Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.66 Durban Aug. 2013
RESEARCH ARTICLE
A novel coumarin Schiff-base fluorescent probe for Mg2+
Xian-Gui Zhou; Ming-Sheng Peng*; Tang-Zhen Feng
College of Chemistry & Chemical Engineering, Key Laboratory of Tropical Medicinal Plant Chemistry of Ministry of Education, Hainan Normal University, Haikou 571158, PR China
ABSTRACT
A novel fluorescent probe for Mg2+ based on coumarin Schiff-base was synthesized and characterized. The sensor displayed high selectivity toward Mg2+ in acetonitrile, and shows 1:1 complex formation with Mg2+ in acetonitrile.
Keywords: Coumarin, Schiff-base, fluorescent probe, magnesium ion.
1. Introduction
The design and synthesis of fluorescent probes with selectivity and sensitivity is an active field of supramolecular chemistry for biological as well as analytical and environmental problems. Mg2+ is one of the most abundant divalent ions in the cell, and it plays a critical role in many biological activities, such as cell proliferation1,2, cell death3, signal transduction4, transporters5 and ion channels.6,7 To date, several fluorescence-based probes for Mg2+ have been developed; however, most of them have shortcomings in practical application, such as difficult synthesis, insufficient selectivity or sensitivity, or interference problems from other metal ions.8,9 Therefore, development of highly sensitive and selective fluorescent probes is necessary.
Coumarin and its derivatives have been widely used as desirable fluorophore and binding moiety. However, there are few reports about the derivatives of coumarin as fluorescent sensors for Mg2+.10-12 In this paper, a highly sensitive and selective fluorescent chemosensor 3 for Mg2+ based on coumarin Schiff-base is demonstrated (Scheme 1), which displays remarkable fluorometric enhancement upon the addition of Mg2+.

2. Experimental
All chemicals were purchased from commercial suppliers and used without further purification. The solvents purified with standard methods. 1H and 13C NMR spectra were recorded in a Broker 400 spectrometer. Chemical shifts are reported in ppm using tetramethylsilane (TMS) as the internal standard. Mass spectra were obtained in Agilent 1100-Bruker Esquire HCT liquid chromatograph-mass spectrometer. UV-vis absorption and fluorescence spectra were measured with a TU-1901 double-beam UV-vis spectrophotometer, RF-53010 PC fluorescence spectrophotometer, respectively.
Synthesis of 8-((1,3,4-Thiadiazol-2-ylimino)methyl)-7-hydroxy-4- methyl-2H-chromen-2-one (3)
1,3,4-Thiadiazol-2-amine (1) was synthesized according to literature.13 8-Formyl-7-hydroxy-4-methylcoumarin (2) was synthesized according to literature.14
Compound 1 (2.45 mmol, 0.500 g) was dissolved in ethanol (20 mL), and compound 2 (2.45 mmol, 0.247 g) was added to the solution. The reaction mixture was refluxed for 8 h under N2 atmosphere, cooled to room temperature. The pale yellow precipitate was filtered and washed with cold ethanol. The crude product was recrystallized from ethanol to give 3 (90 %) as yellow solid; m.p. 226-228 °C; δH (400 MHz, CDCl3): 2.43(3H, s), 6.21(1H, s), 6.91(1H, d, J 9.2 Hz,), 7.73(1H, d, J 8.8 Hz), 10.62(1H, s),12.22(1H, s) ppm; δC (100 MHz, CDCl3): 18.95, 108.66, 111.96, 112.06, 114.27, 132.88, 152.63, 156.14, 159.19, 165.25,193.40 ppm; m/z: 286.1 [M-H]+ (Found: C, 54.40; H, 3.11; N, 14.78; S, 11.10 %. Calc. for C13H9N3O3S(287.29): C, 54.35; H, 3.16; N, 14.63; S 11.16 %).
3. Results and Discussion
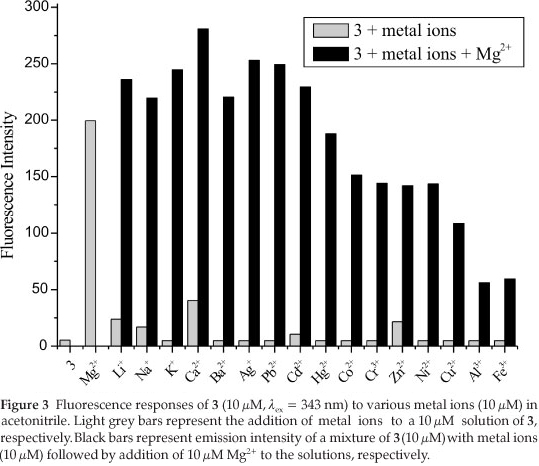
As shown in Fig. 1, the free ligand 3 (10 ^M) alone had a weak fluorescence intensity at 456 nm when it was excited at 343 nm, due to isomerization of the C=N double bond and effect of intramolecular charge transfer (ICT) in Schiff base. Compound with an unbridged C=N structure is often nonfluorescent due to the C=N isomerization, but it may be inhibited by complexation with metal ions.15 Upon addition of Mg2+ (0-300 µM) into the solution of 3 (10µM the fluorescence intensity was increased, and λem red-shifted to 470 nm. At a Mg2+ concentration of 150 µM, the fluorescence intensity reached a maximum and showed a 33.3-fold enhancement, which suggested that 3 may serve as a 'turn-on' sensor for Mg2+.


The selectivity of 3 (10 /M) to various complex at 470 nm decreased, but it still had strong fluorescence intensity. The fluorescence intensity increased with the addition of Li+,Na+,K+,Ca2+,Ba2+, Ag+, Pb2+ and Cd2+. Although Cu2+, Al3+ and Fe3+ could quench fluorescence of 3 via energy or electron transfer16, the quenched fluorescence intensity could be enhanced to 8-fold, 4.5-fold and 4.75-fold, respectively. Therefore, 3 showed a high selectivity for Mg2+ in the presence of these coexistent metal ions in acetonitrile.
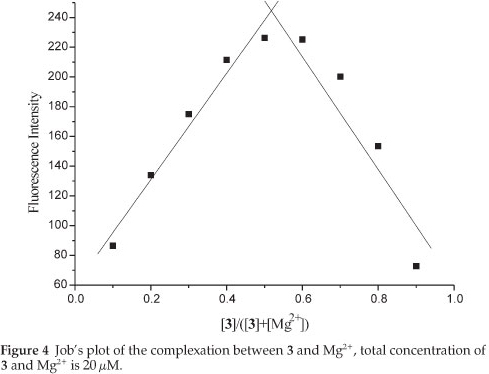
To determine the stoichiometry of the 3/Mg2+ complex, Job's method was used. By keeping the total concentration of Mg2+ and 3 at 20 and changing the molar ratio of Mg2+ (XM; XM=[Mg2+]/{[3] + [Mg2+]}) from 0 to 0.9, the fluorescence intensity of 3 in the absence (F0) and presence (F) of Mg2+ ion was determined, respectively. A plot of (F-F0) versus XM shows that the value goes through a maximum at a molar fraction of about 0.5, indicating a 1:1 stoichiometry complex formation exactly (Fig. 4).
4. Conclusion
A novel coumarin Schiff-base fluorescent probe 3 for Mg2+ has been designed and synthesized. It displayed high selectivity and sensitivity for Mg2+ over the other metal ions. The chemosensor 3 shows 1:1 complex formation with Mg2+ in acetonitrile.
References
1 F.I. Wolf and A. Cittadini, Front. Biosci., 1999, 4, 607-617. [ Links ]
2 H. Rubin, Bioessays, 2005, 27, 311-320. [ Links ]
3 R. Eskes, B. Antonsson, A. Osen Sand, R. Montessuit, C. Richter, R. Sadoul, G. Mazzei, A. Nichols and J.C. Martinou, J. Cell Biol., 1998, 143, 217-224. [ Links ]
4 H.C. Politi and R.R. Preston, Neuroreport, 2003, 14, 659-668. [ Links ]
5 T. Kubota, K. Tokumo, J. Nakagawa, Y. Kitamura, H. Ogawa, Y. Suzuki, K. Suzuki and K. Oka, Biochem. Bioph. Res. Co., 2003, 303, 332-336. [ Links ]
6 M.J.S. Nadler, M.C. Hermosura, K. Inabe, A. Perraud, Q.Q. Zhu, A.J. Stokes, T. Kurosaki, J.P. Kinet, R. Penner, A.M. Scharenberg and A. Fleig, Nature, 2001, 411, 590-595. [ Links ]
7 C. Schmitz, A.L. Perraud, C.O. Johnson, K. Inabe, M.K. Smith, R. Penner, T. Kurosaki, A. Fleig and A.M. Scharenberg, Cell, 2003, 114, 191-200. [ Links ]
8 J. Pesco, J. Salmon, J. Vigo and P. Viallet, Anal. Biochem., 2001, 290, 221-231. [ Links ]
9 L. Prodi, F. Bolletta, M. Montalti and N. Zaccheroni, Tetrahedron Lett., 1998, 39, 5451-5454. [ Links ]
10 D. Ray and P.K. Bharadwaj, Inorg. Chem., 2008, 47, 2252-2254. [ Links ]
11 Y. Suzuki, H. Komatsu, T. Ikeda, N. Saito, S. Araki, D. Citterio, H. Hisamoto, Y. Kitamura, T. Kubota, J. Nakagawa, K. Oka and K. Suzuki, Anal. Chem., 2002, 74, 1423-1428. [ Links ]
12 E. Brunet, M.T. Alonso, O. Juanes, R. Sedano and J.C. Rodriguez-Ubis, Tetrahedron Lett., 1997, 38, 4459-4462. [ Links ]
13 P. Kaur and D. Sareen, Dyes. Pigments, 2011, 88, 296-300. [ Links ]
14 A. Kulkarni, S.A. Patil and P.S. Badami, Eur. J. Med. Chem. , 2009, 44, 2904-2912. [ Links ]
15 J.S. Wu, W.M. Liu, X.Q. Zhuang, F. Wang, P.F. Wang, S.L. Tao, X.H. Zhang, S.K. Wu and S.T. Lee, Org. Lett, 2007, 9, 33-36. [ Links ]
16 A.P.D. Silva, H.Q.N. Gunaratne, T. Gunnlaugsson, A.J.M. Huxley, C.P. McCoy, J.T. Rademacher and T.E. Rice, Chem. Rev. , 1997, 97, 1515-1566. [ Links ]
Received 14 March 2012
Accepted 7 November 2012
* To whom correspondence should be addressed. E-mail: pengmsh@163.com














