environmental protection will be in jeopardy. Water-soluble anionic dyes are found in wastewaters produced by the dyeing industries. They are toxic and need to be removed.19 Among some existing technologies, adsorption is one of the most efficient methods to remove dyes due to its simplicity and easy operational conditions.20-22
Currently, the textile industry produces a huge quantity of dyed wastewater. The colour and the non-biodegradable nature of the spent dye baths constitute serious environmental problems.
Despite a wide range of wastewater treatment techniques available, there is no single process capable of the adequate treatment for these effluents. Adsorption is an efficient method for the removal of dyes from wastewater and activated carbon is one of the most studied adsorptive materials.23-27 It has been found that the performance of the adsorbents depends on their textural properties (porosity, surface area). The pore size distribution determines the fraction of the structure that can be accessed by a molecule of a given size and shape. More recently, some authors started looking at the surface chemistry of activated carbons in order to interpret some results of dye adsorption experi-ments.25-28 It was shown that the surface chemistry of the activated carbon could play a key role in dye adsorption performance.
Adsorption is a major industrial separation technique for the purification of effluent media. It is a mass transfer operation through which a solid material can selectively remove dissolved components from an aqueous solution by attracting the dissolved solute to its surface. Therefore, it involves the interphase accumulation of concentration of substances at a surface or at the inter phase. This separation technique finds wide application in removal of dye from aqueous media. In particular, it finds application in textile, leather, dyeing, cosmetics, plastics, food and paper industries where water recovery is essential. In order to achieve and sustain this efficient recovery of desired water quality, a careful selection of adsorbent is paramount.1,29,30 However, most adsorbent materials in Nigeria are imported from other countries despite of the abundance of raw materials in Nigeria for the production of the required quantity of adsorbents for local industries.30 This situation is a concern and calls for serious efforts to search for adsorbents sourced from local raw materials such as plantain peels, animal hairs, corn cobs, coal, and animal bones, etc.30 A number of these adsorbents have been investigated for the removal of dyes from wastewater. These include amongst others; apple pomace and wheat straw,31 corncob and barley husk,32 maize cob, wood and rice hull,33 banana stalk,34 groundnut hull,35,36 oil palm ash,37,38 cocoa pod husks,39 mango peels,40 rice husk38,41-44 periwinkle shell,45-47 coconut shell,48Imperata cylindrica leaf49 and rubber seed coat.50
Activated carbon has been successfully used in removing coloured organic species and it is the most widely used adsorbent due to its high capacity for the adsorption of organic materi-als;51-52 and its effectiveness and versatility.5,54 One potential adsorbent material is sand, which can be utilized for such purposes as it can also bring unlimited number of economic and environmental benefits to the industrial waste-water treatment. Much work has been done on the use of sand to remove inorganic ions from solutions;55,56 however, little work has been done on sand surfaces for the removal of dyes.57,58 Therefore, in order to minimize the high cost and the difficulty of regeneration, a search for cheap, effective adsorbents such as sand derivatives is desirable.59
The potential of locally available sand was assessed for the removal of coomassie blue (CB), malachite green (MG) and safranin orange (SO) from aqueous solutions.59 Removal of various organic dyes from aqueous solution onto sand surface was carried out at room temperature.59 The conditions of adsorption of these dyes were optimized for maximum removal of these dyes from aqueous solutions in the absence and presence of various ions. It was seen that under these conditions, a maximum of 65-70 % of the dye could be removed from the solution onto the sand surface. The adsorption of the two dyes, namely coomassie blue and safranin orange, decreased substantially in the presence of added ions, namely sulfate, thiosulfate, acetate, potassium, nickel and zinc ions. The adsorption data for all the dyes investigated fitted well to the Freundlich equation.59 Sorp-tion kinetic data revealed that the adsorption kinetics followed a pseudo-second-order equation for all the three dyes investigated (Fig. 1).59 Furthermore, the Morris-Weber equation59 revealed that the diffusion coefficient values were comparable, and that malachite green reaches the equilibrium faster than the other two dyes,59
2. Different Types of Sand and their Uses
Depending on their chemical composition, size, grade and shape, sand is classified60,61 as follows:
2.1. Gypsum Sand (Fig. 2)

Gypsum is a very soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula CaSO4-2H2O.61 It can be used as a fertilizer, is the main constituent in many forms of plaster and is widely mined. As a mineral, it is alabaster, which has been used for sculptures by many cultures including Ancient Egypt, Mesopotamia and the Nottingham alabasters of medieval England. It has a hardness of 2 on the Mohs scale of mineral hardness. It forms as an evaporate mineral and as a hydration product of anhydrite. It is a rare sand type, composed of gypsum grains, because it is moderately soluble in water. Gypsum crystallizes out of concentrated solutions. It can also quite easily go into solution again. Anything soluble is generally not going to last long in sand. Gypsum is a relatively rare constituent of sand. An exception is a large dune field in New Mexico White Sands National Monument that is entirely composed of tabular gypsum grains.60
Gypsum board62 is primarily used as a finish for walls and ceilings, and is known in construction as drywall or plasterboard. It is a plaster ingredient (surgical splints, casting moulds, modelling), and has been used as fertilizer and soil conditioner: in the late 18th and early 19th centuries, Nova Scotia gypsum, often referred to as plaster, was a highly sought fertilizer for wheat fields in the United States. It is also used in ameliorating sodic soils. 63 Gypsum is used as a binder in fast-dry tennis court clay, as alabaster, i.e. a material for sculpture, especially in the ancient world before steel was developed, when its relative softness made it much easier to carve than stone. A wood substitute in the ancient world: for example, when wood became scarce due to deforestation on Bronze Age Crete, gypsum was employed in building construction at locations where wood was previously used.61 A tofu (soy bean curd) coagulant, making it ultimately a major source of dietary calcium, especially in Asian cultures which traditionally use few dairy products, it is also used to add hardness to water used for home brewing64 and a compo- nent of Portland cement used to prevent flash setting of concrete.
2.2. Silica sand (Fig. 3)

The chemical compound silicon dioxide, also known as silica (from the Latin isle), is an oxide of silicon with the chemical formula SiO265-66 and has been known for its hardness since ancient times. 67 Silica is most commonly found in nature as sand or quartz, as well as in the cell walls of diatoms 68-69. Silica sand is almost pure quartz.60 Quartz is a common mineral with the same chemical composition but quartz and silica are not synonyms. There are many light-coloured beach sands around the world but many of them (especially in low latitudes) are made of small pieces of corals and other sea creatures. This sand is calcareous (composed of calcium carbonate) but some biogenic grains are siliceous as well. For example, radiolarians (ameoboid protozoa) and diatoms (algae) have siliceous shells. Sometimes sandstone is said to be siliceous.60 Silica is manufactured in several forms including fused quartz, crystal, fumed silica (or pyrogenic silica), colloidal silica, silica gel and aerogel.70
Silica is used primarily in the production of glass for windows, drinking glasses, beverage bottles, and many other uses. The majority of optical fibres for telecommunications are also made from silica. It is a primary raw material for many white ware ceramics such as earthenware, stoneware, porcelain, as well as industrial Portland cement.68,71 Silica is a common additive in the production of foods, where it is used primarily as a flow agent in powdered foods, or to absorb water in hygroscopic applications. It is the primary component of diatomaceous68-69 earth, which has many uses ranging from filtration to insect control. It is also the primary component of rice husk ash, which is used, for example, in filtration and cement manufacturing. Thin films of silica grown on silicon wafers via thermal oxidation methods can be quite beneficial in microelectronics, where they act as electric insulators with high chemical stability72. In electrical applications, it can protect the silicon, store charge, block current, and even act as a controlled pathway to limit current flow.73.
2.3. Black Sand (Fig. 4)

This is sand that is black in colour. There are two types of black sand.60 The first is composed of volcanic minerals and lava fragments. Such sands are especially common on the coasts of volcanic islands (Hawaii, Canary Islands, Aleutians, etc. - they are black because many volcanic minerals and rocks are dark. Common rock types of volcanic islands are basalt (black when fresh), andesite (usually dark gray) and volcanic glass (often black in colour). Another type of black sand occurs mostly in the continental settings. It is heavy mineral sand, which has a specific gravity above 2.9. There are almost all colours present among the heavy minerals but compared to usually light-coloured quartzose sand, they seem to be dark. While some beaches are predominantly made of black sand, even other colour beaches (e.g. gold and white) can often have deposits of black sand, particularly after storms. Larger waves can sort out sand grains leaving deposits of heavy minerals visible on the surface of erosion scarps.74
Black sands are used by miners and prospectors to indicate the presence of a placer formation. Placer mining activities produce a concentrate that is composed mostly of black sand. Black sand concentrates often contain additional valuables, other than precious metals: rare earth elements, thorium, titanium, tungsten, zirconium and others are often fractionated during igneous processes into a common mineral-suite that becomes black sands after weathering and erosion.74,75
Several gemstones, such as garnet, topaz, ruby, sapphire, and diamond are found in placers and in the course of placer mining, and sands of these gems are found in black sands and concentrates. Purple or ruby-coloured garnet sand often forms a showy surface dressing on ocean beach placers.76
2.4. Desert Sand (Fig. 5)

Deserts cover wide areas, mostly between 10 and 30 degrees N and S of the equator.60 Many of these deserts are sandy, at least partly. Good example is the Sahara - the largest desert in the world. When we imagine desert sand, we probably think of sand dunes with the characteristics that dune sand is generally very well sorted, which means that all the sand grains are roughly the same size. Another characteristic feature of dune sand is the 'frosted' or scratched surfaces of sand grains, which is the result of countless collisions between windblown sand grains. Figure 5 shows desert sand, which is composed almost exclusively of rounded quartz grains.60
Desert sands are an important carbon sink on earth. Scientists discovered that bacteria living in the sands of the Kalahari desert in Africa help gather and store carbon dioxide from the air. Since carbon dioxide is one of the prime causes of global warming, these desert sands may play a critical role in preventing additional carbon dioxide from entering the atmosphere.77 Desert sands are also important but poorly understood depositional systems, which occur throughout much of geological time. Many desert sand deposits also include a minor but significant amount of inter-dune lacustrine and extra-dune fluvial, lacustrine or marine deposit, which provide important evidence for reconstructing the history of the sand body.77-78
2.5. Mixed Carbonate-Silicate Sand (Fig. 6)

These are calcium carbonate, for example aragonite, which has mostly been created, over the past half billion years, by various forms of life, like coral and shellfish. It is, for example, the primary form of sand apparent in areas where reefs have dominated the ecosystem for millions of years, like the Caribbean. As said earlier, the world of sands can be divided into two major parts - mineral sands and biogenic sands. These sand types occur often separately but they can also mix in all proportions and form hybrid carbonate-silicate sand type.79 This sand type is also found in low-latitude beaches as biogenic sands and it is very common. It is usually a mixture of biogenic and volcanic or biogenic and continental sands. Some sand samples are a mixture of organic and inorganic sand grains. Carbonate sands, are made of particles of CaCO3. The other is commonly called the 'siliciclastic sands', where 'silici-' refers to a chemical composition rich in silicate material and '-clastic' refers to the origin of the grains as clasts or fragments of silicate rocks.60,74
Some of the uses of carbonate sand are: (1) in agriculture: sandy soils are ideal for crops such as watermelons, peaches, and peanuts, and their excellent drainage characteristics make them suitable for intensive dairy farming. (2) Aquaria: sand makes a low-cost aquarium base material which some believe is better than gravel for home use. It is also an absolute necessity for salt-water reef tanks, which emulate environments composed largely of aragonite sand broken down from coral and shellfish. (3) Artificial reefs: geotextile bagged sand can serve as the foundation for new reefs. It also important in beach nourishment in which Governments move sand to beaches where tides, storms or deliberate changes to the shoreline erode the original sand.74 (4) Bricks: manufacturing plants add sand to a mixture of clay and other materials for manufacturing bricks. (5) Mortar, which is sand mixed with cement and sometimes lime to be used in masonry construction. (6) Concrete: sand is often a principal component of this critical construction material. (7) Hydraulic Fracturing: the drilling technique for natural gas also known as fracking use 'frac sand'. The rounded silica sand is used as a 'proppant', a material that holds the cracks open after hydraulic fracturing process, etc.
2.6. Biogenic Sand (Fig. 7)

Sand may be composed entirely of tiny skeletons type - sea shells, corals, forams, algae, echinoids, sponges, etc. - and therefore one biogenic sand may greatly differ from another.60 This sand is widespread in low-latitudes (less than 35 °) beaches. Most biogenic sands are light-coloured and their components are usually made of carbonate material, although, some organisms prefer silica. Sand dunes are also stabilized by biogenic soil crusts. These crusts comprise a variety of organisms, including cyano-bacteria, lichens and mosses, which live at the surface of desert soils.79
Biogenic sand is useful in enhancing the aggregation of sand grains, preventing saltation, and reducing wind erosion. Since most sandy soils are located in dry lands where the vegetation is patchy and generally sparse,80 the role of biogenic sand in stabilizing dunes is important and often crucial.81 Despite their significance and vast presence in the Kalahari, Australian and Central Asian deserts, soil crusts have rarely been considered in studies of dune dynamics.82,83
2.7. Volcanic Sand (Fig. 8)

s
Volcanically active regions have their own unique type of generally dark-coloured sand with a characteristic mineral assemblage.60 Volcanic sands have some characteristic minerals and mineral associations which are not present in every volcanic sand but if they are, they suggest that the particular sand could be of volcanic origin. Such minerals are olivine, pyroxene, and magnetite. Volcanic sands that contain relatively high content of dark minerals can be used as a natural fertilizer. The amount of the nutrients from this material available for the plant growth is very low. To increase the release of the nutrients to the environment, the weathering process of the minerals should be acceler-ated.84 Volcanic sand is also used to improve tyre traction. It plays an important role in the special rubber recipe used in Triple Tred Technology. The tyre features three zones - water zone, ice zone and dry zone - that promote superior traction in any kind of weather. This lets drivers confidently take on rain-drenched highways, and icy or snowy roads, or unanticipated situations on dry pavement.85
2.8. Heavy Mineral Sand (Fig. 9)

Mineral sands are different to most commodities; however, they share similarities with other commodity types, such as the importance of quality constraints of iron ore and coal or the importance of physical properties of say diamonds. The term 'mineral sands' normally refers to concentrations of heavy minerals in an alluvial (old beach or river system) environ-ment.86 Occasionally, these deposits are referred to as 'beach sands'. However, mineral sands are also found in large aeolian sand systems or 'dunal sands'. Heavy minerals are present in most sand types. However, they rarely make up more than few per cent of it.87 Sometimes heavy minerals become concentrated enough to form heavy and usually very beautiful sand. The source materials for heavy mineral sands are metamorphic and igneous rocks. Metamorphic rocks are source material for kyan-ite and garnet for example, although the latter may crystallize from magma as well. Magnetite is of igneous origin mostly.60
In today's globalized economy they constitute important resources, employing tens of thousands in the production and international trading of millions of tonnes per year. Heavy minerals are used in the manufacture of very important construction and industrial products. Without ilmenite and rutile there would be no snow-white paints and airplanes; without zircon no control rods for nuclear reactors; without tantalite no transistors; and without cassiterite no tin cans. Some heavy minerals are not easily substituted and are only available in a few countries. Many of them are thus very valuable and rare - wars have been and are still being waged over of them.88
2.9. Ooid Sand (Fig. 10)

Ooids are rounded pellets formed in shallow wave agitated water.60 Ooids are usually marine. Well-known locations where ooid sands are formed are the Persian Gulf, the Gulf of Mexico near the Yucatan Peninsula and the Bahama platform. Ooids are most commonly composed of calcium carbonate (calcite or aragon-ite), but can be composed of phosphate, chert, dolomite or iron minerals, including hematite. Dolomitic and chert ooids are most likely the result of the replacement of the original texture in limestone.74 Oolites are often used in the home aquarium industry because their small grain size (0.2 to 1.22 mm) is ideal for shallow static beds and bottom covering of up to one inch in depth. Also known as 'oolitic' sand, the sugar-sized round grains of this sand pass easily through the gills of gobies and other sand-sifting organisms. Importantly, this unusually smooth sand promotes the growth of bacteria, which are important biofilters in home aquaria. Because of its extremely small grain size, oolitic sand has a lot of surface area, which promotes high bacterial growth.76
2.10. Continental Sand (Fig. 11)

The name says it all. This sand is a common weathering product of the continental landmasses. This sand is usually yellow or light brown and often speckled with dark grains. It is common along passive continental margins and therefore the most widespread sand type on most of the European coasts for example.60 Continental sands are important in bathymetric features for commercial and recreational fishing areas if they provide important fish habitat; however, there is little evidence to refute or support that possibility. Adults, settled juveniles, and larvae of a number of fish species have been documented on sand ridges and in the immediate vicinity of sand ridges, indicating that these features are used by multiple fish at various life history stages.89 Although sand ridges may provide habitat for important fish species, sand ridges from Massachusetts to North Carolina including ridges off New Jersey90 have gained attention as potential locations from which to extract sand and gravel for ongoing beach nourishment projects and to provide construction materials.91
3. Sand Materials used as Adsorbents for Dye Treatment
3.1. Gypsum
Gypsum is a commonly available commodity used for treating industrial effluents.92 The abundance of gypsum in nature, besides its low cost, was the main factor in studying the potentials of this material as an adsorbent. Moreover, no pretreatment of this material is required as compared to other adsorbents such as activated coal or inorganic substances. Attention is focused on the use of gypsum as an alternative low-cost adsorbent for the removal of methylene blue (MB) from aqueous solutions due to the reason that many textile manufacturers use this and it releases aromatic amines (e.g. benzidine, methylene) and is a potential carcinogen.93 Effluents containing dyes are difficult to treat because most of these chemicals are not prone to aerobic digestion.94 There are several reported methods for the removal of pollutants from effluents; however, there is no single process which is capable of treating these effluents because of the complexity of the matrix.95 Practically, a combination of different processes is often used to achieve the desired water quality in the most economical way. Liquid-phase adsorption is one of the most studied methods for the removal of pollutants from wastewater since it generally will produce a high-quality treated effluent.8-9,96 The treatment of wastewaters by the adsorption process is an excellent choice especially if the sorbent is inexpensive and does not require an additional pre-treatment step before its application. Few reports are available on the use of gypsum as adsorbent for dye removal.92
Commercially obtained gypsum powder (unbranded) was used as an adsorbent material because of its availability in huge quantities, its cheapness and its application without prior treatment. The surface area of the gypsum sample was found by the nitrogen adsorption method using the Quantasorb Autosorb Automated gas sorption system (Quantochrome corporation).97 After dye adsorption, a significant change is observed in the structure of this adsorbent. The adsorbent appears to have a rough surface and pores containing a new shiny and bulky particle.92
Adsorption experiments were carried out by adding a fixed amount of gypsum (0.25 g) to a series of 250 mL conical flasks filled with 100 mL diluted solutions (5-25 mg L-1) of methylene blue dye. The conical flasks were then sealed and placed in a water-bath shaker and shaken at 100 rpm within a required time at 298 K. After pre-determined time intervals, the flasks were then removed from the shaker, and the final concentration of dye in the solution was measured at maximum wavelength of the dye solution (668 nm) by a CARY 50 UV/VIS spectrophotometer, using a 1-cm quartz cell.92
Since the solution pH has a considerable effect on dye removal, the pH of the solution was also changed to monitor the adsorption behaviour of dyes on gypsum samples. Changes in adsorption were then used to calculate the concentration and adsorption of the dye used in the study.92 The amount of dye adsorbed (mg g-1) increased with increase in time and then reached equilibrium. The initial dye concentration provides the necessary driving force to overcome the resistances to the mass transfer of MB between the aqueous and solid phases.58 A similar phenomenon was observed for the adsorption of methylene blue (MB) dye onto banana stalk waste98 pomelo (C. grandis) peel13 and castor seed shell.99
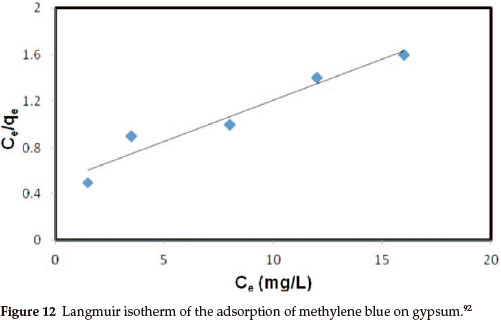
The adsorption of MB on gypsum was also studied as a function of contact time in order to find out the equilibrium time for maximum adsorption. The results showed that equilibrium time required for the adsorption of MB on gypsum ranged from 20 to 40 min. However, the samples were left for 40 min to ensure complete equilibrium. An equilibrium adsorption time of 135 min was reported for the adsorption of methylene blue onto wheat shells100 and 150 min for the adsorption of methylene blue on fallen phoenix tree leaves.101 Muhammad A. Rauf and co-workers studied the thermodynamics of the adsorption of some dyes.92 The pH of the dye solution plays an important role in the adsorption process, particularly on adsorption capacity. The amount of dye adsorption at equilibrium, i.e. qe (in mg g-1), was found to be maximum at natural pH (pH = 7.5). The adsorption amount was less in acidic media but remained almost constant in basic conditions. The observed low adsorption rate of MB on the gypsum at pH < 7.5 may be because the surface becomes positively charged, thus making (H+) ions compete effec- tively with dye cations causing a decrease in the amount of dye adsorbed. A similar behaviour was observed for MB adsorption on other adsorbents.102-104 Analyses of data using various adsorption models such as adsorption isotherms was used to relate the adsorbate concentration in the bulk and the adsorbed amount at the interface at equilibrium.92 In this regard the adsorption data were analysed by fitting them to different equations such as the Langmuir, Freundlich and Tempkin equations. The Langmuir isotherm graph followed a linear equation approach (Fig. 12).105 This suggests that the adsorption of MB on gypsum may be best described by the pseudo-second-order kinetic model.92 Similarly, the Weber and Morris plot, however, was also used to investigate the intra-particle diffusion mechanism. The equation used in this case is as follows:
qt = k1 t1/2 + C ,
where, k1 (mg g-1 min1/2) is the intra-particle diffusion rate constant. If intra-particle diffusion is rate-limited, the plot of qt versus t1/2 is linear and passes through the origin. In this case intra-particle diffusion is the sole rate-limiting step.59 However, if the linear plot does not pass through the origin some degree of boundary layer control is indicated and also that the intra-particle diffusion was not the only rate-controlling step.106
3.2. Bentonite-Sand Mix
Bentonite is composed of 74 % clay-sized (2 lm), 9 % sand (4.75 to 0.075 mm) and17 % silt-sized (0.075 to 0.002 mm) fractions. On treatment with HDPy+ ions, the HDPy+B specimen coagulates to sand-sized particles (sand content = 98 %, silt content = 2 %).107 Gaomiaozi (GMZ) bentonite has been extracted from the northern Chinese Nei Mongolia autonomous region, 300 km northwest from Beijing. There are 160 million tons with 120 million tons Na-bentonite reserves in the deposit and the mining area is about 72 km2. In China, GMZ bentonite has been selected as one of the candidates of buffer/backfill material for the geological disposal of highly radioactive waste.108-110 GMZ bentonite has attracted great interest in China because of its outstanding properties, such as its prominent high swelling and sealing abilities,111 cation exchange capacity and strong adsorption capacity.112
3.3. Ooid Sand
The utility of ooid sand, also called riverbed sand, lies in availability in ample amounts; it can be an economically viable alternative to costly adsorbents.113 Different thermodynamic parameters such as change in standard free energy (ΔG°), enthalpy (ΔH°) and entropy (ΔS°) have been determined.114-116
The values of ΔG° were found to be negative at all temperatures which indicate that the adsorption process is spontaneous in nature. As the temperature increases, the values of ΔG° decreases, indicating less driving force127 at elevated temperatures. The ΔG° addresses the possibility and feasibility of any reaction and more negative values of ΔG° reflect a more energetically favourable adsorption process.118 The value of the enthalpy change ΔH° was found to be negative for this system which confirms the exothermic nature of the process of adsorption. Equilibrium studies have demonstrated that the Langmuir model fits better than the Freundlich model for the adsorption equilibrium data in the examined concentration range. The value of the adsorption capacity of ooid sand was found to be significant, which indicates that it can be successfully used for the removal of Ni (II). Further, as the adsorbent is naturally available, it incurs no extra financial burden on the users and hence it can always be recommended for the treatment of Ni (II)-contain-ing waters and wastewaters.113
3.4. Desert Sand
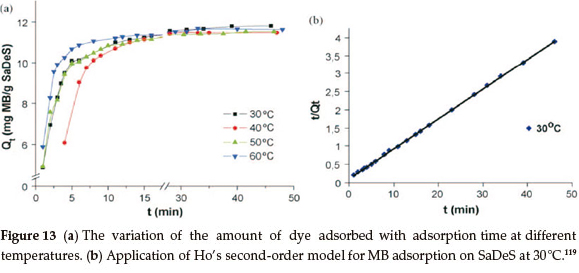
Desert sand contains active components that can strongly adsorb positively charged organic material from an aqueous solution. An example is sand in the present Sahara Desert (SaDeS) in Tozeur, Tunisia, the active component of which was related with kyanite, a negatively charged mineral that imparts electrostatic attraction towards cationic material.119 In recent years, Sahara Desert sand has become the subject of investigations related to its influence on the lower atmosphere and its effects on the oceanographic equilibrium. Many of these studies have reported that SaDeS has the capacity of adsorbing and carrying volatile organics.120-125 Although this fact is known, there are not many studies in the literature treating adsorption itself, while there are no studies focusing on removal of industrial wastes by the use of SaDeS.126 In view of the limited supply of water in countries of northern Africa, where SaDeS is abundant, use of this natural material for cleaning waste waters may be of great value 119
It has been noted that washed SaDeS loses its active component and its original microstructure is lost. More than 90 % of methylene blue may be removed from a 3.5 x 10-5 M25mL solution with the use of 20 mg of adsorbent. The adsorption is found to be pseudo-second-order.132 The Pseudo-first-order and pseudo-second-order models have been widely used in understanding dye kinetics.103,127-129 These models were tested for the adsorption of MB on SaDeS and the best model was selected depending on the linear regression correlation coefficient. The pseudo-first-order model has been described by Lagergren,130 whereas the pseudo-second-order model was also applied using Ho's pseudo-second-order model.131
The thermodynamic parameters, i.e. the Gibbs free energy, enthalpy change and entropy change, were calculated and found to be -6411 J mol-1, -30360 Jmol-1 and -76.58 Jmol-1 K, respectively.15,132 These values indicate that the adsorption of MB onto desert sand has a decreased randomness at the solid-solution interface, and is an exothermic spontaneous process at low temperatures.133 It is therefore proposed that natural SaDeS is a good candidate as a low-cost adsorbent to be used for the removal of dyes from water119 (Fig. 13a, b).
3.5. Silica and Quartz Sand
These are natural sands, eroded from mountain rock, which is mined from where it was deposited. The host rock determines the exact mineral composition. Due to its chemical hardness, it is therefore found to be extremely resistant to weathering and breakdown. The sorbent was sediment obtained from Guanting Reservoir (Beijing, China), which contained 25 % sand, 67 % silt, 8 % clay, and 2.06 % organic carbon.134 The neutral red (NR) dye in aqueous solution present as a pollutant material in textile waste water6 was removed by adsorption on sand. This removal of NR dye from the aqueous solution onto the sand surface was carried out at room temperature (298 K). It was noted that the local sand sample used as an adsorbent was initially characterized for its textural properties including surface area, mean pore radius and total pore volume.135 These properties were examined from the low-temperature adsorption of nitrogen on sand samples at 77 K. The conditions of maximum adsorption of the dye were optimized. It was seen that under optimized conditions, up to 85 % ot the dye could be removed from the solution onto the sand surface. The experimental data were fitted to the Freundlich isotherm which showed that adsorption was monolayer in nature.136 The rate constant for adsorption of the NR dye was found to be 3.85 min-1 using the Lagergen equation.55 Furthermore, in order to determine the actual rate-controlling step involved in the dye adsorption process, the adsorption data were further analysed by using the kinetic expression given by Boyd et al.137 This equation revealed that the adsorption process was physical in nature and that the dye did not actually diffuse in the sand.136 This indicates that a particle diffusion mechanism is not operative and hence does not control the kinetics of NR dye adsorption on sand.136 The adsorption of the dye decreased in the presence of all the added ions; the effect was more pronounced in the presence of chloride, sodium and copper ions.
4. Challenges and Future Prospects
The world is currently facing the worst environmental crisis in its entire history. Within the space of a few decades, the huge increase in the production of waste and growing environmental concern have become one of the most challenging topics, and critical consideration should now be given towards the recovery of contaminated resources. Since industrial effluents are always contaminated with various additives such as inorganic salts, it is therefore important to study the effect of these ions on the adsorption property of dye solutions. The adsorption of dye in the presence of anions (added as sodium salts) and cations (added in the nitrate form) should be carried out. One major problem is that the shape, size and grading of sands are impossible to measure and observe collectively since they differ from type to type and use, and so attention should be given to this aspect, both technologically and scientifically. This will go a long way in reducing the amount of stress and time it consumes.
The interaction between adsorbate and the adsorbent should be studied to establish the relationships and roles of functional groups in the dye adsorption process. Critical investigation is needed in studying the adsorptive properties and the molecular structure/surface group (both the size, shape and grading) of the adsorbents. The efficiency of these sand materials with real industrial dye effluents should also be investigated.
Another challenge is that the adsorbate-adsorbent mixture is difficult to separate after adsorption; this limits the practical application of sand materials. Research is currently in progress to find new, modified and practical methods that can not only separate sand materials from water effectively, but also improve the dye removal rate. The process of recycling, regeneration and reuse of sand materials is important.
5. Conclusion
Despite the various drawbacks and challenges identified, great progress in this area can be expected in the near future. Low-cost adsorbents have found use at least at laboratory scale for the treatment of coloured effluents with different degrees of successes. Some materials like periwinkle shells, cocoa pod husks, rubber seed coat, ackee apple seeds, groundnut hull, papaya seeds, etc., have shown considerable adsorption capacity. However, the applications of these materials are limited practically because the adsorbents are not available in large amounts. This shows that, despite having a significant capacity for dye adsorption, most of these materials are not produced in large quantities. Consequently, they are not available in sufficient bulk to be commercialized for full-scale application. Different types of sand can act as an alternative to activated carbon. To be useful, they must have both a high adsorption capacity and be available in bulk at low or no cost. Different sand types, which are available in bulk, have a considerable adsorption capacity for anionic, cationic and nonionic dye removal.
Acknowledgement
The corresponding author acknowledges the support obtained from Third World Academy of Science (TWAS) in the form of a grant; Research Grant number: 11-249 RG/CHE/AF/AC_1_ UNESCO FR: 3240262674
References and Notes
1 M.M. Abd El-Latif, M.F. El-Kady, A.M. Ibrahim and M.E. Ossman, Am. J. Sci., 2010, 6(5), 280-292. [ Links ]
2 F. Kargi and S. Ozmihci, Enzyme Microb. Technol., 2004, 35, 267-271. [ Links ]
3 G.M. Walker, S. Hensen, J.A. Hanna and S.J. Allen, Water Res., 2003, 37, 2081-2089. [ Links ]
4 P.M. Malik and S.K. Saha, Sep. Purif. Technol., 2003, 31, 241- 250. [ Links ]
5 K.C. Chen, J.Y. Wu, D.J. Liou and S.C. Hwang, J. Biotechnol, 2003, 10, 57-68. [ Links ]
6 S.H. Lin and F.C. Peng, Water Res., 1996, 30, 587-593 [ Links ]
7 M.A. Rauf, S. Ashraf and S.N. Alhadrami, Dyes Pigm, 2005, 66, 197-200. [ Links ]
8 S Al-Asheh, F. Banat and L. Abu-Aitah, Adsorp. Sci. Technol., 2003, 21, 451-462. [ Links ]
9 M.A. Khraisheh and M.S. Alg-Houti, Adsorp., 2005, 11, 547-549. [ Links ]
10 I. Janos, H. Buchtova and M. Ryznarova, Water Res., 2003, 37, 4938-4944. [ Links ]
11 D. Mohan, K.P. Singh, G. Singh and K. Kumar, Ind. Eng. Chem. Res, 2002, 41, 3688-3695. [ Links ]
12 G. McKay and S.J. Allen, J. Sep. Process Technol., 1983, 4, 1-7. [ Links ]
13 S. Wang and H. Li, Dyes Pigm., 2007, 72, 308-314 [ Links ]
14 I. A.W. Tan, B.H Hameed and A.L. Ahmad, J. Chem. Eng., 2007, 127,111-119. [ Links ]
15 M.J. Iqbal and M.N. Ashiq, J. Hazard. Mater., 2007, 139, 57-66. [ Links ]
16 E.Y. Ozmen and M. Yilmaz, J. Hazard. Mater., 2007, 2, 42. [ Links ]
17 M. Lehocky and A. Mracek, Czech. J. Phys, 2006, 56, 1277-1282. [ Links ]
18 R.G. Harris, J.D. Wells and B.B. Johnson, Colloid. Surf. A, 2001, 180, 131-140. [ Links ]
19 M. Nakamura, H. Saitoh, Y. Maejima, S.I. Yamagiwa and S. Kaneko, Fresenius J. Anal. Chem., 1989, 335, 573-575. [ Links ]
20 E. Eren, J. Turkey, Hazard. Mater., 2009, 162, 1355-1363. [ Links ]
21 B.K. Nandi, A. Goswami and M.K. Purkait, Appl. Clay. Sci., 2009, 42, 583-590. [ Links ]
22 A.B. Karim, B. Mounir, M. Hachkar, M. Bakasse and A. Yaacoubi, J. Hazard. Mater., 2009, 168, 304-309. [ Links ]
23 G.M. Walker and L.R. Weatherley, Sep. Sci. Technol., 2000, 35, 1329-41. [ Links ]
24 C. Pelekani and VL.Snoeyink, Carbon, 2001, 39, 25-37. [ Links ]
25 M.F.R. Pereira, S.F. Soares, J.J.M. Orfao and J.L. Figueiredo, Carbon, 2003, 41, 811-21. [ Links ]
26 K.P. Singh, D. Mohan, S. Sinha, G.S. Tondon and D. Gosh, Ind. Eng. Chem. Res., 2003, 42, 1965-76. [ Links ]
27 H. Valdes, M. Sanchez-Polo, J. Rivera-Utrilla and C.A. Zaror, Langmuir, 2002, 18, 2111-6 [ Links ]
28 Y. Al-Degs, M.A.M. Khraisheh, S.J. Allen and M.N. Ahmad, Wat. Res., 2000, 34, 927-35. [ Links ]
29 T.A. Albarins and T.M. Hela, Removal of Dyes from Aqueous Solutions by Adsorption on Mixture of Fly Ash Soil in Batch and Column Techniques. Department of Chemistry, University of Loannins, Loanina, Greece, 1993. [ Links ]
30 M.C. Menkiti and O.D. Onukwuli, J. New York Sci., 2011, 4, (2)91-95. [ Links ]
31 T. Robinson, B. Chandran and P. Nigam P, Wat. Res., 2002, 36, 2824-2830. [ Links ]
32 T. Robinson, G. McMullan, R. Marchant R and P. Nigam, Bioresour. Technol., 2001, 77, 247-253. [ Links ]
33 K.S. Low, C.K. Lee and B.F. Tan, Biotechnol. Appl. Biochem., 2000, 87, 233-239. [ Links ]
34 O.S. Bello, M.A. Ahmad and N. Ahmad, Chem. Ecol., 2012, 28(2), 153-67. [ Links ]
35 O.S. Bello, T.A. Fatona, F.S. Falaye, O.M. Osuolale and V.O. Njoku, Environ. Eng. Sci., 2012, 29(3), 186-94. [ Links ]
36 Z. Zawani, C.A. Luqman and T.S.Y. Chong, Euro. J. Sci. Res., 2009, 37(1), 63-71. [ Links ]
37 M.B. Hasan, Adsorption ofReactive Azo Dyes on Chitosan/Oil Palm Ash Composite Adsorbent: Batch and Continuous Studies, M.Sc. thesis, Universiti Sains Malaysia, 2008. [ Links ]
38 K.Y. Foo and B.H. Hameed, J. Hazard. Mater, 2009, 172, 523-531. [ Links ]
39 O.S. Bello and M.A. Ahmad, Toxicol. Environ. Chem., 2011, 93(7), 1298-1308. [ Links ]
40 O.S. Bello and M.A. Ahmad, Adsorption of dyes from aqueous solution using chemical activated mango peels. 2nd International Conference on Environmental Science and Technology (ICEST), 2011. [ Links ]
41 N.E.M. Yahaya, M.F. Pakir, M. Latiff, I. Abustan, O.S. Bello and M.A. Ahmad, Int. J. Environ. Technol., 2010, 10(06), 132-36. [ Links ]
42 N.E.M. Yahaya, M.F. Pakir, M. Latiff, I. Abustan, O.S. Bello and M.A. Ahmad Int. J. Environ. Technol. 10(06) 2010, 47-51. [ Links ]
43 N.E.M. Yahaya, M.F. Pakir, M. Latiff, I. Abustan, O.S. Bello and M.A. Ahmad, Int. J. Environ. Technol., 2011, 11(01), 207-11. [ Links ]
44 N.E.M. Yahaya, M.F. Pakir, M. Latiff, I. Abustan, O.S.Bello and M.A. Ahmad, Int. J. Environ. Technol, 2011, 11(01), 248-52. [ Links ]
45 O.S. Bello and M.A. Ahmad, Sep. Sci. Technol., 2011, 46, 2367-79. [ Links ]
46 O.S. Bello and M.A. Ahmad, Chem. Ecol., 2011, 27(5), 481-92. [ Links ]
47 O.S. Bello, I.A. Adeogun and J.C. Ajaelu, Chem. Ecol., 2008, 24(4), 285-95. [ Links ]
48 O.S. Bello and M.A. Ahmad, Sep. Sci. Technol., 2012b, 47(6), 903-12. [ Links ]
49 O.S. Bello and B. Semire, Toxicol. Environ. Chem., 2012; 94(6), 1114-1124. [ Links ]
50 O.S. Bello and M.A. Ahmad, Bulgarian J. Sci. Edu, 2012, 21(3), 389-395. [ Links ]
51 R.S. Joan, F.C. Wu and R.L. Tseng, 1997, Environ. Technol., 1997, 18, 525-531. [ Links ]
52 L. Markovska, V. Meshko, V. Noveski and M. Marinovski, J. Serb. Chem. Soc., 2001, 66, 463^75. [ Links ]
53 B. Chen, C.W Hui and G. McKay, Langmuir, 2001, 17, 740-748. [ Links ]
54 M.K. Purkait, A. Maiti, S. DasGupta and S. De, J. Hazard. Mater., 2007, 145, 287-295. [ Links ]
55 M.A. Rauf, M.J. Iqbal, I. Ellahi and S.M. Hasany, Adsorp. Sci. Technol., 1996, 13(2), 97-104. [ Links ]
56 S.I.H. Taqvi, S.M. Hasany and M.I. Bhanger, J. Hazard. Mater., 2007, 141, 37-44. [ Links ]
57 S.B. Bukallah, M.A. Rauf andS.S. Al Ali, Dyes Pigm., 2007, 74, 85-87. [ Links ]
58 M.A. Rauf, I. Shehadi and W.W. Hassan, Dyes Pigm., 2007, 75, 723-726. [ Links ]
59 M.F. Rauf, S.B. Bukallah, F.A. Hamour, A.S. Nasir, J. Chem. Eng., 2008, 137, 238-243 [ Links ]
60 http://www.sandatlas.org [ Links ]
61 K. Cornelis and S.H. Cornelius Jr., Manual ofMineralogy, 20th edn, John Wiley, 1985, 352-353. [ Links ]
62 A.E.S. Van Driessche, L.G.Benning, J.D. Rodriguez-Blanco, M. Ossorio, P. Bots andJ.M. García-Ruiz, Science, 2012, 336(6077), 69-72. [ Links ]
63 J.D. Oster and H. Frenkel, Soil Sci. Soc. Am. J., 1980, 44(1), 41-45. [ Links ]
64 L.C. Urquhart, Civil Engineering Handbook, McGraw-Hill Book Company, New York, 1959. [ Links ]
65 B.J. Skinner and D.E. Appleman, Am. Mineral., 1963, 48, 854-867. [ Links ]
66 A.F. Holleman and E. Wiberg, Inorganic Chemistry, Academic Press, San Diego, 2001. [ Links ]
67 N.N. Greenwood and A. Earnshaw, Chemistry of the Elements, Oxford Pergamon Press, New York, 1984, 393-399. [ Links ].
68 R.K. Iler, The Chemistry of Silica, Plenum Press, New York, 1979, ISBN 0-471-02404-X. [ Links ]
69 T.W. Lynn Jr., Technol. Culture, 1961, 2, (2), 97-111. [ Links ]
70 D. Robert and N. Yoshio, Handbook ofSemiconductor Manufacturing Technology, CRC Press, Boca Raton, 2007. [ Links ]
71 A.F. Wright and M.S. Lehmann, J. Solid State Chem., 1981, 36(3), 371-80. [ Links ]
72 G.A. Lager, J.D. Jorgensen and F.J. Rotella, J. Appl. Phys, 1982, 53(10), 6751-6756. [ Links ]
73 R. Michael, IEEE Spectr., 2007. [ Links ]
74 L.C. Peck, Geol. Surv. Bull, 1964, 21, 1170. [ Links ]
75 C.S. Cockell and J.A. Raven, Philos. Trans. R. Soc. A., 2007 365 (1856), 1889-1901. [ Links ]
76 R. Atkinson and F. Atkinson, Rocks & Minerals (The Observer's Books of London: Bloomsbury), 1992, [1979], 161-162. [ Links ]
77 M. Sarnthein, Nature, 1978, 272, (5648), 43-46. [ Links ]
78 N. Lancaster Episodes, 1988, 11(1), 79. [ Links ]
79 J. Belnap and O.L. Lange, Structure and Functioning of Biological Soil Crusts, Springer, 2001, 80. [ Links ]
80 K. PyeandH. Tsoar, Aeolian Sand and Dunes, 2ndedn.,Springer, 1990, [ Links ]
81 M. Veste, S.W. Breckle and W. Wucherer, Sustainable Land Use in Deserts, Springer, 2001, 357-367. [ Links ]
82 H. Yizhaq, Phys. Rev. Lett, 2007, 98, 188001. [ Links ]
83 O. Duran, Phys. Rev. Lett., 2006, 97, 188001. [ Links ]
84 I. Iskandar and J. Irwanti, Tanah dan Lingkungan, 2003, 5(1), 23-28. [ Links ]
85 A Zojomo, J. Mater. Online, 2006, 2, 1-9. [ Links ]
86 J. Greg, BEc, LLB, FAIM, MAICD, Iluka Res. Ltd, [2013], 1-26 [ Links ]
87 B. Hou and I.R. Warland, MESAJ., 2005, 37, 4-12. [ Links ]
88 T.E. Gerner Jr, Geogia. Geol. Surv. Inf. Circ, 1978, 49(8), 25-36. [ Links ]
89 K.W. Able, M.P. Fahay, D.A. Witting, R.S. McBride and S.M. Hagan, Estuar. Coast. Shelf Sci., 2006, 66, 280-290. [ Links ]
90 M.R. Byrnes, R.H. Hammer, T.D. Thibaut and D.B. Snyder, J. Coast. Res., 2004, 20(1), 25-43. [ Links ]
91 B.S. Drucker, W. Waskes and M.R. Byrnes J. Coast. Res., 2004, 20, (1), 1-5. [ Links ]
92 Muhammad, A, Rauf, I, Shehadeh, A. Amal & A. Al-Zamly, World Acad. Sci. Eng. Technol., 2009, 55, 609-613. [ Links ]
93 M. Boeningo, Carcinogenicity and Metabolism of Azodyes Especially Derived from Benzidine, DNHS (NIOSH) Publication, U.S. Government Printing Office, Washington DC, 1994, 80-119. [ Links ]
94 I.A. Alaton, B.H. Gursoy and J.E. Schimidt, Dyes Pigm., 2008, 78, 117-130. [ Links ]
95 S.M. Ghoreishi and R. Haghighi, J. Chem. Eng., 2003, 95, 163-169. [ Links ]
96 A.R. Cestari, E.F.S. Viera and S.A. Mota, J. Hazard. Mater., 2008, 160, 337-343. [ Links ]
97 N.A. Seaton, J.P.R.B. Walton and N. Quirke, Carbon., 1989, 27, 853-861. [ Links ]
98 B.H. Hameed, A.T.M. Din and A.L. Ahmed, J. Hazard. Mater., 2007, 141, 819-825. [ Links ]
99 Z.N. Ni, S.J. Xia, L.G. Wang, F.F. Xing and G.X. Pan, J. Colloid. Interf. Sci., 2007, 316, 284-291. [ Links ]
100 A.E, Ofomaja and Y.S. Ho, Dyes Pigm., 2007, 74, 60-66. [ Links ]
101 M.V Sureshkumar and C. Namasivayam, Colloid. Surf. A., 2008, 317, 277-283. [ Links ]
102 B.H. Hameed, J. Hazard. Mater., 2009, 162, 344-350. [ Links ]
103 Y. Bulut and H. Aydön, Desalin, 2006, 194, 259-267. [ Links ]
104 F.A. Pavan, A.C. Mazzocato and Y. Gushikem, Bioresour. Tech., 2008, 99, 3162-3165. [ Links ]
105 I. Langmuir, J. Am. Chem. Soc., 1918, 40, 1361-1403. [ Links ]
106 W.J. Weber and J.C. Morris, J. Sanit. Eng. Div., Am. Soc. Chem. Eng., 1963, 89, 31-59. [ Links ]
107 S. Chegrouche, A. Mellah and S. Telmoune, Water Res., 1997, 31, 1733-1737. [ Links ]
108 M. Sudhakar, S. Rao and S. Sivachidambaram, Environ. Earth Sci., 2013, 68(2), 559-566. [ Links ]
109 Y.M. Liu and, Z.R. Chen, Acta Mineral Sinica., 2001, 21, 541-543 (in Chinese). [ Links ]
110 S.T. Yang, J.X. Li, Y. Lu, Y.X. Chen and X.K. Wang, Appl. Radiat. Isot, 2009, 67, 1600-1608. [ Links ]
111 J.X. Li, J. Hu, G.D. Sheng, G.X. Zhao and Q. Huang, Colloid. Surf. A., 2009, 349 195-201. [ Links ]
112 B. Qin, Z.H. Chen, Y.M. Liu and J. Wang, Chin. J. Geotech. Eng., 2008, 30, 1005-1010. [ Links ]
113 W.M. Ye,M. Wan, B. Chen, Y.G. Chen and Y. Cui, J. Central South Univ. Technol., 2009, 16, 821-826 [ Links ]
114 N. Rajic, D. Stojakovic, M. Jovanovic,N.Z. Logar, M. Mazaj and V Kaucic, Appl. Surf. Sci., 2010, 257, 1524-1532. [ Links ]
115 H.D. Choi, W.S. Jung, J.M. Cho, B.G. Ryu, J.S. Yang and K. Baek, J. Hazard. Mater., 2009, 166, 642-646. [ Links ]
116 VK. Gupta, A. Mittal, L. Kurup and J. Mittal, J. Colloid Interf. Sci., 2006, 304(1), 52-57. [ Links ]
117 J.C. Moreno-Piraján andL. Giraldo, J. Anal. Appl. Pyrolysis., 2011, 90, 42-47. [ Links ]
118. A.N. Kosasih, J. Febrianto, J. Sunarso, Y.H. Ju, N. Indraswati and S. Ismadji, J. Hazard. Mater., 2010, 180, 366-374. [ Links ]
119 Y. Sandeep, V. Srivastava, S. Banerjee, G. Fethiye & Y. Sharma, Environ. Sci. Pollut. Res., 2012, 80-93. [ Links ]
120 C. Varlikli, V. Bekiari, M. Kus, N. Boduroglu, I. Oner, P. Lianos, G. Lyberatos and I. Siddik, J. Hazard. Mater., 2009, 170, 27-3420. [ Links ]
121 A.S. Goudie andN.J. Middleton, Earth Sci. Rev., 2001, 56, 179-204. [ Links ]
122 Y.rel,U. Dayan,R. Rabi, Y. RudichandM. Stein,Environ. Sci. Technol., 2006, 40, 2996-3005. [ Links ]
123 M. Kocak, N. Mihalopoulos and N. Kubilay, J. Atmos. Environ., 2007, 41, 7351-7368. [ Links ]
124 A.C. Saydam and I. Polar, Ecol. Environ, 1999, 36, 742-6. [ Links ]
125 C.R. Usher, A.E. Michel and V.H. Grassian, Chem. Rev., 2003, 103, 4883-4939. [ Links ]
126 G. Pan, M.D. Krom and B Herut, Environ. Sci. Technol., 2002, 36, 3519-3524. [ Links ]
127 A. Zertal, M. Jacquet, B. Lavedrine and T. Sehili, Chemosphere, 2005, 58, 1431-1437. [ Links ]
128 A. Gurses, C. Dogar, M. Yalcrn, M. Acikyildiz, R. Bayrak and S.J. Karaca, J. Hazard. Mater., 2006, 131, 217-228. [ Links ]
129 N. Kannan and M.M. Sundaram, Dyes Pigm, 2001, 51: 25^0. [ Links ]
130 M. Dogan and M. Alkan, Chemosphere, 2003, 50, 517-528. [ Links ]
131 S. Lagergren, Handlingar, 1898, 24, 1-39. [ Links ]
132 Y.S. Ho and G. McKay, J. Chem. Eng., 1998, 70, 115-124. [ Links ]
133 V. Ponnusami, S. Vikram and S.N. Srivastava, J. Hazard. Mater., 2008, 152, 276-286. [ Links ]
134 M. Zhaoa, Z. Tang and P. Liu, J. Hazard. Mater, 2008, 158, 43-51. [ Links ]
135 H.T. Qing and X.T. Hong, Chemosphere, 2004, 56, 31-38 [ Links ]
136 M.A. Rauf, A.S. Ihsan and H.W. Walaa, Dyes Pigm., 2007, 75, [ Links ]
137 W. Shaobin and H. Li, Dyes Pigm., 2007, 72, 308-14. [ Links ]
Received 21 February 2013
Revised 7 March 2013
Accepted 13 March 2013
* To whom correspondence should be addressed. E-mail: osbello06@gmail.com














