Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.66 Durban Aug. 2013
RESEARCH ARTICLE
Investigation into the aroma of rosemary using multi-channel silicone rubber traps, off-line olfactometry and comprehensive two-dimensional gas chromatography-mass spectrometry
Leandri van der WatI; Martin DoveyII; Yvette NaudéI; Patricia B.C. ForbesI, *
IDepartment of Chemistry, Faculty of Natural and Agricultural Sciences, University of Pretoria, Lynnwood Road, Hatfield, Pretoria, 0083, South Africa
IIKerry Ingredients South Africa (Pty) Ltd, Block 1 & 2 Nguni Park, 4-6 Lucas Drive, Hillcrest, 3610, South Africa
ABSTRACT
Multi-channel polydimethylsiloxane rubber traps were used to sample the headspace of rosemary samples (two essential oils from different sources, one oleoresin and one dried herb) followed by comprehensive two-dimensional gas chromatography -time of flight mass spectrometry (GCxGC-TOFMS) or GC-MS analyses. The aroma of different headspace samples was characterized using a custom-built olfactory apparatus. The differences between the aroma profiles were evident from bubble plots of the perceived aroma at different temperatures. The samples were heat-treated to simulate cooking of food products, and were then reassessed to determine any changes in the aroma profile. It was found that the intense menthol and cooling aromas subsided in all the samples with heating. GCxGC-TOFMS allowed for separation of the numerous components in the headspace samples. Many terpenes and aliphatics were thus tentatively identified and the relative peak areas were compared to better understand the mixture that contributes to the rosemary aroma.
Keywords: Rosemary, multi-channel silicone rubber traps, GCxGC-TOFMS, essential oil, off-line olfactometry.
1. Introduction
Rosemary has been a popular culinary herb for centuries due to its camphoraceous, fresh and cooling aroma. Recently, it has been found that rosemary does indeed possess many of the properties it was rumoured to have in ancient times such as antimicrobial and antioxidant characteristics.1,2 The herb has many varieties, of which Rosmarinus officinalis L. is most often used in culinary applications. Of this species, rosemary of Tunisian origin is considered to be of the finest quality.3
The many important uses of rosemary, the drive towards natural products (of the 'organic' type) and the fact that the food industry is generating millions of dollars worldwide, means that a study of the aroma of rosemary is most relevant. It would be highly advantageous to the industry if the popular aroma of rosemary could be produced at a fraction of the cost of the fine essential oils. This is the challenge of many flavour and perfume chemists today, and what this study has been taking steps towards achieving: to produce a cost-effective yet nature-identical rosemary additive.
This study was based on the premise that an understandingof the key odourants of rosemary can be used to create its aroma. The long-term goal is to be able to either modify a cheaper oleoresin based product, or to create the aroma from individual aroma compounds. The work described here concentrated on method development and first results are presented as a starting point for professional sensory science studies.
The whole aroma of rosemary has been described as 'camphory, cooling and eucalyptus' with citrus, bark, spice and woody notes also being present.4 Guillen and co-workers reported that essential oil character varies with geographical location and environmental conditions of cultivation.5 Other factors that may influence the composition of herb samples are species and variety, as well as the time of harvest and processing method. It has been reported that rosemary essential oils contain camphor, 1,8-cineole (eucalyptol), camphene and myrcene in highest concentrations, with sabinene, terpinene, linalool, borneol and caryophyllene at lower concentrations.5 The compounds contained in rosemary can be classified into four major groups namely terpenes (isoprenoids), aliphatics, benzenoids and unclassified (miscellaneous) compounds.6 It has further been suggested that verbenone is responsible for the characteristic odour of rosemary.6
Investigations have been conducted on the effects of drying herbs such as basil7 and rosemary.8 However, these studies were motivated by understanding the changes in composition and not by the goal of reconstitution of the herb. The temperature-induced changes in the aroma profile in this study were used directly in order to identify the major rosemary odourants across a broader temperature range. This has significant implications on the end result of reconstitution when applied to cooking applications.
There are significant data to suggest that the aroma of foodstuffs arises as a result of only a small fraction of compounds present in the volatiles profile, resulting in intensive dataprocessing to distinguish the odour-active compounds within the whole range of volatiles present.9 The critical step of sample preparation in flavour analysis must be chosen carefully to ensure reproducibility, sensitivity and no chemical modification. Techniques commonly used are liquid/liquid extraction, solid-phase extraction, extraction with liquid CO2, and headspace extraction.10 Headspace extraction techniques are solvent-free and do not sample non-volatile compounds with no odour activity, which simplifies the chromatography considerably. Extraction of volatiles from the food matrix may lead to decomposition or artefacts depending on the extraction method, and for this reason headspace extraction is simpler, cheaper and more environmentally friendly in the context of this study.9
The volatile nature of odour-active compounds makes the essential oil samples perfect candidates for gas chromatography. Analytical detectors are not as sensitive as the human nose for aroma compounds due to the influence of odour thresholds and thus analytical techniques have been built around olfactometric analysis such as gas chromatography- olfactometry (GC-O) and the electronic nose.9 The lack of sensitivity, limited reproducibility and single-compound identification in GC-O minimizes the efficacy of the technique.9 The complex nature of organic compounds in aroma analysis is well studied using GCxGC-TOFMS as a result of its superior separation capabilities.11
Multi-channel open tubular traps (MCTs) with PDMS (polydimethylsiloxane) channels (Fig. 1) have been reported to concentrate semi-volatile compounds well and maintain good gaseous flow rates due to their open tubular design.12-14 These traps were used for headspace sampling of the herb samples for both chromatographic and olfactory analysis. Headspace sampling is convenient in aroma analysis by virtue of the fact that the aroma is solvent-free and there are no solvent-odourant interactions to consider. The approach is very simple and cost-effective; no purge-and-trap methods need to be employed due to the full aroma of the herb preparations. The MCT is then thermally desorbed directly into the GC-MS, or is inserted directly into the custom-built heating device for off-line olfactory assessment.
The work reported here is on the identification of likely key odorants in the headspace of four rosemary samples using GC-MS and GCxGC-TOFMS, as well as the olfactory analysis of all four samples at room temperature and heated to different temperatures.
2. Experimental
2.1. Rosmarinus officinalus L. Samples
Samples comprised an essential oil of Tunisian origin, a South African oil, an oleoresin from the same local producer and a batch of finely ground dried herb. All samples were stored sealed in a dark, cool place when not in use.
2.1.1. Heat Treatment of Samples
In addition to sampling the headspace of samples maintained at room temperature, all four samples were heated to investigate the effect of temperature on the overall aroma profile. 1.5 mL of each of the liquids was placed into three separate 25.0 mL clean stoppered volumetric flasks. For the dried herb, 1.5 g was transferred into a clean 25.0 mL volumetric flask, and the neck plugged with some quartz wool and then stoppered. Samples were placed in a water bath for1hat50 °C. After cooling to room temperature, the headspace was extracted as described in section 2.2. Samples were similarly obtained on fresh sample aliquots after heating at 75 °C for 1 h, and lastly after heating at 95 °C for 1 h.
2.2. Headspace Sampling using MCTs
Polydimethylsiloxane (PDMS) multi-channel traps were prepared according to the method described by Ortner and Rohwer.12 The glass desorption tube (17.8 cm long, 4 mm I.D., 6 mm O.D.) fits a commercial thermal desorber (Gerstel TDS 3), and 22 channels of silicone tubing (0.3 mm I.D. and 0.64 mm O.D., Sil-tec, Technical Products, GA, USA) were inserted into the tube. The length of the PDMS section inside the trap was 55 mm. The traps were conditioned before use.
Headspace samples were obtained by attaching a clean syringe with rubber tubing to the MCT. The contents of the headspace were then trapped on the PDMS channels as the volume of headspace was pulled through the trap into the syringe. All of the headspace extractions were performed at room temperature (20 °C), including the samples that were heated. Once the desired volumes were extracted, as detailed in the sections to follow, each trap was sealed on each end with glass stoppers by means of Teflon connectors, and was stored in a cool dark place until analysis. For the dried herb samples, the volatiles were not easily captured at room temperature, so an approach of heating 2.5 g of dried herb in a vial covered with quartz wool, in a water bath at 35 °C for 1 h was undertaken. The headspace extraction using this method for the dried herb proved better in yielding results in the olfactory analysis.
2.3. Olfactory Analysis
The first analysis was performed on all four samples individually as sampled at room temperature. 250 mL of headspace was extracted and collected onto the traps for the two essential oils and the oleoresin. 500 mL of the headspace was collected for the dried herb sample, due to its lower whole aroma intensity. The custom-built off-line sniffing apparatus consisted of a thermostated, temperature-controlled oven, with a sniffing port for a blank trap as well as one for the trap loaded with the sample of interest. The traps were heated from room temperature to 200 °C at 10 °C min-1 with a nitrogen gas flow of 20 mL min-1. Sniffing was done in a ventilated room by a non-smoking person. The temperature at which a specific aroma was perceived as well as the intensity was noted. Each rosemary preparation was sampled and analysed in duplicate. Roasted coffee beans were used to refresh the senses between analyses. The time taken for each analysis was approximately 17 min.
The same method was followed for the analysis of the four heat-treated samples (described in section 2.1.1). Here only 100 mL of the headspace was extracted and collected for the two essential oils and the oleoresin. For the dried herb, 200 mL headspace was collected.
2.4. GC-TOFMS
5 mL of the headspace of all four samples were collected onto PDMS traps at room temperature prior to analysis by GC-TOFMS (LECO Pegasus 4D, LECO Corporation, St Joseph, MI, USA) run in one-dimensional mode for the initial analysis. The system was combined with a Gerstel thermal desorber (TDS 3) and an Agilent 7890 GC.
The TDS transfer line temperature was set to 280 °C and the PDMS traps were heated from 30 °C to 250 °C at 30 °C min-1 to thermally desorb the headspace samples. The desorption flow rate was 80 mL min-1 at a vent pressure of 30 psi using helium (Helium UHP, Afrox, Gauteng, South Africa). Cryogenic focusing was performed using liquid nitrogen at -100 °C. After desorption, a splitless injection (purge on after 2 min, purge flow of 30 mL min-1 in solvent vent mode) was performed by heating the Cold Inlet System (CIS) to 250 °C and maintained at that temperature for the entire GC run.
A polyethylene glycol based ZB Wax column (60 m x 0.25 mm ID x 0.25 µm df, Phenomenex, Separations, Randburg, South Africa) was used as the primary column. The secondary column was a 95 % dimethylpolysiloxane: 5 % phenyl-arylene based ZB-5MS (1.190 m x 0.1 mm ID x 0.1 µm df, Restek Corporation, Bellefonte, PA, USA). The primary oven was programmed from 40 °C (2 min) at 5 °C min-1 to 245 °C (5 min), and the GC run time was 48 min. The secondary oven was programmed as for the primary oven but with an offset of +20 °C. The system was unmodulated, and thus the separation was essentially one-dimensional. The carrier gas was helium (helium UHP, Afrox, Gauteng, South Africa) and the velocity of the gas was 1.5 mL min-1 in the constant flow mode.
The MS transfer line was set to 280 °C, and the ion source temperature was 230 °C. The electron energy was 70 eV in the electron impact ionization mode, the mass acquisition range was from 45-150 amu, the detector voltage was held at 1650 V Ten spectra s-1 were acquired.
2.5. GCxGC-TOFMS
Volumes of 5 mL of the headspace of all four samples were collected onto PDMS traps at room temperature and were analysed using the same instrumental setup described in 2.4 now run in GCxGC mode. Thermal desorption from the PDMS trap was performed as in 2.4.
The GC conditions were the same as those detailed in Section 2.4 except here the secondary oven was offset by +10 °C and the modulator temperature offset was 30 °C. The period of modulation was 4 s (hot pulse time 1 s) and 100 spectra s-1 were collected. Tentative identification of compounds was based on comparison of mass spectra with a NIST mass spectral library, using a minimum similarity value of 80 % as a match criterion.
3. Results and Discussion
Analytical techniques can be used to bridge subjective, physiological perceptions of aromas and science. This study focuses on one-dimensional gas chromatography coupled to a mass spectrometer (GC-MS) which provides the separation characteristic of chromatography and the identification tool characteristic of mass spectrometry. Coupling the results of the one-dimensional GC-MS to the results obtained from the off-line olfactory analysis provides data that indicates the identity of compounds responsible for recorded odours perceived at given temperatures, since the temperature of elution from the GC oven can be determined from the retention times of identified compounds. A different sort of chromatogram based on volatility can then be pieced together by aligning the data from the analytical science and the sensory science. Comprehensive two-dimensional chromatography coupled to a time of flight mass spectrometer (GCxGC-TOFMS) provides identities of compounds and highly efficient separation. This technique was thus used for the tentative identification of compounds in the headspace using mass spectral libraries. Qualitative information based on relative peak areas provided useful comparative data.
The PDMS traps were highly effective at sampling the aroma of the rosemary samples. The smell of the silicone rubber channels could only be perceived at high temperatures >190 °C. The smell of the silicon rubber was easily identified when using a blank PDMS trap as a reference in the other sniffing port. No deterioration of aromas was observed when headspace extractions had been sealed in a PDMS trap and stored in a cool place for a few days prior to olfactory analysis.
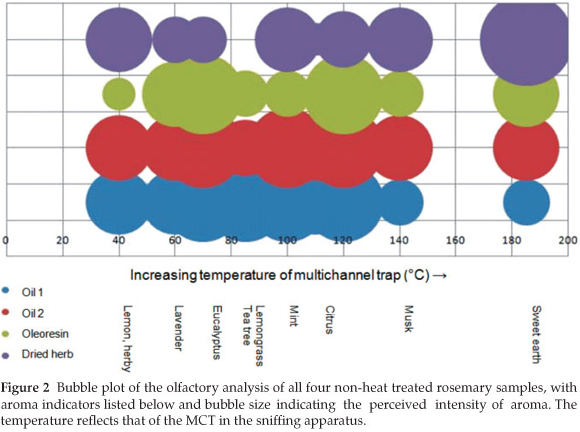
During the olfactory analysis it was found that the essential oil samples had a finer aroma in agreement with Boelens.6 The aroma of the dried herb was considerably different from the liquid samples; having a grassier, farmyard and woody aroma. This is due to the loss of many aroma active compounds in the preparation of the dried herb from fresh rosemary. Overall, the oils had a dominant menthol aroma, with prominent eucalyptus, bark and camphor notes. An overall impression of the aroma of samples maintained at room temperature is given in Fig. 2.
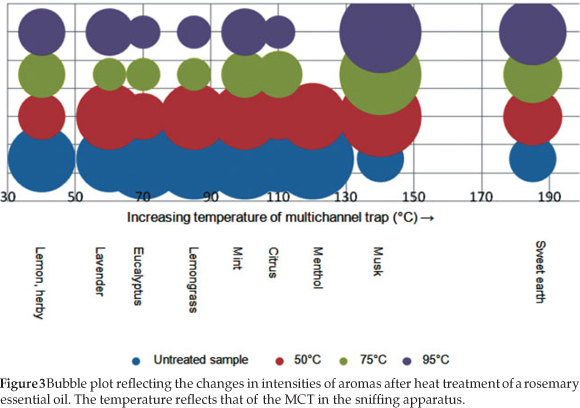
Overall in the heat-treated samples, the intensities of the aromas perceived at low temperatures decreased, and the intensity of the aromas perceived at higher temperatures was increased relative to the initial olfactory analysis of non-heat-treated samples. Heating the samples was valuable since the overwhelming menthol aroma subsided in the oleoresin and oils, thus finer aromas such as citrus peel, smoked ham and must were perceived. The loss in fresh pine, lemongrass and eucalyptus smells suggest, as with the dried herb results, that the amount of eucalyptus and pine aromas in the sample were not entirely crucial to the true aroma of rosemary. This is especially true for applications such as cooking where the rosemary products will be heated. The dried herb aroma did not change significantly with the heat treatment, as a result of the processing the herb undergoes during manufacture. A bubble plot of the change in intensities of aromas as the samples were heat-treated is given in Fig. 3.
The GC retention times and correlating oven temperature of the compounds tentatively identified by GC-MS coupled to the olfactory analysis pinpointed which compounds were responsible for the perceived aromas. The possible compounds responsible for given aromas at specific temperatures are listed in Table 1.
A chromatogram from the GCxGC-MS analysis is given in Fig 4. The compounds identified in the four samples that were likely to be responsible for the whole aroma of the herb are listed in Table 2, with the identified differences between samples given in Table 3. Siloxanes, as a result of the PDMS traps, could be easily identified and removed from the data. Slight column bleed was observed due to the age of the columns. The efficient separation in both dimensions illustrates the usefulness and power of GCxGC-MS in complex odour analyses. The 2D chromatograms were slightly overloaded and wrap around was observed. The difficulty in working with aromas is that odour thresholds always need to be considered. A compromise needs to be met in terms of overloading so that some of the compounds present in low concentrations, which are highly odour-active, can be positively identified at the expense of overloading. Dilution studies should thus be considered in future work.
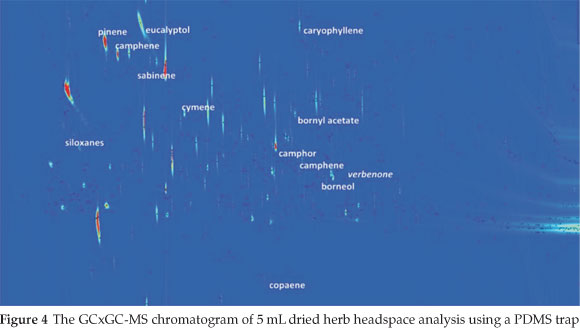
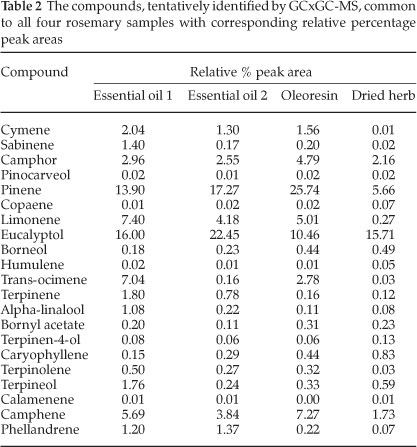
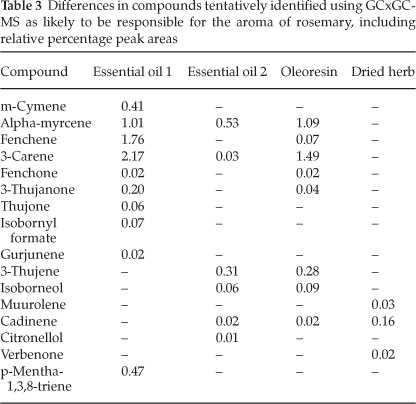
Compounds with the character of the aromas perceived in the olfactometry analysis were present in the samples' headspace. Compounds of note that were particularly distinguishable during the olfactory analysis were eucalyptol, pinene (α-or β-isomers), camphor and limonene. The relative percentage areas are given to provide an indication of the relative concentration in the sample. The difference in relative peak area between the essential oils and the dried herb proved why there was much less of a pine needle and fresh aroma in the dried herb. The apparent loss of limonene in the dried herb was also perceived during the olfactory analysis by the lack of peppery and citrus fruit aromas such as grapefruit and citrus rind that had been smelt in the liquid samples. The lower relative percentage area for phellandrene in the oleoresin and the dried herb compared to the essential oils pointed toward the lack of pepper (lavender) and citrus - green aromas (such as lemongrass, and citrus rind). Curiously, the dried herb sample was the only sample containing verbenone, which has been reported to bear the characteristic odour of rosemary.6 Verbenone standards should be used in future work to confirm this finding.
Having tentatively identified likely key odourants in the samples, further studies using standards need to be undertaken prior to blending of compounds to recreate the aroma. GC-FID is a useful separation tool and can also be used to trap individual aroma compounds, as described in the aroma studies of UHT milk volatiles.14 These trapped peaks can be subsequently analysed with the olfactory apparatus at a later stage, allowing for longer assessment of the odour and thus more accuracy and less nose fatigue. Trapping of the peaks also accommodates the study of synergistic, antagonistic and additive effects between compounds. This forms a part of the future work leading to the recreation of rosemary aroma ab initio based on the relative percentage peak areas as a starting point.
4. Conclusion
The use of the PDMS multi-channel traps to extract headspace from four different samples of rosemary was successful for both GC and olfactory analysis. The MCTs were effective at retaining the aromas of the samples without interfering with the olfactory analysis. The MCTs also did not compromise the integrity of the GCxGC-MS analysis, with the efficacy of the technique seen in the sufficient separation of compounds known to be odour-active. The beauty of this sampling technique is the ease with which the headspace can be sampled, and the 'clean' analysis since liquid sample introduction is avoided. The interference of solvent and formation of artefacts was avoided by using this simple, cheap and environmentally friendly sample preparation technique.
The aroma of rosemary is the result of many complex interactions of hundreds of compounds. The most prevalent aroma compounds were determined using GC-MS and GCxGC-MS. These were found to be mainly terpenes, with high concentrations of compounds such as eucalyptol, camphor and pinene. These results are all in line with the previous work on the aroma profiles of essential oils.3,6,15 Small discrepancies were found between the composition of the essential oils and the oleoresin. These differences are useful in directing the improvement of the oleoresin aroma profile.
The techniques used in this study were sensitive enough to recognize compounds of both high and low concentration. It was important that separation be efficient enough so that the compounds of low concentration with low odour threshold values could be tentatively identified, such as gurjunene, calamene and thujone. Two-dimensional gas chromatography provided the separation required to identify the preliminary list of odorants.
The compounds tentatively identified by GC-MS generally have aromas that are aligned with the fresh, cooling aroma of the rosemary samples. The few exceptions such as trans-ocimene, caryophyllene and citronellol are compounds that could possibly be involved in synergism or masking. Trapping of peaks using the GC-FID fraction collection setup would be useful for uncovering the effects that are difficult to predict and identify in such complex mixtures.
References
1 I. Takaki, L.E. Bersani-Amado, A. Vendruscolo and S.M. Sartoretto, J. Med. Food, 2008, 11 (4), 741-746. [ Links ]
2 B. Adorjan and G. Buchbauer, Flavour Fragr. J., 2010, 25(6), 407-426. [ Links ]
3 S. Ouahada and B. Benveniste, Perfumer Flavorist, 2008, 25, 24-25. [ Links ]
4 A. Kamath, M.R. Asha, S. Narasimhan and D. Rajalakshmi, Flav. Fragr. J., 2001, 16, 401-407. [ Links ]
5 M.D. Guillén, N. Cabo and J. Burillo, J. Sci. Food Agric., 1996, 70 (3), 359-363. [ Links ]
6 M.H. Boelens, Perfumer Flavorist, 2000, 25, 10-23. [ Links ]
7 M.C. Diaz-Moroto, E.S. Palomo, L. Castro and M.A. Gonzalez-Vinas, J. Sci. Food Agric., 2004, 84, 2070-2076. [ Links ]
8 M.C. Diaz-Moroto, M.S. Perez-Coello and E. Sanchez-Palomo, J. Sens. Stud. 2007, 22, 3448. [ Links ]
9 S.M. van Ruth, Biomol. Eng., 2001, 17, 121-128. [ Links ]
10 P. Werkhoff, S. Brennecke, W. Bretschneider and H. Bertram, Modern methods for isolating and quantifying volatile flavor and fragrance compounds, in Flavour, Fragrance and Odor Analysis, (R. Marsili, ed.), Marcel Dekker Inc., New York, 2002, pp. 140-141. [ Links ]
11 J.F. Holland and B.D. Gardner, The advantages of GC-TOFMS for flavour and fragrance analysis, in Flavour, Fragrance and Odor Analysis, (R. Marsili, ed.), Marcel Dekker Inc., New York, 2002, pp. 107-108. [ Links ]
12 E.K. Ortner and E.R. Rohwer, J. High Res. Chromatog., 1996, 19, 339-344. [ Links ]
13 D. Sivakumar, Y. Naudé, E. Rohwer and L. Korsten, J. Sci. Food Agric., 2008, 88, 1074-1081. [ Links ]
14 Y. Naudé, M. van Aardt and E.R. Rohwer, J. Chromatog. A, 2009, 1216 (14), 2798-2804. [ Links ]
15 M.H. Boelens, Perfumer Flavorist, 1985, 10, 21- 32. [ Links ]
Received 3 August 2012
Revised 29 October 2012
Accepted 28 November 2012
* Author for correspondence. E-mail: patricia.forbes@up.ac.za





![Synthesis and characterization of new bis-symmetrical adipoyl, terepthaloyl, chiral diimido-di-L-alanine diesters and chiral phthaloyl-L-alanine ester of tripropoxy p-tert-butyl calix[4]arene and study of their hosting ability for alanine and Na+](/img/en/next.gif)








