Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Chemistry
versão On-line ISSN 1996-840X
versão impressa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.66 Durban Ago. 2013
RESEARCH ARTICLE
One-pot, facile synthesis of quinoxaline derivatives from bis-aryl α-hydroxyketones and o-arenediamines using KMnO4/CuSO4
Ahmad Khorramabadi-zad*; Mohammad Azadmanesh; Somaye Mohammadi
Faculty of Chemistry, Bu-Ali Sina University, Hamedan 65174, Iran
ABSTRACT
KMnO4/CuSO4, a readily available reagent combination, was found to be effective for the high-yield synthesis of quinoxaline derivatives from bis-aryl o-hydroxyketones and o-arenediamines in hot ethanol.
Keywords: Quinoxalines, bis-aryl α-hydroxyketone, oxidation, Lewis acids, KMnO4, CuSO4.
1. Introduction
Quinoxaline derivatives are an important class of nitrogen-containing heterocycles in medicinal chemistry.1-3 For example, quinoxalines are a part of various antibiotics such as echinomycin, leromycin and actinomycin, which are known to inhibit the growth of Gram-positive bacteria and are also active against various transplantable tumours.4 In addition, they are well known for their application in dyes,5 efficient electroluminescent materials,6 organic semiconductors,7 building blocks for the synthesis of anion receptors,8 cavitands,9 dehydroannulenes10 and DNA cleaving agents.11 Different methods for the preparation of quinoxaline derivatives have been published in the literature.12 The general method for the synthesis of quinoxalines is the condensation of o-arenediamines with 1,2-dicarbonyl compounds in refluxing ethanol in the presence of acetic acid.13 Improved methods have been reported using different catalysts, including I2,14-15 sulfamic acid,16 Montmorillonite K-10,17 polyaniline-sulfate salt18 H6P2W18O62.24H2O,19 InCl3,20 MnCl2,21 CuSO4.5H2O,22 Zn[(L)proline],23 Ga(OTf)3,24 PEG-400,25 keggin heteropoly acid,26 IBX27 and SBA-Pr-SO3H28 have been explored.
The above cited references mostly use 1,2-diketones as starting materials, which are themselves prepared from a-hydroxyketones. Therefore, a more appropriate protocol for the synthesis of quinoxalines would be the in situ oxidation of the latter. However, a survey of the literature shows that there are few reports concerning this strategy, utilizing for example the reagents CAN,29,30 Pd(OAc)231 and MnO2.32 Notably, each of these reagents has its own drawback, namely, CAN contains a heavy metal cation which is not environmentally friendly, the reaction time in the presence of Pd(OAc)2 is about 24 hours and, in the case of MnO2, which is commercially available, it needs to be activated (e.g. with KOH in methanol).32
According to a recently published review,33 iron-exchanged molybdophosphoric acid,34 manganese octahedral molecular sieves35 and ruthenium on charcoal36 (in the presence of randomly methylated cyclodextrin) have also been used for the synthesis of quinoxalines from a-hydroxyketones. However, catalysts or co-catalysts used in cited references are either expensive or have to be prepared by sophisticated methods.
Hence, some of the synthesis protocols reported above suffer from one or more disadvantages, such as harsh reaction conditions, poor yields, prolonged reaction times, corrosive reagents, difficult work-ups and often expensive acid catalysts. On the other hand, a literature survey shows that KMnO4/CuSO4 mixture is a good choice for the oxidation of alcohols, oxidative coupling of primary aromatic amines, as well as conversion of aryl and alkyl sulfides into their corresponding sulfones.37 Based on the above discussion therefore, it was decided to use KMnO4/CuSO4 for the preparation of substituted quinoxalines from bis-aryl a-hydroxyketones and o-arenediamines (Scheme 1).

2. Results and Discussion
As was mentioned above, the KMnO4/CuSO4 system may be used for the oxidation of bis-aryl a-hydroxyketones to bis-aryl diketones. In fact, control tests confirmed that the co-presence of KMnO4 and CuSO4 is necessary for the reaction to proceed. In our hands, when use was made of this mixture in hot EtOH media, a black precipitate of MnO2 was generated in situ by the action of KMnO4 on hot EtOH during the reaction. Formation of black precipitate of MnO2 could be proved by qualitative tests. This freshly prepared and active MnO2 is a mild oxidant which can easily oxidize benzylic hydroxyls into the corresponding carbonyl groups, for which three routes have been proposed.38
In conclusion, this is the first report in which use is made of a KMnO4/CuSO4 mixture for the one-pot, direct synthesis of quin-oxaline derivatives from bis-aryl a-hydroxyketones. The advantages of this procedure, in addition to its being a one-pot reaction, are the use of a green solvent, a cheap and readily available oxidizing agent, and high product yields when starting from bis-aryl a-hydroxyketones.
In order to show the efficiency of the present method, a comparison of reaction times and yields is provided in Table 1 for the preparation of substituted quinoxalines from bis-aryl a-hydroxyketones and aryl 1,2-diamine. As is seen in this table, the present method (entry 1) is superior to that of the other ones in terms of both reaction times and yields, with the additional advantage of being less expensive.
3. Experimental
Melting point apparatus Stuart model SMP3 was used for measuring melting points. IR spectra were recorded on a PerkinElmer series II spectrometer. 1H-NMR and 13C-NMR spectra were recorded in CDCl3 using a Bruker DRX-300 spectrometer at 300 and 75 MHz, respectively. All chemicals and solvents were purchased from Aldrich, Merck and Fluka, and were used as received. Melting points and spectral data of all products are fully consistent with those of the reported ones.
3.1. Preparation of KMnO4/CuSO4 Mixture
The optimized amounts of KMnO4 and CuSO4 i.e. 5 mmol and 1 mmol, respectively, were ground together in a mortar to obtain a purple powder.
3.2. General Procedure for the Synthesis of Quinoxalines
Bis-aryl a-hydroxyketone (1.0 mmol), aromatic o-arenedia-mine (1.0 mmol) and the mixture of KMnO4/CuSO4 (0.35 mmol) were stirred and heated at reflux in ethanol. Progress of the reaction was monitored b y TLC. After completion of the reaction, the mixture was poured in cold water (50 mL), the precipitated solid was filtered, washed several times with water, dried and recrystallized from ethanol. The products were characterized by spectral and physical data. The experimental results are summarized in Table 2.
3.3. Spectral Data of Quinoxaline Derivatives
2,3-Diphenylquinoxaline (1): δh (CDCl3, 300 MHz): 7.35-7.37 (m, 6H), 7.56-7.59 (m, 4H), 7.74-7.77 (m, 2H), 8.20-8.23 (m, 2H); δc (CDCl3, 75 MHz): 128.3,128.9,129.2, 129.9, 130.0,139.1,141.2, 153.5; IR (KBr, cm-1): 3056,1478,1442,1396,1347,1142,1058,978, 770, 697, 598, 539.
6-Methyl-2,3-diphenylquinoxaline (2): δh (CDCl3, 300 MHz): 2.63 (3H, s), 7.36 (m, 6H), 7.53-7.55 (m, 4H), 7.60-7.63 (d, 1H), 7.98 (s, 1H)), 8.08-8.11 (d, 1H); δc (CDCl3, 75 MHz): 22.0, 128.0, 128.3, 128.7, 128.7, 129.9, 132.4, 139.2, 139.7, 140.6, 141.3, 152.6, 153.3; IR (KBr, cm-1): 3083,3054,3026,1619,1484,1445,1345,1201,1138, 1060, 1023, 979, 833, 775, 703, 695, 595, 545.
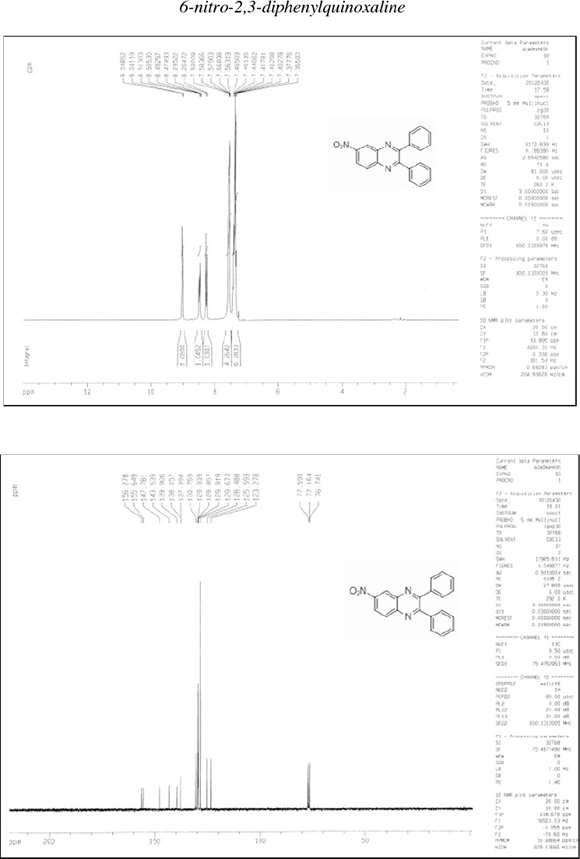
6-Nitro-2,3-diphenylquinoxaline (3): δh (CDCl3, 300 MHz): 7.35-7.46 (m, 6H), 7.56-7.59 (m, 4H), 8.26-8.29 (d, 1H), 8.47-8.51 (dd, 1H), 9.04-9.05 (d, 1H); δc (CDCl3, 75 MHz): 123.3, 125.6, 128.5, 129.7, 129.8, 129.8, 129.9, 130.7, 137.9, 138.0, 139.9, 143.5, 147.8, 155.6, 156.3; IR (KBr, cm-1): 3056, 1618, 1521, 1399, 1346, 1338, 1055, 1025, 979, 768, 743, 699, 598.
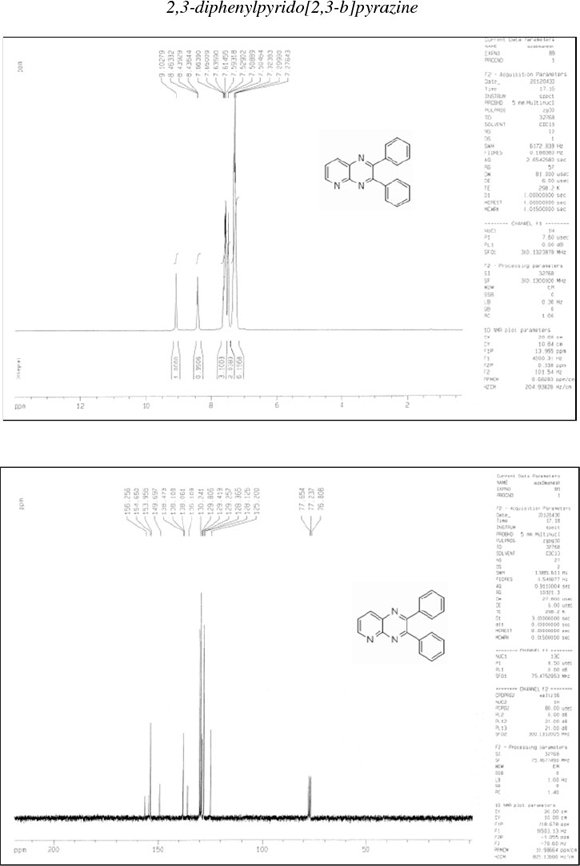
2,3-Diphenylpyrido[2,3-b]pyrazine (4): δh (CDCl3, 300 MHz): 7.28-7.32 (t, 6H), 7.50-7.53 (d, 2H), 7.59-7.66 (m, 3H), 8.44-8.46 (d, 1H), 9.11 (s, 1H); δc (CDCl3,75 MHz): 125.2,128.1,128.4,129.2, 129.4, 129.8, 130.2, 136.1, 138.0, 138.1, 138.5, 149.7, 153.9, 154.6, 156.2; IR (KBr, cm-1): 3057,1593,1548,1432,1389,1241,1190,1021, 974, 800, 781, 698, 612, 543.
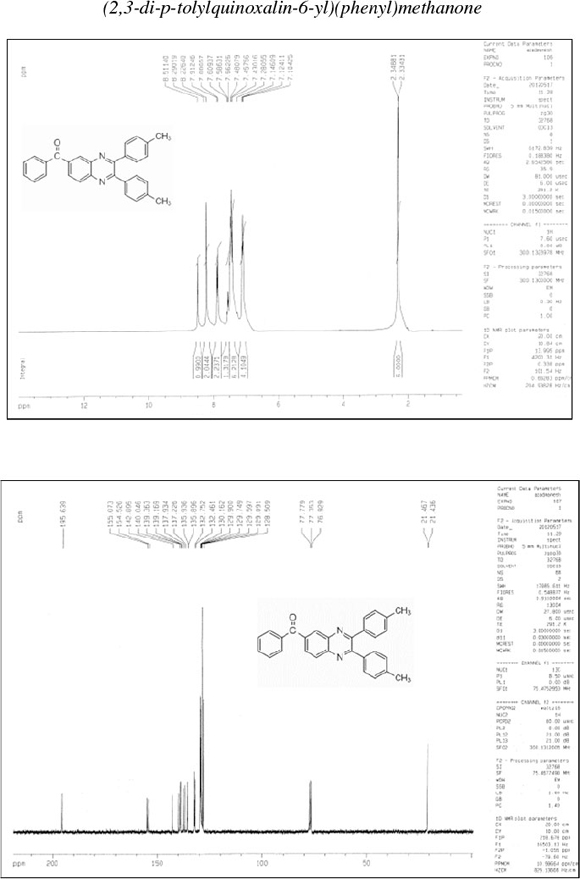
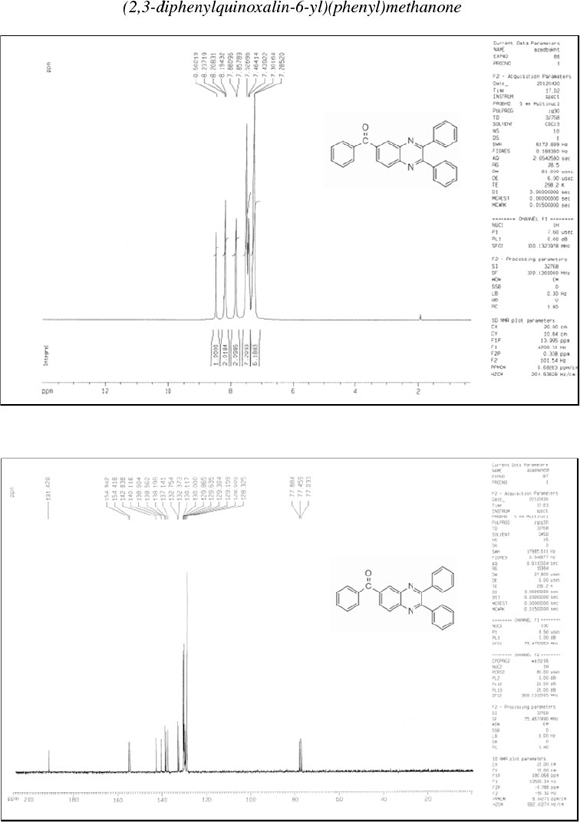
(2,3-Diphenylquinoxalin-6-yl)(phenyl)methanone (5): δh (CDCl3, 300 MHz): 7.28-7.30 (m, 6H), 7.44-7.53 (m, 7H), 7.86-7.88 (d, 2H), 8.19-8.21 (d, 2H), 8.50 (s, 1H); δc (CDCl3, 75 MHz): 128.3, 128.5, 129.1, 129.3, 129.6, 129.9, 130.0, 130.1, 132.3, 132.7, 137.1, 138.2, 138.6, 138.6, 140.1, 142.8, 154.4, 154.9, 191.4; IR (KBr, cm-1): 3057, 3035, 1733, 1660, 1598, 1446, 1402, 1346, 1309, 1265, 1198, 1124, 1057, 1022, 980, 890, 846, 770, 715, 696, 600, 542.
2,3-Bis(4-chlorophenyl)quinoxaline (6): δh (CDO3, 300 MHz): 7.32-7.34 (d, 4H), 7.45-7.48 (d, 4H), 7.75-7.78 (dd, 2H), 8.13-8.16 (dd, 2H); δc (CDCl3, 75 MHz): 128.7, 129.1, 130.4, 131.2, 135.3, 137.2, 141.2, 151.8; IR (KBr, cm-1): 3062, 1592, 1555, 1490, 1394, 1343, 1219, 1089, 1012, 976, 844, 830, 761, 588, 539, 461.
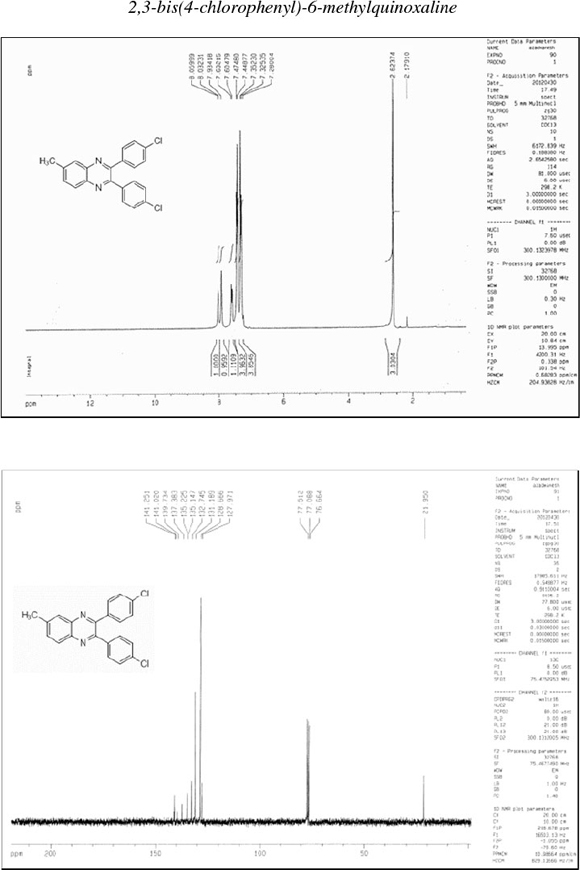
2,3-Bis(4-chlorophenyl)-6-methylquinoxaline (7): δh (CDO3, 300 MHz): 2.62 (s, 3H), 7.32-7.35 (d, 4H), 7.45-7.47 (d, 4H), 7.60-7.63 (d, 1H), 7.93 (s, 1H), 8.03-8.06 (d, 1H); δc (CDCl3, 75 MHz): 21.9,128.0,128.7,131.2,132.7,135.2,135.2,137.4,139.7, 141.0,141.2; IR (KBr, cm-1): 3070,2948,1620,1594,1484,1343,1089, 1013, 978, 833, 729, 547.
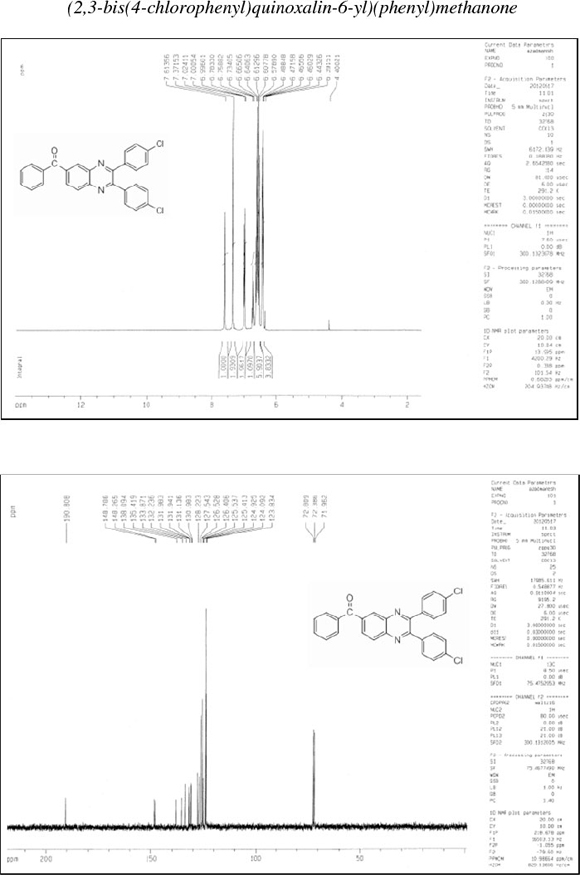
[2,3-Bis(4-chlorophenyl)quinoxalin-6-yl](phenyl)methanone (8): δh (CDCl3,300 MHz): 6.44-6.49 (m, 4H), 6.58-6.66 (m, 6H), 6.73-6.78 (m, 1H), 7.00-7.02 (d, 2H), 7.37 (s, 2H), 7.61 (s, 1H); δc (CDCl3, 75 MHz): 123.8,124.1,124.9,125.4,125.5,126.4,126.5,127.5,128.2, 131.0, 131.1, 132.0, 132.2, 133.9, 135.4, 138.1, 148.3, 148.8, 190.8; IR (KBr, cm-1): 3066,1651,1594,1492,1447,1408,1334,1270,1199, 1177, 1092, 1052, 978, 893, 833, 788, 594, 540, 483; MS (m/z): 454 (M+).
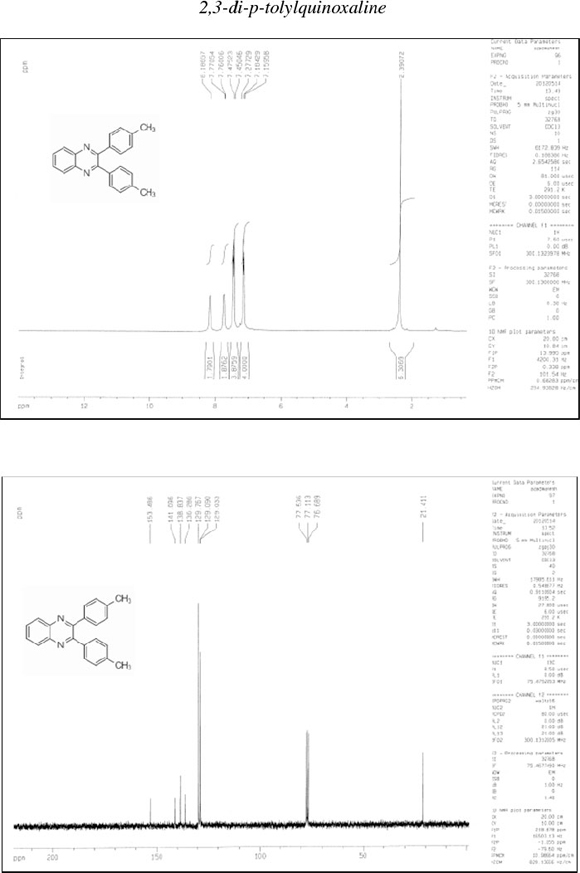
2,3-Di-p-tolylquinoxaline (9): δh (CDCl3, 300 MHz): 2.39 (s, 6H), 7.16-7.18 (d, 4H), 7.45-7.47 (m, 4H), 7.76-7.77 (d, 2H), 8.19 (d, 2H); δc (CDCl3, 75 MHz): 21.41, 129.0, 129.1, 129.8, 136.3, 138.8, 141.1,153.5; IR (KBr, cm-1): 3030,2911,1612,1475,1344,1212,1185, 1056, 1020, 977, 820, 762, 723, 595, 546,529.
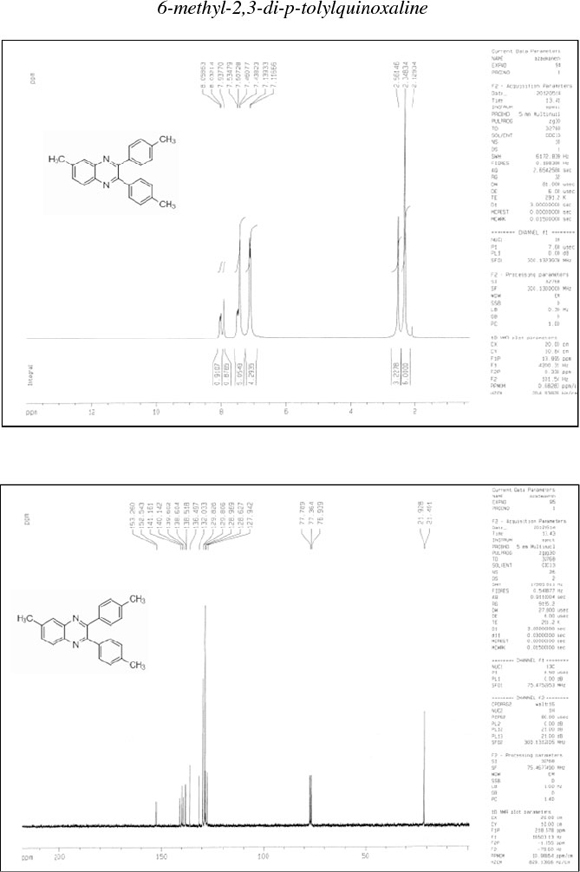
6-Methyl-2,3-di-p-tolylquinoxaline (10): δh (CDCl3, 300 MHz): 2.35 (s, 6H), 2.56 (s, 3H), 7.12-7.14 (d, 4H), 7.44-7.53 (m, 5H), 7.94 (s, 1H)), 8.03-8.06 (d, 1H); δc (CDCl3, 75 MHz): 21.4, 21.9,127.9, 128.6, 129.0, 129.8, 129.8, 132.0, 136.5, 138.5, 138.6, 139.6, 140.1, 141.1, 152.5, 153.3; IR (KBr, cm-1): 3026, 1610, 1512, 1484, 1394, 1342, 1203, 1055, 979, 818, 748, 588, 545, 505.
(2,3-Di-p-tolylquinoxalin-6-yl)(phenyl)methanone (11): δh (CDQ3, 300 MHz): 7.10-7.15 (t, 4H), 7.43-7.48 (t, 6H), 7.56-7.61 (t, 1H), 7.89-7.91 (d, 2H), 8.23 (s, 2H), 8.51 (s, 1H); δc (CDCl3, 75 MHz): 21.4,21.5,128.5,129.1,129.6,129.7,129.9,130.2,132.5,132.7,135.9, 135.9, 137.2, 137.9, 139.2, 139.4, 140.0, 142.9, 154.5, 155.1, 195.6; IR (KBr, cm-1): 3033,2917,1665,1610,1579,1454,1398,1305,1182, 1053, 988, 882, 721, 704, 598, 548, 500.
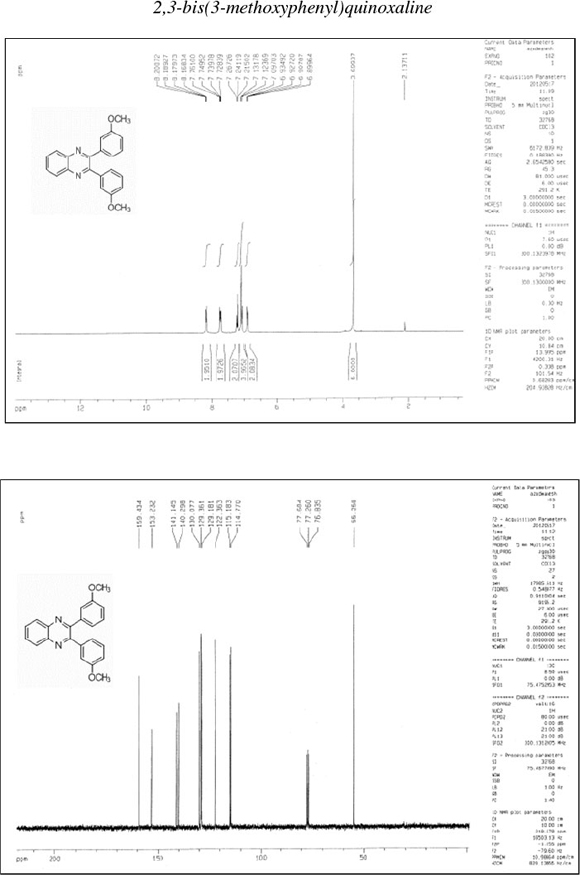
2,3-Bis(3-methoxyphenyl)quinoxaline (12): δh (CDCl3, 300 MHz): 3.70 (s, 6H), 6.90-6.93 (dd, 2H), 7.10-7.13 (d, 4H), 7.21-7.27 (m, 2H), 7.73-7.76 (dd, 2H), 8.16-8.20 (dd, 2H); δc (CDCk, 75 MHz): 55.2,114.8,115.2,122.4,129.2,129.4,130.1,140.3,141.1, 153.2,159.4; IR (KBr, cm-1): 3053,2945,1608,1579,1452,1430,1291, 1234, 1042, 997, 868, 790, 755, 585.
Acknowledgements
We gratefully acknowledge the support of this work by the Research Council of Bu-Ali Sina University.
References
1 A. Jaso, B. Zarranz, I. Aldana and A. Monge, J. Med. Chem, 2005, 48, 2019-2025. [ Links ]
2 A. Dell, D.H. William, H.R. Morris, G.A. Smith, J. Feeney and G.C.K. Roberts, J. Am. Chem. Soc., 1975, 97, 2497-2502. [ Links ]
3 C. Bailly, S. Echepare, F. Gago and M.J. Waring, Anti-Cancer Drug Des., 1999, 14, 291. [ Links ]
4 S. Sato, O. Shiratori and K. Katagiri, J. Antibiot., 1967, 20, 270. [ Links ]
5 N.D. Sonawane and D.W. Rangnekar, J. Heterocycl. Chem., 2002, 39, 303-308. [ Links ]
6 K.R. Justin Thomas, V. Marappan, T.L. Jiann, C. Chang-Hao and T. Yu-ai, Chem. Mater., 2005, 17, 1860-1866. [ Links ]
7 S. Dailey, J.W. Feast, R.J. Peace, R.C. Saga, S. Till and E.L. Wood, J. Mater. Chem., 2001, 11, 2238-2243. [ Links ]
8 L.S. Jonathan, M. Hiromitsu, M. Toshihisa, M.L. Vincent and F. Hiroyuki, Chem. Commun, 2002, 862-863. [ Links ]
9 P.C. Peter, Z. Gang, A.M. Grace, H. Carlos and M.G.T. Linda, Org. Lett., 2004, 6, 333-336. [ Links ]
10 O. Sascha and F. Rudiger, Synlett, 2004, 1509-1512. [ Links ]
11 L.S. Hegedus, M.G. Marc, J.W. Jory and P.B. Joseph, J. Org. Chem., 2003, 68, 4179-4188. [ Links ]
12 A.E.A. Porter, A.R. Katritsky and C.W. Rees, in Comprehensive Heterocyclic Chemistry, Pergamon, Oxford, 1984. [ Links ]
13 J.D. Brown, in The Chemistry of Heterocyclic Compounds: Quinoxalines, Supplement II, (C.E. Taylor and P. Wipf), John Wiley and Sons, New Jersey, 2004. [ Links ]
14 R.S. Bhosale, S.R. Sarda, S.S. Ardhapure, W.N. Jadhav, S.R. Bhusare and R.P. Pawar, Tetrahedron Lett, 2005, 46, 7183-7186. [ Links ]
15 S.V. More, M.N.V. Sastry, C.C. Wang and C.F. Yao, Tetrahedron Lett., 2005, 46, 6345-6348. [ Links ]
16 H.R. Darabi, S. Mohandessi, K. Aghapoor and F. Mohsenzadeh, Catal. Commun, 2007, 8, 389-392. [ Links ]
17 T.K. Huang, R. Wang, L. Shi and X.X. Lu, Catal. Commun, 2008, 9, 1143-1147. [ Links ]
18 C. Srinivas, C.N.S.S.P. Kumar, V Jayathirtha Rao and S. Palaniappan, J. Mol. Catal. A: Chem., 2007, 265, 227-230. [ Links ]
19 M.M. Heravi, K. Bakhtiari, F.F. Bamoharram and M.H. Tehrani, Monatsh. Chem, 2007, 138, 465-467. [ Links ]
20 P. Hazarika, P. Gogoi and D. Konwar, Synth. Commun., 2007, 37, 3447-3454. [ Links ]
21 M.M. Heravi, K. Bakhtiari, H.A. Oskooie and S. Taheri, Heteroat. Chem., 2008, 19, 218-220. [ Links ]
22 M.M. Heravi, S. Taheri, K. Bakhtiari and H.A. Oskooie, Catal. Commun, 2007, 8, 211-214. [ Links ]
23 M.M. Heravi, M.H. Tehrani, K. Bakhtiari and H.A. Oskooie, Catal. Commun, 2007, 8, 1341-1344. [ Links ]
24 J.-J. Cai, J.-P. Zou, X.-Q. Pan and W. Zhang, Tetrahedron Lett, 2008, 49, 7386-7390. [ Links ]
25 X.Z.Zhang,J.X.Wang,Y.J.SunandH.W.Zhan,Chin. Chem.Lett.,2010, 21, 395-398. [ Links ]
26 T.-K. Huang, L. Shi, R. Wang, X.-Z. Guo and X.-X. Lu, Chin. Chem. Lett., 2009, 20, 161-164. [ Links ]
27 M.M. Heravi, K. Bakhtiari, M.H. Tehrani, N.M. Javadi and H.A. Oskooie, ARKIVOC, 2006, xvi, 16-22. [ Links ]
28 G. Mohammadi Ziarani, A. Badiei and M. Haddadpour, Int. J. Chem., 2011, 3, 87-94. [ Links ]
29 S.V. More, M.N.V. Sastry and C.F. Yao, Green Chem., 2006, 8, 91-95. [ Links ]
30 A. Shaabani and A. Maleki, Chem. Pharm. Bull, 2008, 56, 79-81. [ Links ]
31 R.S. Robinson and R.J.K. Taylor, Synlett, 2005, 1003-1005 [ Links ]
32 S.A. Raw, C.D. Wilfred and R.J.K. Taylor, Chem. Commun., 2003,22862287. [ Links ]
33 Y.V.D. Nageswar, K. Harsha Vardhan Reddy, K. Ramesh and S. Narayana Murthy, Org. Prep. Proced. Int., 2013, 45, 1-27. [ Links ]
34 K.T.V Rao, P.S.S. Prasad and N. Lingaiah, J. Mol. Catal A: Chem., 2009, 312, 65-69. [ Links ]
35 S. Sithambaram, Y. Ding, W. Li, X. Shen, F. Gaenzler and S.L. Suiib, Green Chem., 2008, 10, 1029-1032. [ Links ]
36 A.V Kumar, V.P. Reddy and K.R. Rao, Synlett, 2010, 17, 2571-2574. [ Links ]
37 A. Shaabani and D.G. Lee, Tetrahedron Lett, 2001, 42, 5833-5836. [ Links ]
38 G. Tojo, M.I. Fernandez and M. Fernandez, Oxidation of Alcohols to Aldehydes and Ketones: A Guide to Current Common Practice (Basic Reactions in Organic Synthesis), 2005. [ Links ]
39 V Jeena and R.S. Robinson, Beilstein J. Org. Chem, 2009, 5, 1-4. [ Links ]
40 M.R. Islami and Z. Hassani, ARKIVOC, 2008, 15, 280-287. [ Links ]
41 H.I.X. Mager and W. Berends, Rec. Trav. Chim, 1965, 84, 314. [ Links ]
42 W.X. Guo, H.L. Jin, J.-X. Chen, F. Chen, J.-C. DingandH.-Y. Wu, J. Braz. Chem. Soc., 2009, 20, 1674-1679. [ Links ]
43 K. Niknam, D. Saberi and M. Mohagheghnejad, Mol. Cells, 2009, 14, 1915-1926. [ Links ]
44 J. Ji and K.-I. Lee, J. Korean Chem. Soc., 2005, 49, 150-154. [ Links ]
Received 31 December 2012
Revised 25 February 2013
Accepted 17 April 2013
* To whom correspondence should be addressed. E-mail: khoram@gmail.com