Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.66 Durban Aug. 2013
RESEARCH ARTICLE
Synthesis and crystal structure of ethyl 2-(benzo[d]oxazol-2-yl)-5-oxo-3-(o-tolylamino)-2,5-dihydroisoxazole-4-carboxylate
A. Poursattar Marjani; J. Khalafy*; N. Noroozi Pesyan
Department of Chemistry, Faculty of Science, Urmia University, Urmia 57154, Iran
ABSTRACT
The title compound ethyl 2-(benzo[d|oxazol-2-yl)-5-oxo-3-(o-tolylamino)-2,5-dihydroisoxazole-4-carboxylate (5) was synthesized and studied by the single crystal X-ray diffraction method. Its structure was confirmed by IR, 1H and 13C NMR spectroscopy, microanalyses, and X-ray single crystal structure determination. Compound 5 crystallized in the monoclinic system, space group P21/c, a = 11.9936(3) Å, b = 13.9638(3) Å, c = 11.5126(4) Å, α = γ = 90°, β = 108.939(3) °, V = 1823.70(9) Å3,Z = 4, R1 = 0.050 and wR2 = 0.130. Compound 5 shows an intramolecular hydrogen bond between N9-H...O3 atoms. The H-bond and donor-acceptor (N9...O3) distances were obtained 2.165 and 2.798 Å, respectively. The crystal structure of 5 also shows a weak interaction between O1 and O26 atoms with distance of 2.985 Å. The benzo[d]oxazole and o-toluidine rings moieties are not collimated together on isoxazolone ring. The torsion angles of N9-C8-N7-C17, O6-N7-C17-O18 equal -49.63 ° and 68.67 °, respectively.
Keywords: Isoxazolone; 2-chlorobenzoxazole; N-substituted isoxazolone; single crystal X-ray structure; hydrogen bond.
1. Introduction
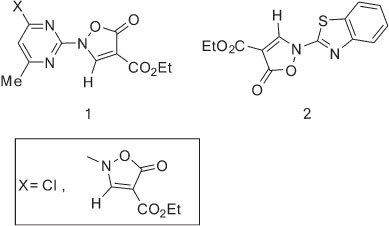
The synthesis of some mono- and bis-isoxazolinyl derivatives of pyrimidine 1 and benzothiazole 2 as central nervous system-active compounds and their base-catalyzed rearrangements to the corresponding annelated heterocycles have been reported.1,2
We reported earlier the syntheses of imidazoles,3 imidazopyridines,4-7 imidazobenzothiazoles,8 indoles,9,10 imidazopyrimidine,10,11 imidazoquinoxalines,12 pyrimidoquinolines13-15 and aminoisoxazole16,17 starting from isoxazol-5(2H)-ones. al structure of ethyl 2-(benzo[d]oxazol-2-yl)-5-oxo-3-(o-tolyl-amino)-2,5-dihydroisoxazole-4-carboxylate (5).
2. Experimental
2.1. Materials and Instruments
Freshly distilled solvents were used throughout, and anhydrous solvents were dried according to Perrin and Armarego.21 Melting points were determined on a digital melting point apparatus (Electrothermal) and remain uncorrected. Infrared spectra were recorded on a Thermo Nicolet (Nexus 670) FT-IR spectrometer, using KBr disks. 1H and 13C NMR spectra were recorded with a Bruker spectrometer at 300 and 75.5 MHz, respectively. The spectra were measured in CDCl3 using TMS as the internal standard. Elemental microanalyses of the compounds studied were performed using a Carlo-Erba 1104 instrument.
2.2. Ethyl 2-(benzo[d]oxazol-2-yl)-5-oxo-3-(o-tolylamino)-2,5-dihydroisoxazole-4-carboxylate (5)
A mixture of the isoxazolone7(4) (100 mg, 0.38 mmol) and 2-chlorobenzoxazole (58 mg, 0.38 mmol) were heated under nitrogen in 120 °C for 30 min. The mixture was left to cool to room temperature; it was then extracted with chloroform. The solution was filtered and passed through a short plug of silica. Removal of the solvent gave a pale yellow oil, which was crystallized from ethanol to give the title compound as a white needles (89 mg, 65 %), mp 131-132 °C. FT-IR (KBr, cm-1): 3265,3076,2981, 1781, 1635, 1486, 1387, 1352, 1286, 1232, 1026, 795, 752; δH (300 MHz, CDCl3): 1.42 (3H, t, J 7.2 Hz, CH3), 2.36 (3H, s, CH3), 4.41 (2H, q, J 7.2 Hz, CH2), 6.83 (1H, d, J 8.1 Hz, ArH), 6.88 (1H, t, J 8.1 Hz, ArH), 7.00 (1H, t, J 8.4 Hz, ArH), 7.13 (1H, dd, J1 7.1 Hz, J21.5 Hz, ArH), 7.22-7.34 (2H, m, ArH), 7.36 (1H, dd, J1 7.1 Hz, J21.5 Hz, ArH), 7.45 (1H, dd, J1 7.1 Hz, J2 1.5 Hz, ArH), 9.73 (1H, s, NH, removed by D2O addition) ppm; <5C (75 MHz, CDCl3): 14.43, 17.82, 61.06, 78.28, 110.68, 120.49, 123.65, 125.38, 126.59, 126.81, 127.66, 130.91, 133.08, 133.58, 139.38, 149.69, 151.19, 163.61, 165.05,165.14 ppm; (Found: C, 63.36; H, 4.54; N, 11.01 %. Calc. for C20H17N3O5 (379.37); C, 63.32; H, 4.52; N, 11.08 %).
Single crystals of 5 were prepared by recrystallization from a mixture of n-hexane/ethanol over a period of two weeks. The pale brown crystals were filtered off, washed with a cold mixture of n-hexane/ethanol and dried in vacuum over P4O10 (mp. 131-132 °C).
2.3. Crystal Structure Determination of 5 (Table 1 and Fig. 1)

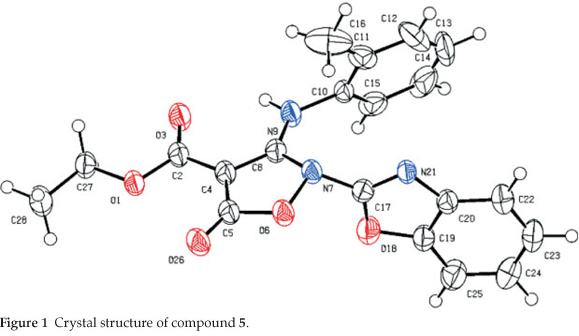
The crystal structure of 5 is shown in Fig. 1. For the crystal structure determinations, single-crystals of 5 were used for data collection on an Oxford Diffraction Gemini E diffractometer. Measurements were made at 150 K with graphite mono-chromated Cu Κα radiation (λ = 1.54184 A). The computing details; data collection: Gemini (Oxford Diffraction, 2006)22; cell refinement: CrysAlis RED, (Oxford Diffraction, 2002)22; data reduction: CrysAlis RED, (Oxford Diffraction, 2002)22; pro- gram(s) used to solve structure: SIR9223; program(s) used to refine structure: CRYSTALS24; molecular graphics: CAMERON25 and software used to prepare material for publication: CRYS-TALS.24 The crystallographic data for structure 5 were deposited to the Cambridge Crystallographic Data Centre (entry no. CCDC-874765) and are available free of charge upon request to CCDC, 12 Union Road, Cambridge, UK (Fax: +44-1223-336033, e-mail: deposit@ccdc.cam.ac.uk).
3. Results and Discussion
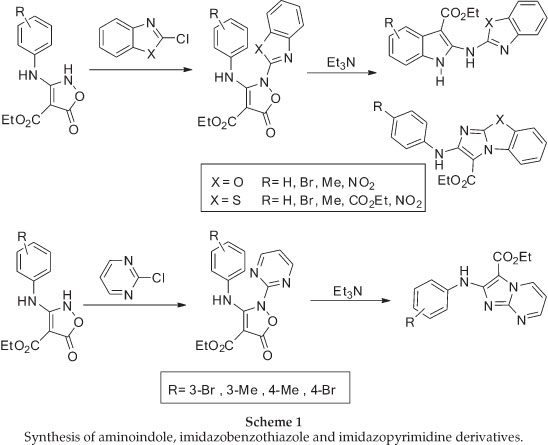
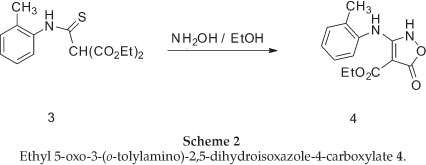
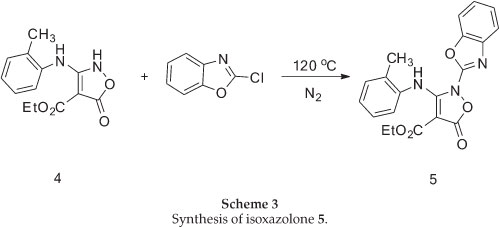
The required isoxazolone (5) was prepared by N-arylation of the 2H-isoxazolone (4), which in turn was made by a modification of Worrall procedure.26,27 Thus, the reaction of the sodium salt of diethyl malonate in ethanol with 2-methylphenyl isothiocyanates gave the thiocarbamate (3) in high yield, and this was converted to the corresponding isoxazolone (4) by reaction with hydroxylamine in ethanol under reflux conditions (Scheme 2).
N-arylation of 4 with 2-chlorobenzoxazole upon heating with- out solvent under nitrogen atmosphere at 120 °C, afforded the corresponding N-benzoxazole derivative 5 in 65% yield in comparison with a previous method10 in chloroform under reflux conditions which was 54% (Scheme 3).
3.1. Description of Crystal Structure of 5
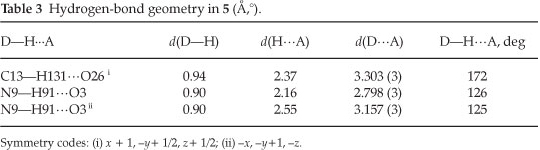
The crystal of 5 is built up from molecules, the structure of which is shown in Fig. 1. The crystal structure of 5 and its crystal packing diagram are shown in Figs. 1 and 2, respectively. Also the structure of 5 from different side views is shown in Fig. 3. A summary of the crystal data and experimental details is given in Table 1. While selected bond lengths, angles and torsion angles for 5 are shown in Table 2. The crystal structure of compound 5 shows an intramolecular hydrogen bond between N9-H...O3 atoms due to the formation of a six membered ring almost planar (torsion angles of C8-C4-C2-O3 and C4-C8-N9-H91 are equals to -4.18 ° and 1.43 °, respectively). The H-bond and donor-acceptor (N9...O3) distances were obtained 2.165 and 2.798 Å, respectively. The N7 atom on the isoxazolone ring has pseudo pyramidal conformation and both N21 on the benzoxazole and N9 on the toluidine (as a secondary amine) moieties have a planar conformation due to aromatization of the benzoxazole ring moiety and resonance of N9 lone pair with carbonyl group of isoxazolone (also with tolyl ring), respectively.
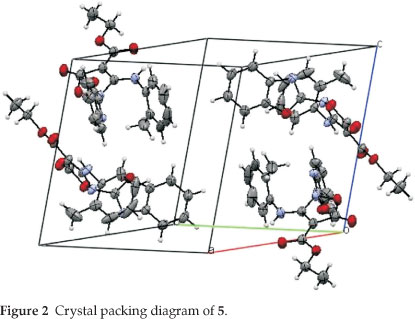
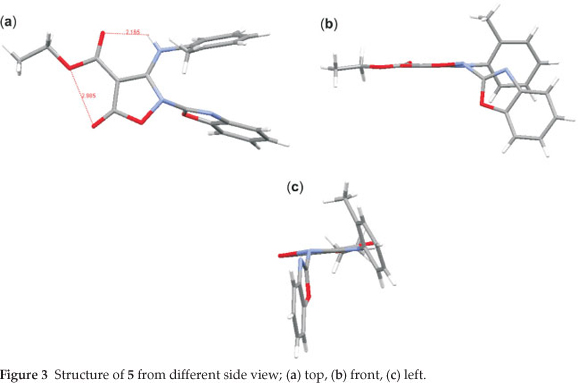
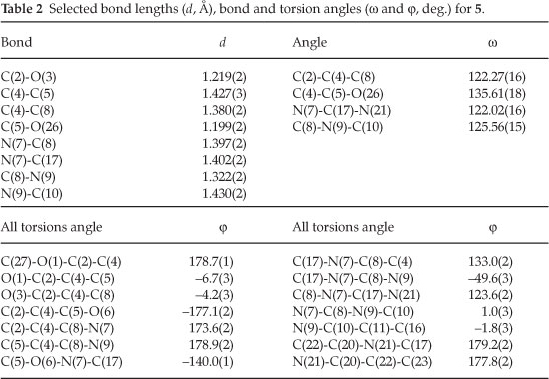
The crystal structure of 5 also shows a weak interaction between O1 and O26 atoms with distance of 2.985 A. The benzo[d]oxazole and o -toluidine rings moieties are not collimated together on isoxazolone ring. The torsion angles of N9-C8-N7-C17, O6-N7-C17-O18 are equal to -49.63 ° and 68.67 °, respectively. The ethoxy carbonyl ester group lies in plane of isoxazolone ring. The ethoxy carbonyl ester group lies in the plane of the isoxazolone ring. The geometry of the hydrogen bonds are shown in Table 3.
4. Conclusions
We have developed a new and facile solvent-less method for preparing ethyl 2-(benzoxazol-2-yl)-3-(o-tolylamino)-5-oxo-2,5-dihydroisoxazole-4-carboxylate (5). The X-ray single crystal diffraction analysis showed that the molecular structure of 5 possesses of an intramolecular hydrogen bond from resonance-assisted N-H- · Ό bond distance of 2.165 Å. This structure also showed a weak interaction between O1 and O26 atoms with distance of 2.985 Å.
Acknowledgements
The authors are grateful to Urmia University for financial support, to Dr. M.I.M. Tahir (University Putra Malaysia, Malaysia) for his help with X-ray crystallography and to Dr. A.R. Dadras (Urmia University, Iran) for his valuable comments.
References
1 T.V Hung, WK. Janowski and R.H. Prager, Aust. J. Chem., 1985, 38, 931-937. [ Links ]
2 R.H. Prager, T.K. Rosenzweig and Y. Singh, Aust. J. Chem., 1992, 45, 1825-1832. [ Links ]
3 J. Khalafy, R.H. Prager and J.A. Smith, J. Chem. Res (M)., 1999,518-536. [ Links ]
4 J. Khalafy and R.H. Prager, J. Sci. I. R. Iran., 2000, 11, 32-38. [ Links ]
5 J. Khalafy, D. Setamdideh and K. Akbari Dilmaghani, Molecules., 2002, 7, 907-916. [ Links ]
6 J. Khalafy, A.R. Molla Ebrahimlo, R. Eisavi and K. Akbari Dilmaghani, ARKIVOC., 2005, xiv, 59-70. [ Links ]
7 M.M. Baradarani, J. Khalafy, S. Khadivi and A. Poursattar Marjani, Chem. Heterocycl. Comp., 2008, 44, 594-599. [Khim. Geterotsikl. Soedin, 2008, 44, 751-757]. [ Links ]
8 J. Khalafy, A.R. Molla Ebrahimlo and K. Akbari Dilmaghani, J. Chin. Chem. Soc., 2004, 51, 1347-1352. [ Links ]
9 J. Khalafy, A. Poursattar Marjani and A.R. Molla Ebrahimlo, J. Braz. Chem. Soc., 2006, 17, 570-576. [ Links ]
10 A. Poursattar Marjaniand J. Khalafy, Turk. J. Chem., 2010, 34, 847-858. [ Links ]
11 A.R. Molla Ebrahimlo and J. Khalafy, ARKIVOC, 2008, xiii, 65-71. [ Links ]
12 A. Poursattar Marjani, J. Khalafy and R.H. Prager, Chem. Heterocycl. Comp., 2012, 48, 931-935. [Khim. Geterotsikl. Soedin., 2012, 48, 1001-1005].
13 A. Poursattar Marjani, J. Khalafy, A.R. Molla Ebrahimlo and R.H. Prager, Bull. Korean Chem. Soc., 2011, 32, 2183-2186. [ Links ]
14 A. Poursattar Marjani, J. Khalafy and A.R. Molla Ebrahimlo, Synth. Commun., 2011, 41, 2475-2482. [ Links ]
15 A. Poursattar Marjaniand J. Khalafy, Chem. Heterocycl. Comp., 2011, 47, 96-100. [ Links ]
16 J. Khalafy, K. Akbari Dilmaghani, L. Soltani and A. Poursattar Marjani, Chem. Heterocycl. Comp, 2008, 44, 729-734. [Khim. Geterotsikl. Soedin., 2008, 44, 907-912].
17 J. Khalafy, A. Poursattar Marjani and A. Rostamzadeh, Heterocycl. Commun., 2011, 17, 65-68. [ Links ]
18 T.-N. Pham, R. Tuteja, A. Ocham and A. Falaschi, Biochem. Biophys. Res. Commun., 1997, 236, 636-640. [ Links ]
19 M. Stiborova, C.A. Bieler, M. Wiessler andE. Frei, Biochem. Pharmacol., 2001, 62, 1675-1684. [ Links ]
20 P. Ajay Babu, M. Laxmi Narasu and K. Srinivas, ARKIVOC, 2007, ii, 247-265. [ Links ]
21 D.D. Perrin and W.L.F. Armarego, Purification of Laboratory Chemicals, Pergamon Press, Oxford, UK, 1988. [ Links ]
22 Oxford Diffraction Ltd., CrysAlis CCD, CrysAlis RED and CrysAlis PRO, Abingdon, 2006. [ Links ]
23 A. Altomare, G. Cascarano, C. Giacovazzo, A. Guagliardi, M.C. Burla, G. Polidori and M. Camalli, J. Appl. Cryst., 1994, 27, 435-436. [ Links ]
24 P.W. Betteridge, J.R. Carruthers, R.I. Cooper, C.K. Prout and D.J. Watkin, J. Appl. Cryst., 2003, 36, 1487. [ Links ]
25 D.J. Watkin, C.K. Prout and L.J. Pearce. CAMERON, Chemical Crystallography Laboratory. Oxford, 1996. [ Links ]
26 D.E. Worrall, J. Am. Chem. Soc., 1918, 40, 415-423. [ Links ]
27 D.E. Worrall, J. Am. Chem. Soc., 1923, 45, 3092-3095. [ Links ]
Received 16 July 2012
Revised 10 October 2012
Accepted 13 November 2012
* Author for correspondence. E-mail: jkhalafi@yahoo.com / j.khalafi@urmia.ac.ir














