Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.66 Durban Aug. 2013
RESEARCH ARTICLE
Synthesis of novel bibrachial lariat ethers (BiBLEs) containing [1,2,4]triazolo[3,4-b][1,3,4]thiadiazines
Sattar Ebrahimi*
Department of Chemistry, Malayer Branch, Islamic Azad University, Malayer, Iran
ABSTRACT
A practical and regioselective method for the synthesis of cis-diastereomers of bibrachial lariat ethers (BiBLEs) bearing ester and amide groups is reported. The novel bibrachial lariat ethers (BiBLEs) 3a-d with neutral side chains were preparedby reaction of the corresponding aza-crown macrocycles 1a-b with ethyl chloroacetate and chloroacetamide.
Keywords: macrocycle, bibrachial, lariat ethers, aza-crown, 1,3,4-thiadiazines.
1. Introduction
The first synthetic crown ether was discovered by Pederson.1 Since then, various structural changes have been made to the basic crown ether skeleton in an attempt to enhance the selectivity of these rings and the capacity of complexation with metal ions. When hard and soft donor atoms were added into the structure of the macrocycles, it has been noted that the complexation properties were increased.2,3 In addition, insertion of heterocyclic and aromatic compounds, have improved the complexation ability of the macrocyclic structures.4,5 These changes may be due to increased number of cation complexing functional groups via their soft or hard donor atoms.6
Lariat ethers are macrocycles which have one or more side arms that contain electron-donating groups.7,8 The donor groups in these side arms have been extensively used to enhance the stability of the lariat ether for cation compelaxation by giving three-dimensionality to the binding.9 Lariat ethers may thus exhibit an enhanced complexation capacity for metal ions as compared with the parent macrocycles.10 These valuable properties prompted us to synthesize a new series of 18-20 bibrachial lariat ethers (BiBLEs) containing 1,3,4-thiadiazine rings.
2. Experimental
2.1. General
All products were characterized using IR, 1H NMR and 13C NMR spectra as well as the mass spectral data. All yields refer to isolated products. IR spectra were performed on a galaxy series FT-IR 5000 spectrophotometer using KBr discs. NMR spectra were recorded on Brucker spectrophotometer (300 MHz) in DMSO-d6 using TMS as an internal standard. Mass spectra were recorded on an Agilent Technologies (HP) 5973 Network mass selective detector under electron impact (EI).
2.2. General Procedure for the Synthesis of Compounds 3a-d
Sodium hydride (2.5 mmol) was added to a solution of compounds 1a-b13 (0.5 mmol) in absolute ethanol (10 mL) at room temperature. Salt formation was allowed to proceed at room temperature for 10 min and ethyl chloroacetate or chloro-acetamide (1.1 mmol) was added and the solution stirred for 5 h at room temperature. After the completion of the reaction, the solvent was removed under vacuum and extracted with ethyl acetate. The organic layer was washed with water (3 × 10 mL), dried (Na2SO4), and evaporated under vacuum. The residue was crystallized from ethyl acetate and petroleum ether to give compounds 3a-d.
Diethyl-8,12-dioxa-33,37-dithia-20,21,23,24,30,31,35,36-octa-azaheptacyclo(27.52.219,22.02,7.013,18.021,25.032,36)octatriaconta-2,4,6, 13(18),14,16,22,24,29,31-decaene-34,38-dicarboxylate (3a)
75% yield, IR: ν 3246 (NH stretch), 3060 (aromatic CH stretch), 2950 (aliphatic CH stretch), 1730 (C=O), 1610 (C=N), 1250 (Ar-O), 1160 (R-O) cm-1; 1H NMR: δ 7.35 (d, 2H, HaromJ = 5.0 Hz), 7.31 (s, 2H, 2 NH, D2O exchange), 7.14 (d, 2H, HaromJ = 8.1 Hz), 7.02 (t, 2H, Harom. J = 7.0 Hz), 6.80 (d, 2H, Harom. J = 7.1 Hz), 4.61 (br, 4H, 2 × OCH2), 4.44 (d, 2H, 2 N-CH, J = 7.9 Hz), 4.34 (d, 2H, 2S-CH, J = 8.0 Hz), 3.96 (q, 4H,2xOCH2, J = 7.0 Hz), 2.78 (br, 4H, 2 xCH2), 2.00 (br, 2H, CH2), 1.94 (br, 2H, CH2), 0.99 (br, 6H, 2 × CH3); 13C NMR: δ 168.9, 156.7, 152.6, 141.2, 130.6, 128.1, 124.5, 121.4, 113.0, 66.5, 62.0, 44.2, 40.3, 27.7, 24.0, 22.0, 14.0; DEPT: δ 130.6 (CH), 128.1 (CH), 121.4 (CH), 113.0 (CH), 66.5 (CH2), 62.0 (CH2), 44.2 (CH), 40.3 (CH), 27.7 (CH2), 24.0 (CH2), 22.0 (CH2), 14.0 (CH3); MS (EI): m/z = 692 (M+), 677, 662, 647, 602, 278,146 (base peak), 120,91; Anal. Calcd. for: C32H36N8O6S2: C, 55.48; H, 5.24; N, 16.17; S, 9.26; Found: C, 55.22; H, 5.17; N, 15.96; S, 9.02.
Diethyl-8,13-dioxa-35,39-dithia-21,22,24,25,32,33,37,38-octa-azaheptacyclo(29.5.2.220,23.02,7.014,19.022,26.034,38)tetraconta-2,4,6,14 (19),15,17,23,25,31,33-decaene-36,40-dicarboxylate (3b)
70% yield, IR: ν 3260 (NH stretch), 3050 (aromatic CH stretch), 2930 (aliphatic CH stretch), 1732 (C=O), 1601 (C=N), 1246 (Ar-O), 1160 (R-O) cm-1; 1H NMR: δ 7.30 (d, 2H, HaromJ = 5.6 Hz) 7.28 (s, 2H,2xNH, D2O exchange), 7.11 (d, 2H, HaromJ = 7.8 Hz), 7.03 (t, 2H, Harom.J = 7.1 Hz), 6.82 (d, 2H, Harom. J = 7.1 Hz), 4.58 (br, 4H, 2 × OCH2), 4.40 (d, 2H, 2 N-CH, J = 8.0 Hz), 4.35 (d, 2H, 2S-CH, J = 8.0 Hz), 3.88 (q, 4H,2xOCH2, J = 7.1 Hz), 2.70 (br, 4H, 2xCH2), 1.90 (br, 4H,2xCH2), 1.19 (br, 4H,2xCH2), 1.04 (br, 6H, 2xCH3); 13C NMR: δ 168.8,156.1,152.8,141.5,130.2,128.0,124.4, 121.0, 113.1, 65.9, 62.3, 44.0, 40.5, 25.2, 23.5, 23.0, 14.0; DEPT: δ 130.2 (CH), 128.0 (CH), 121.0 (CH), 113.1 (CH), 65.9 (CH2), 62.3 (CH2), 44.0 (CH), 40.5 (CH), 25.2 (CH2), 23.5 (CH2), 23.0 (CH2), 14.0 (CH3); MS (EI): m/z = 720 (m+), 705, 690, 675, 630, 292,146 (base peak), 120.91; Calcd. for: C34H40N8O6S2: C, 56.65; H, 5.59; N, 15.54; S, 8.90; Found: C, 56.51; H, 5.50; N, 15.35; S, 8.73.
8,12-Dioxa-33,37-dithia-20,21,23,24,30,31,35,36-octaazaheptacyclo(27.5.2.219,22.02,7.013,18.021,25.032,36)octatriaconta-2,4,6,13(18),14, 16,22,24,29,31-decaene-34,38-dicarboxamide (3c)
65% yield, IR: ν 3310, 3185 (NH stretch), 3040 (aromatic CH stretch.), 1678 (C = O), 1255 (Ar-O), 1105 cm-1; 1H NMR: δ 7.80 (s, 2H, NH2,D2O exchange), 7.39 (d, 2H, HaromJ = 6.1 Hz), 7.34 (d, 2H, HaromJ = 7.0 Hz), 7.23 (s, 2H, 2 NH, D2O exchange), 7.12 (d, 2H, HaromJ = 7.8 Hz), 7.01 (t, 2H, HaromJ = 6.5 Hz), 6.68 (s, 2H, NH D2O exchange), 4.52 (br, 2H,2xOCH2), 4.44 (br, 2H,2xOCH2), 4.40 (d, 2H, 2 N-CH, J = 7.8 Hz), 4.24 (d, 2H, 2 S-CH, J = 8.0 Hz), 2.80 (br, 4H,2xCH2), 2.12 (br, 2H, CH2), 1.96 (br, 2H, CH2); 13C NMR: δ 169.5, 156.7, 152.3, 142.1, 130.4, 127.8, 125.0, 121.0, 113.4, 66.4, 43.5, 40.1, 27.5, 24.0, 22.1; DEPT: δ 130.4 (CH), 127.8 (CH), 121.0 (CH), 113.4 (CH), 66.4 (CH2), 43.5 (CH), 40.1 (CH), 27.5 (CH2), 24.0 (CH2), 22.1 (CH2); MS (EI): m/z = 634 (M+), 605, 576, 339, 278, 146, 119, 91, 57, 43 (base peak); C28H30N10O4S2:C, 52.98; H, 4.76; N, 22.07; S, 10.10; Found: C, 52.72; H, 4.66; N, 21.88; S, 9.95.
8,13-Dioxa-35,39-dithia-21,22,24,25,32,33,37,38-octaazahepta-cyclo(29.5.2.220,23.02,7.014,19.022,26.034,38)tetraconta-2,4,6,14(19),15,17, 23,25,31,33-decaene-36,40-dicarboxamide (3d)
65% yield, IR: ν 3314, 3180 (NH stretch), 3060 (aromatic CH stretch.), 1682 (C = O), 1250 (Ar-O), 1110 cm-1; 1H NMR: δ 7.78 (s, 2H, NH2,D2O exchange), 7.41 (d, 2H, HaromJ = 6.1 Hz), 7.31 (d, 2H, HaromJ = 7.0 Hz), 7.25 (s, 2H,2xNH, D2O exchange), 7.10 (d, 2H, Harom'J = 7.8 Hz), 6.99 (t, 2H, HaromJ = 6.5 Hz), 6.71 (s, 2H, NH D2O exchange), 4.48 (br, 2H, OCH2), 4.37 (br, 2H, OCH2), 4.38 (d, 2H, 2N-CH, J = 7.8 Hz), 4.30 (d, 2H, 2 S-CH, J = 8.0 Hz), 2.75 (br, 4H,2xCH2), 2.00 (br, 4H, 2 × CH2), 1.23 (br, 4H, 2 × CH2); 13C NMR: δ 169.7, 156.6, 152.5, 142.1, 130.5, 127.8, 124.9, 121.1, 113.1, 66.1, 43.6, 40.0, 25.3, 25.1, 23.0; DEPT: δ 130.5 (CH), 127.8 (CH), 121.10 (CH), 113.1 (CH), 66.1 (CH2), 43.6 (CH), 40.0 (CH), 25.3 (CH2), 25.1 (CH2), 23.0 (CH2); MS (EI): m/z = 662 (M+), 633, 604, 339, 292, 146, 119, 91, 57, 43 (base peak); Anal. Calcd. for: C30H34N10O4S2: C, 54.36; H, 5.17; N, 21.13; S, 9.68; Found: C, 54.16; H, 5.09; N, 20.96; S, 9.55.
3. Results and Discussion
In continuation of our interest in developing the synthesis of new macrocycles and lariat ethers,11-14 we report herein a simple and efficient method for the regioselective synthesis of novel bibrachial lariat ethers (BiBLEs) 3a-d. In this paper we demonstrate a novel method to introduce 1,2,4-triazolo[3,4-b][1,3,4]-thiadiazines rings into macrocycles. Aza-crown ether compounds 1a-b were prepared according to the published method.13 The functionalities in these aza-crown ethers made them valuable key precursors for the formation of different fused heterocyclic compounds. The available macrocycles 1a-b encouraged us to study their transformation into the lariat ethers containing ester or amide groups. Thus, the novel lariat compounds 3a-d with neutral side arms were prepared by reaction of the corresponding aza-crown macrocycles 1a-b with ethylchlroacetate and chloroacetamide. Stirring of compounds 1a-b with ethyl chloroacetate or chloroacetamide in the presence of sodium hydride for 5 h afforded good yields of the corresponding novel bibrachial lariat ethers 3a-d (Scheme 1).
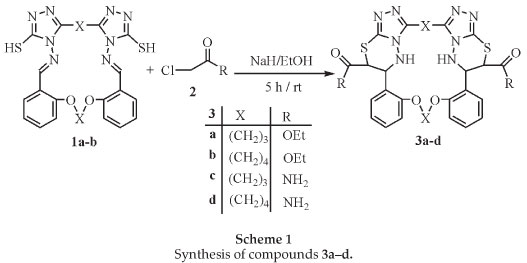
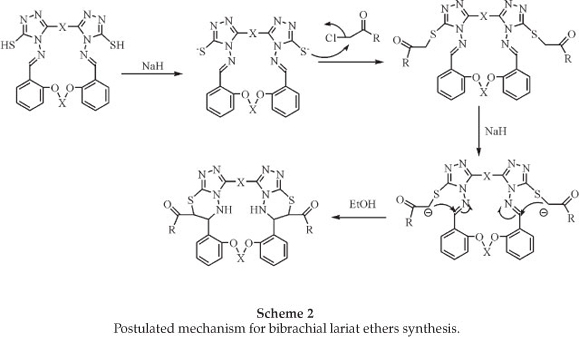
The reaction proceeds via intramolecular cyclocondensation of the active methylene group with the imine group. A proposed mechanism for the reaction is outalined in Scheme 2.
The expected compounds were obtained in good yields. The reaction of 1a-b with ethyl chloroacetate and chloroacetamide in the presence sodium hydride was regioselective and afforded only the cis isomer after ring closure. The isolated compounds 3a-d were obtained as cis-diastereomers. This fact was confirmed by 1H NMR spectroscopy data. The stereochemistry of the products was determined from the coupling constant between the two vicinal methine protons. In the 1H-NMR spectra of compounds 3a-d, the coupling constant (3JN-CHiCH-S ≈ 7.8-8.0 Hz) is typical for the cis configuration.11,14
The IR, 1H NMR and 13C NMR spectra of 3a-d confirmed the success of the cyclization by demonstrating the disappearance of the signals corresponding to the SH and CH=N protons and the appearance of signals assigned to the methine and NH protons. The infrared spectra of the aza-crown 1a-b showed absorption bands at 2766 cm-1 due to SH groups which were absent in the IR spectra of compounds 3a-d. Similarly, the 1H NMR spectra of the compounds 1a-b showed two characteristic absorptions (singlet at: δ 9.6 ppm) attributed to the CH=N groups, and another at δ 13.5 ppm, assigned to the SH, which disappeared after formation of compounds 3a-d. In addition, absence of the 13C NMR and DEPT spectroscopy signals due to the CH=N groups and appearance of the aliphatic carbon relative to the thiadiazine ring confirmed the formation of compounds 4a-d.
4. Conclusion
In conclusion, a new series of bibrachial lariat ethers (BiBLEs) having pendant groups containing a strong donor group was prepared. These macrocycles can be used as promising polydentate ligands with three-dimensional binding character.
Acknowledgement
We are thankful to Malayer Branch, Islamic Azad University for their financial support.
References
1 C.J. Pederson, J. Am. Chem. Soc. 1967, 89, 7017-7036. [ Links ]
2 A.H.M. Elwahy, A.A. Abbas and Y.A. Ibrahim, J. Chem. Res., 1996, 182-183, and references cited therein. [ Links ]
3 E.D. Richard and D.G. Milton, J. Am. Chem. Soc., 1976, 98, 762-767. [ Links ]
4 N. Foroughifar, A. Mobinikhaledi, S. Ebrahimi, H. Moghanian, M.A. Bodaghi Fard and M. Kalhor, Tetrahedron Lett., 2009, 50, 836-839 and references cited therein. [ Links ]
5 H.A. Muathen, N.A.M. Aloweiny and A.H.M. Elwahy, J. Heterocyclic. Chem., 2009, 46, 656-663. [ Links ]
6 G.R. Newkome, J.D. Sauer, J.M. Rober and D.C Hager,. Chem. Rev. 1977, 77, 513-597. [ Links ]
7 H. Sharghi, R. Khalifeh and A.R. Salimi Beni, J. Iranian Chem. Soc., 2010, 7, 275-288. [ Links ]
8 E. Weber and F. Vogtle, eds., Topics in Current Chemistry: Host Guest Complex Chemistry I, Springer, New York, USA, 1981, p. 14. [ Links ]
9 I.M. Kolthoff, Anal. Chem., 1979, 51(5), 1R. [ Links ]
10 G.W Gokel, D.M. Dishong and C.J Diamond, J. Chem. Soc. Chem. Commun., 1980, 1053-1054. [ Links ]
11 N. Foroughifar, A. Mobinikhaledi and S. Ebrahimi, Synthesis, 2009, 2557-2560. [ Links ]
12 N. Foroughifar, A. Mobinikhaledi and S. Ebrahimi, Synth. Commun. 2010, 40, 2421-2428. [ Links ]
13 S. Ebrahimi and H. Moghanian, Heterocycl. Commun. 2012, 18, 29-31. [ Links ]
14 S. Ebrahimi, A. Mobinikhaledi and N. Foroughifar, J. Heterocyclic Chem. 2012, 49, 955-958. [ Links ]
15 A.H.M. Elwahy, A.A. Abbas and A.M.A. Ahmed, J. Heterocyclic Chem., 2005, 42, 93-101. [ Links ]
Received 25 June 2012
Revised 2 November 2012
Accepted 10 March 2013
* E-mail: seyonesi@gmail.com / seyonesi@iau-malayer.ac.ir














