Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.66 Durban Aug. 2013
RESEARCH ARTICLE
Zn(BH4)2/2NaCl: A novel reducing system for efficient reduction of organic carbonyl compounds to their corresponding alcohols
Davood Setamdideh*; Leila Khaledi
Department of Chemistry, Faculty of Sciences, Mahabad Branch, Islamic Azad University, Mahabad, 59135-443, Iran
ABSTRACT
Zn(BH4)2/2NaCl, obtained by the reaction of ZnCl2 and NaBH4 ,is a stable, efficient and selective reducing system in dry-THF. The Zn(BH4)2/2NaCl system (0.5-1 mmol) reduces a variety of carbonyl compounds to their corresponding alcohols in CH3CN at room temperature in high to excellent yields.
Keywords: Reduction, carbonyl compounds, Zn(BH4)2, chemoselective, regioselectivity, NaCl.
1. Introduction
Reduction is one of the most fundamental and useful reaction in organic synthesis. For the past 50 years, sodium borohydride has played an important role in modern synthetic organic chem-istry.1 This reagent is used widely and has gained commercial status, in spite of its poor solubility in organic solvents and low reactivity.2 To overcome these drawbacks and for controlling its reducing power, soluble metal borohydrides have been realized through the variation of alkali-metal cations and transition metal cations in the hydride complex such as LiBH4,3KBH4,4Ca(BH4)2, 5Cu(BH4)2, 6Zn(BH4)2, 7Ti(BH4)3, 8Zr(BH4)4 and others.8b LiBH4, Ca(BH4)2 and Zn(BH4)2 are the modified borohydride agents which have better solubility in aprotic solvents, so their uses and applications are interesting in organic synthesis. Among these reagents, zinc borohydride is unique because of the better coordination ability of Zn2+, which imparts selectivity in hydride-transferring reactions. Zinc borohydride is moderately stable in ethereal solution and has many applications in organic synthesis.9 Zinc borohydride, as a nonconventional hydride transferring agent, has been reported as an efficient chemo-, regio- and stereoselective reducing agent in several complex substrates. It can be used in a range of aprotic solvents such as, THF, Et2O and DME.10 Several combination reducing systems of Zn(BH4)2 such as Zn(BH4)2/TMEDA, 11aZn(BH4)2/Me3SiCl, 11bZn(BH4)2/ TFA/DME, 11cZn(BH4)2/H2O,11dZn(BH4)2/Al2O3,11e and Zn(BH4)2/C11f are interesting and have been used for different reduction purposes. Also, Zn(BH4)2 has been modified as stable tertiary amino or phosphinoligand complexes such as [Zn(BH4)2 (dabco)],12 [Zn(BH4)2(pyz)]n, 13[Zn(BH4)2(PPh3)] and [Zn(BH4)2(PPh3)2],14 [Zn(BH4)2(bpy)],15 [Zn(BH4)2(py)],16 [Zn(BH4)2XP4],17 [Zn(BH4)2(nmi)]18a and [Zn(BH4)2(nic)]18b. In this context we wish to introduce Zn(BH4)2/2NaCl as a new combination reducing system for fast and efficient reduction of a variety of carbonyl compounds such as aldehydes, ketones, acyloins, a-diketones and α, β-unsaturated carbonyl compounds to their corresponding alcohols at room temperature.
2. Experimental
2.1. General
All substrates and reagents were purchased from commercial sources (Merck and Sigma-Aldrich) and used without further purification. IR and 1H NMR spectra were recorded on Perkin-Elmer FT-IR RXI and (250, 300 & 400) MHz Bruker spectrometers, respectively. The products were characterized by their Ή NMR or IR spectra and compared with authentic samples (melting points or boiling points). Organic layers were dried over anhydrous sodium sulfate. All yields refer to isolated pure products. TLC (silica gel 60 F254 aluminum sheet) was applied for the purity determination of substrates, products and reaction monitoring.
2.2. Preparation of Zn(BH4)2/2NaCl as Combination Reducing System
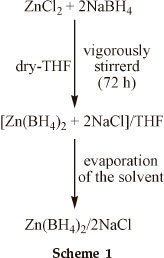
In a round-bottomed flask (500 mL) equipped with a magnetic stirrer bar and covered with foil, a solution of ZnCl2 (5.452 g, 0.04 mol) and NaBH4 (3.177 g, 0.084 mol) was prepared in dry THF (250 mL). The mixture was vigorously stirred for 72 hours at room temperature according to a literature procedure.18a,b Then the solvent was evaporated and the Zn(BH4)2/2NaCl was afforded as a grey solid (8.629 g, 100 %) as shown in Scheme 1.
2.3. Typical Procedure for the Reduction of Aldehydes with Zn(BH4)2/2NaCl System in CH3CN
In a round-bottomed flask (10 mL) equipped with a magnetic stirrer bar, a solution of benzaldehyde (0.106 g, 1 mmol) was prepared in CH3CN (3 mL). To this solution, Zn(BH4)2/2NaCl (0.105 g, 0.5 mmol) was added and the mixture was stirred at room temperature for 1 min. The reaction was monitored by TLC (eluent; Hexane/EtOAc: 10/1). After completion of the reaction, distilled water (5 mL) was added to the reaction mixture and stirred for 5 min. The mixture was extracted with CH2Cl2 (3x8 mL) and dried over anhydrous Na2SO4. Evaporation of the solvent followed by column chromatogra-phy of the resulting crude material over silica gel (eluent; Hex-ane/EtOAc: 10/1) afforded liquid benzyl alcohol (0.102 g, 94 %, Table 2, entry 1).
2.4. Typical Procedure for the Reduction of Ketones with Zn(BH4)2/2NaCl System in CH3CN
In a round-bottomed flask (10 mL), equipped with a magnetic stirrer bar, a solution of acetophenone (0.121 g, l mmol) was prepared in CH3CN (3 mL). To this solution, Zn(BH4)2/2NaCl (0.210 g, 1 mmol) was added. The resulting mixture was stirred at room temperature for 60 min. The reaction was monitored by TLC (eluent; Hexane/EtOAc: 10/1). After completion of the reaction, distilled water (5 mL) was added to the reaction mixture and stirred for 5 min. The mixture was extracted with CH2Cl2 (3 x 8 mL) and dried over anhydrous Na2SO4. Evaporation of the solvent followed column chromatography of the resulting crude material over silica gel (eluent; Hexane/EtOAc: 10/1) afforded crystals of 1-phenylethanol (0.l1 g, 93 % yield, Table 2, entry 11).
2. 5. Typical Procedure for the Reduction of α-Diketones and Acyloins with Zn(BH4)2/2NaCl System in CH3CN
In a round-bottomed flask (10 mL) equipped with a magnetic stirrer bar, a solution of benzil (0.21 g, l mmol) was prepared in CH3CN (3 mL). To this solution, Zn(BH4)2/2NaCl (0.210 g,1 mmol) was added. The resulting mixture was stirred at room temperature for 10 min. The reaction was monitored by TLC (eluent; Hexane/EtOAc: 10/1). After completion of the reaction, distilled water (6 mL) was added to the reaction mixture and stirred for 5 min. The mixture was extracted with CH2Cl2 (3 x 10 mL) and dried over anhydrous Na2SO4. Evaporation of the solvent followed by short column chromatography of the resulting crude material over silica gel (eluent; Hexane/EtOAc: 10/1) afforded crystals of 1,2-diphenyl ethane-1,2-diol (0.20 g, 95 % yield, Table 2, entry 19).
2.6. Typical Procedure for Competitive Reduction of Aldehydes and Ketones with Zn(BH4)2/2NaCl System in CH3CN
In a round-bottomed flask (10 mL) equipped with a magnetic stirrer bar, a solution of benzaldehyde (0.106 g, l mmol) and acetophenone (0.121 g, 1 mmol) was prepared in CH3CN (3 mL). To this solution, Zn(BH4)2/2NaCl (0.105 g, 0.5 mmol) was added and the mixture was stirred at room temperature. The reaction was monitored by TLC (eluent; Hexane/EtOAc: 10/1). After 5 min, the reaction was quenched by the addition of distilled water (5 mL) and stirred for 5 min. The mixture was extracted with CH2Cl2 (3 x 15 mL) and dried over anhydrous Na2SO4. Evaporation of solvent followed by short column chromatogra-phy of the resulting crude material over silica gel (eluent; Hex-ane/EtOAc: 10/1) afforded liquid benzyl alcohol as a sole product (0.105 g, 97 %) and acetophenone (0.112 g, 93 %) an unused substrate (Table 3, entry 1).
2.7. Typical Procedure for Regioselective 1,2-Reduction of Conjugated Carbonyl Compounds with Zn(BH4)2/2NaCl System in CH3CN
In a round-bottomed flask (10 mL) equipped with a magnetic stirrer bar, a solution of benzylideneacetone (0.146 g, 1 mmol)) was prepared in CH3CN (3 mL). To this solution Zn(BH4)2/2NaCl (0.210 g, 1 mmol) was added. The resulting mixture was stirred at room temperature. The reaction was monitored by TLC (eluent; Hexane/EtOAc: 10/1). After completion of the reaction within 40 min, distilled water (5 mL) was added to the reaction mixture and stirred for 5 min. The mixture was extracted with CH2Cl2 (3 x 10 mL) and dried over anhydrous Na2SO4. Evaporation of the solvent followed by short column chromatography of the resulting crude material over silica gel (eluent; Hexane/EtOAc:10/1) afforded liquid 4-phenyl-3-buten-2-ol (0.l43 g, 96 % yield, Table 2, entry 24).
3. Results and Discussion
The Zn(BH4)2 reduces some carbonyl compounds10a with some peculiar chemoselectivities such as the reduction of aldehydes over aromatic ketones10b and the reduction of ketones and conjugated aldehydes over enones.10c In order to determine the most appropriate reaction conditions and evaluate the efficiency of the addition of NaCl, we first examined the reduction of benzaldehyde as a model compound as shown in Table 1. Among the tested aprotic and protic solvents i.e. n-hexane, CHCl3,CH2Cl2,Et2O, DME, CH3CN, THF, CH3OH, C2H5OH and solvent-free conditions, benzaldehyde reduction was better in CH3CN. The optimization study showed that using 0.5 molar equivalents of Zn(BH4)2/2NaCl in CH3CN (3 mL) is the best conditions as shown in Scheme 2.

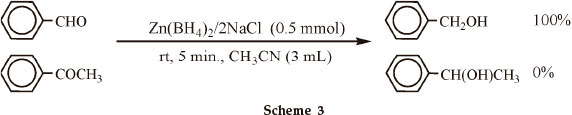
This procedure was also applied to the reduction of various aliphatic and aromatic aldehydes (Table 2, entries 1-10). All reductions were completed within 1-5 min by 0.5 molar equivalents of Zn(BH4)2/2NaCl with excellent yield of products (93-98 %). Our next attempt was the reduction of ketones to the corresponding secondary alcohols. We optimized the reaction conditions with acetophenone as model compound (Table 1, entries 14-17). Due to the lower reactivity of ketones relative to aldehydes, the reduction require a higher molar amounts of Zn(BH4)2/2NaCl. The reduction reactions were carried out with 1 molar equivalents of Zn(BH4)2/2NaCl at room temperature in CH3CN. All reductions were completed within 30-120 min with high to excellent yield of products (92-98 %) as shown in Table 2 (entries11-18). Since the reduction of aldehydes and ketones is dependent on molar ratio Zn(BH4)2/2NaCl and aldehydes reduce faster than ketones by this reducing system, therefore, we thought that this system has a chemoselectivity towards reduction of aldehydes over ketones. Therefore, a 1:1 mixture (1 mmol of each) of benzaldehyde and acetophenone was treated with 0.5 molar equivalents of Zn(BH4)2/2NaCl at room temperature in CH3CN as shown in Scheme 3. The selectivity ratio for the reduction of aldehyde was 100 %. The usefulness of this chemoselectivity of the reduction was further examined with the reduction of benzaldehyde in the presence of other ketones as shown in Table 3.
Reductions of α-diketones and acyloins to the corresponding vicinal diols are an interesting subject in organic synthesis.19 In this context, we decided to use the Zn(BH4)2/2NaCl system for the reduction of α-diketones and acyloins. Reduction of α-diketones to their corresponding vicinal diols took place with 1 molar equivalents of Zn(BH4)2/2NaCl at room temperature in CH3CN. Vicinal diols were obtained in excellent yields (93-95 %) (Table 2, entries 19 & 20). All attempts to reduce α-diketones into acyloins were not satisfactory. The reduction of acyloins to their corresponding vicinal diols (92-96 %) was also obtained successfully by 1 molar equivalents of Zn(BH4)2/2NaCl at room temperature in CH3CN (Table 2, entries 21 & 22). Preparation of allyl alcohols from conjugated carbonyl compounds is an important synthetic method in organic synthesis.20 However, regioselective 1,2-reduction of α, β-unsaturated aldehydes and ketones with metal hydride reducing agents is often difficult to achieve due to the competing 1, 4-reduction.21 On the other hand, need for allylic alcohols has led to the development of several specific reagents and some of them are commercially available.13 In this context, we also investigated the potential of the 1,2-reduction of α, β-unsaturated aldehydes and ketones with the Zn(BH4)2/ 2NaCl system. The reduction of cinnamaldehyde with 0.5 molar equivalents of the Zn(BH4)2/2NaCl exclusivity afforded the 1,2-reduction product after 5 min at room temperature in CH3CN. In this reaction, cinnamyl alcohol was obtained in 97 % yield (Table 2, entry 23). Under this protocol, reduction of conjugated ketones such as benzylidenacetone (Table 2, entry 24) and chalcone (Table 2, entry 25) were achieved efficiently with 1 molar equivalents of Zn(BH4)2/2NaCl at room temperature in CH3CN in excellent yields (95-97 %).
In order to show the efficiency of this reducing system, we compared our results with other reported reducing systems (Table 4). In all cases, the Zn(BH4)2/2NaCl has a greater potential for the reduction of organic carbonyl compounds. Only Zn(BH4)2(bpy) (Table 4, entry 8) has some advantages over Zn(BH4)2/2NaCl. But the synthesis of Zn(BH4)2(bpy) is more costly than Zn(BH4)2/2NaCl. Also, in our experience,18 reducing complexes (Table 4, entries 6-13) release a ligand during reduction reactions. The isolation of organic ligands is costly and time-consuming exercise. Whereas, the Zn(BH4)2/2NaCl has no such limitations and does not require any other additives. The stability and shelf-life of the Zn(BH4)2/2NaCl was investigated by comparing the reduction reactions of benzaldehye as model compound under different conditions as shown in Table 5. Experiments show that, in adverse conditions, this reducing system is stable and its reducing power remains significant (Table 5, entry 3). The effect of the NaCl is not clear, but most probably NaCl protects Zn(BH4)2 from the direct effect of oxygen, humidity and light.22 It is notable that NaCl does not interfere with the reduction and extraction processes. In the work-up process, water is added to the reaction mixture to hydrolyze of the resulting borates. The NaCl is dissolved in water and the resulting brine increases the yield of products, since the solubility of alcohols decreases in brine.
4. Conclusion
In this investigation, we have shown the reducing properties of Zn(BH4)2/2NaCl remained intact for several months. Consequently, it is much easier to use than other similar reducing agents. Also, we have shown that the Zn(BH4)2/2NaCl reduces a variety of carbonyl compounds to their corresponding alcohols in high to excellent yields. Reduction reactions were carried out with 0.5-1 molar equivalents of Zn(BH4)2/2NaCl at room temperature in CH3CN without any other additive. The chemoselective reduction of aldehydes over ketones was accomplished successfully with this reducing system. In addition, regioselectivity of this system was also investigated with exclusive 1,2-reduction of conjugated carbonyl compounds to their corresponding allylic alcohols in high to excellent yields. Reduction of acyloins and α-diketones by this reducing system also produced the corresponding vicinal diols. Moreover, features such as: a) high efficiency of the reduction reactions, b) shorter reaction times, c) ease of preparation, handling and storage, d) very good stability, e) low reaction temperature, f) easy work-up procedure and g) possible to produce commercially make this reducing system an attractive protocol for the reduction of carbonyl compounds, therefore it could be a useful addition to the present methodologies.
Acknowledgements
The authors gratefully acknowledge financial assistance by the research council of the Islamic Azad University branch of Mahabad.
References
1 (a) H. O. House, In Modern Synthetic Reaction. Benjamine, Menlo Park, CA, 1972. [ Links ] (b) S. D. Burk and R.L. Danheiser, Handbook of Reagents for Organic Synthesis, Oxidising and Reducing Agents. Wiley-VCH, New York, 1999. [ Links ] (c) J. Seyden-Penne, Reductions by the Alumino and Borohydrides in Organic Synthesis. Wiley-VCH, New York, 1997. [ Links ] (d) M. Hudlicky, Reductions in Organic Chemistry. Ellis Horwood, Chichester, 1984. [ Links ]
2 H.C. Brown and S. Krishnamurthy, Tetrahedron, 1979, 35, 567-607. [ Links ]
3 (a) H. C. Brown,S. Narasimhan and Y. M. Choi, J. Org. Chem. 1982, 47, 4702-4708. [ Links ] (b) K. Oai and A. Ookawa, J. Org. Chem. 1986, 51, 4000-4005. [ Links ] (c) A. Arase, Y. Nunokawa, Y. Masuda and Y. M. Hoshi, J. Chem. Soc. Chem. Commun. 1991, 205-206. [ Links ]
4 I. Nishiwaki and F. Fujiyama, Synthesis 1972, 569-571. [ Links ]
5 H. Fujii, K. Oshima and K. Utimoto, Chem. Lett. 1991, 1847-1848. [ Links ]
6 (a) M.E. Osborn, J.F. Pegues and L.A. Paquett, J. Org. Chem. 1980, 45, 167-168. [ Links ] (b) G.W.J. Fleet and P.J.C. Harding, Tetrahedron Lett. 1981, 22, 675-678. [ Links ] (c) J.A. Cowan, Tetrahedron Lett. 1986, 27, 1205-1208. [ Links ]
7 (a) W.J. Gensler, F. Johnson andA.D.B. Sloan, J. Am. Chem. Soc. 1960, 82, 6074-6081. [ Links ] (b) P.G. Crabbe, A. Garcia and C. Rius, J. Chem. Soc., Perkin Trans. 1, 1973, 810-816. [ Links ]
8. (a) K. Ravikumar, S.S. Baskaran and S. Chandrasekaran, Tetrahedron Lett. 1993, 34, 171-174. [ Links ] (b) T.J. Marks andJ.R. Kolb, Chem. Rev. 1977, 77, 263-293. [ Links ]
9 S. Narasimhan and R. Balakumar, Aldrichim. Acta. 1998, 31, 19-26. [ Links ]
10 (a) B.C. Ranu, Synlett. 1993, 885-892. [ Links ] (b) B.C. Ranu and R. Chakraborty, Tetrahedron Lett. 1990, 31, 7663-7664. [ Links ] (c) D.C. Sarkar, A.R. Das and B.C. Ranu, J. Org. Chem. 1990, 55, 5799-5801. [ Links ]
11 (a) H. Kotsuki, Y. Ushio, N. Yoshimura and M. Ochi, Bull. Chem. Soc. Jpn. 1988, 61, 2684-2686. [ Links ] (b) H. Kotsuki, Y. Ushio, N. Yoshimura and M. Ochi, J. Org. Chem. 1987, 52, 2594-2596. [ Links ] (c) B.C. Ranu and A.R. Das, J. Chem. Soc. Perkin Trans. 1: 1992, 1561-1562. [ Links ] (d) D. Setamdideh, B. Khezri, M. Rahmatollahzadeh and A. Aliporamjad, Asian J. Chem. 2012, 8, 3591-3596. [ Links ] (e) D. Setamdideh, B. Khezri and M. Rahmatollahzadeh, J. Serb. Chem. Soc. 2013, 78, 1-13. [ Links ] (f) D. Setamdideh andM. Rahmatollahzadeh,J. Mex. Chem. Soc. 2012, 56, 169-175. [ Links ]
12 H. Firouzabadi, M. Adibi and B. Zeynizadeh, Synth. Commun. 1988, 28,1257-1273. [ Links ]
13 B.TamamiandM.M.Lakouraj, Synth. Commun. 1995, 25,3089-3096. [ Links ]
14 H. Firouzabadi, M. Adibi and M. Ghadami, Phosphorus, Sulfur, Silicon Rel. Elem. 1998, 142, 191-220. [ Links ]
15 B. Zeynizadeh, Bull. Chem. Soc. Jpn. 2003, 76, 317-326. [ Links ]
16 (a) B. Zeynizadeh and F. Faraji, Bull. Korean Chem. Soc. 2003, 24, 453-459. [ Links ] (b) B. Zeynizadeh and K. Zahmatkesh, J. Chin. Chem. Soc. 2003, 50, 267-271. [ Links ] (c) B. Zeynizadeh and K. Zahmatkesh, J. Chin. Chem. Soc. 2004, 51, 801-806. (d) B. Zeynizadeh and K. Zahmatkesh, J. Chin. Chem. Soc. 2005, 52, 109-112. [ Links ]
17 H. Firouzabadi, B. Tamami and N. Goudarzian, Synth. Commun. 1991, 21, 2275-2285. [ Links ]
18 (a) B. Zeynizadeh and D. Setamdideh, Asian J Chem. 2009, 21, 3603-3610. [ Links ] (b) D. Setamdideh and M. Rafig E-J. Chem. 2012, 9, 2338-2345. [ Links ]
19 (a) W. Kreiser, Ann. Chem. 1971, 745, 164-168. (b) V H. [ Links ] Pechmann and F. Dahl, Chem. Ber. 1890, 23, 2421-2427. [ Links ] (c) T. L. Ho and G. A. Olah, G. A. Synthesis 1976,815-816. [ Links ] (d) T. Mori, T. Nakahara and H. Nozaki, Can. J. Chem. 1969, 47, 3266-3269. [ Links ] (e) R. Mayer, G. Hiller, M. Nitzschke, and J. Jentzsch, Angew. Chem. 1963, 75, 370-373. [ Links ] (f). M. B. Rubin and J.M. BenBassat, Tetrahedron Lett. 1971, 12, 3403-3406. [ Links ]
20 (a) H. Firouzabadi and G.R. Afsharifar, Bull. Chem. Soc. Jpn., 1995, 68, 2595-2602. [ Links ] (b) H. FirouzabadiandM. Adibi, Synth. Commun. ,1996, 26, 2429-2441. [ Links ] (c) H. Firouzabadi and M. Adibi, Phosphorus, Sulfur, Silicon Relat. Elem. , 1998, 142, 125-147. [ Links ]
21 (a) N.M. Yoon and J. Choi, Synlett., 1993, 135-136. (b) T. [ Links ]B. Sim, J.H. Ahn and N.M. Yoon, Synthesis, 1996,324-326. [ Links ] (c) G. Bram, E.D. Incan and A. Loupy, J. Chem. Soc. Chem. Commun, 1981, 1066-1067. [ Links ]
22 B. Elvers, Ullmann's Encyclopedia of Industrial Chemistry, 5th edn., Vol. A24, Wiley, New York, 1991. [ Links ]
23 (a) Dictionary of Organic Compounds, 5th edn., Chapman & Hall, New York, 1982; [ Links ] (b) Aldrich Catalogue of Fine Chemicals, Aldrich Chemical, Milwaukee, 2003. [ Links ]
Received 7 January 2013
Revised 26 March 2013
Accepted 8 May 2013
* To whom correspondence should be addressed. E-mail: davood.setamdideh@gmail.com














