Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Animal Science
versão On-line ISSN 2221-4062
versão impressa ISSN 0375-1589
S. Afr. j. anim. sci. vol.53 no.5 Pretoria 2023
http://dx.doi.org/10.4314/sajas.v53i5.14
SHORT COMMUNICATION
Preservation of mannanase Eupenicillium javanicum (BS4) in solid state fermentation using glycerol and polyol
T. HaryatiI, #; T. PurwadariaII; F. PaulinII; A. P. SinuratI; F. SaputraI
IResearch Centre for Animal Husbandry, Research Organization for Agriculture and Food, National Research and Innovation Agency, Bogor 16915, Indonesia
IIFaculty of Biotechnology, Atma Jaya Catholic University of Indonesia, South Jakarta 12930, Indonesia
ABSTRACT
The digestibility of palm kernel meal can be increased using mannanase. Enzyme application in the field requires a preservation process. The aim of this study was to evaluate the stability of liquid Eupenicillium javanicum BS4 mannanase resulting from solid state fermentation with the addition of a preservative (glycerol, sorbitol, or mannitol) at storage temperatures of 4 °C and at room temperature. Enzymes were produced using solid state fermentation with coconut cake as substrate and E. javanicum BS4 inoculum. The preservation evaluated the stability of the enzyme in three conditions: in submerged culture and solid state fermentation, filtration and non-filtration treatments, and different concentrations of the preservative. Each treatment was added with each preservation agent and included a negative control. Enzyme activity (U/mL) and saccharification activity (U/mL) were observed during incubation. The half-life of the enzyme activity was determined by the log curve of the enzyme activity. The half-life of the enzyme under solid state fermentation was longer than enzyme-submerged culture. Filtration treatment produced more stable results. The addition of mannitol and sorbitol (polyol) at a concentration of 20% gave similar results during incubation. Increasing the concentration of mannitol was not possible at it caused crystallization. Although the enzyme activity and saccharification fluctuated over the incubation period, 30% sorbitol was the best preservative. The results of saccharification can be used as a parameter in the field for determining enzyme addition to feed containing high palm kernel meal.
Keywords: glycerol, mannanase, polyol, preservation, solid state fermentation
Introduction
Feed is the largest expenditure component in the livestock industry. Imported feed, corn, and soybean meal still dominate the use of poultry feed in Indonesia. The use of imported feed makes livestock production costs very high. The use of alternative feed can reduce production costs. Alshelmani et al. (2021) reported that the use of fermented palm kernel cake (PKC) for chickens could improve body weight, growth, and feed conversion compared to non-fermented PKC. Therefore, palm oil cake can be considered as an alternative feed ingredient. However, the use of PKC-based feed is inefficient for chicken feed because it contains 38.4% mannan, which cannot be digested by chickens (Adrizal et al., 2011).
To increase the digestibility of mannan, mannanase can be used to hydrolyse mannan. Mannan is a polysaccharide hydrolysed by endo ß-mannanase (1,4-ß-D-mannan mananohydrolase [EC 3.2.1.78]) and exo-ß-mannosidase (ß-D-mananopiranoside hydrolase [EC 3.2.1.25]) to mannose. Purwadaria et al. (2003) produced mannanase using Eupenicillium javanicum BS4 submerged in minimal media containing coconut meal as an inducer in Erlenmeyer flasks. Enzyme storage can result in a decrease in enzyme activity. Prevention of decreased enzyme activity can be achieved using preservation methods. Enzyme preservation can be done using two methods, solid or liquid preservation (Purwadaria et al., 2003; Rodríguez-Fernández et al., 2013). Rodríguez-Fernández et al. (2013) used variations in glycerol with storage time to preserve liquid phytase resulting from solid state fermentation. The results of the study indicated that the storage temperature and concentration of the preservative affected the stability of the enzyme. Glycerol and polyols (sorbitol and mannitol) are compounds that can inhibit the rate of enzyme reactions. In the current study, preservation agents were used (glycerol, mannitol, and sorbitol) to maintain enzyme activity when stored at room temperature and at 4 °C. Liquid enzymes were used as research objects because they are easier to apply directly to feed. This study aimed to evaluate the stability of liquid E. javanicum BS4 mannanase resulting from solid state fermentation with the addition of preservative agents (glycerol, sorbitol, and mannitol) at storage temperatures of 4 °C and room temperature. The results of saccharification can be used as a parameter in the field for determining enzyme addition to feed containing high palm kernel meal.
Material and Methods
This study consisted of two main stages, namely enzyme production and enzyme preservation, which consisted of three experiments with three repetitions. The first experiment compared enzymes from submerged culture and from solid state fermentation using a 20% concentration of the preservative agent. The next experiment used enzymes from solid state fermentation with filtration and non-filtration treatments. The final stage carried out preservation by comparing two concentrations of preservative, namely 20% and 30%. During storage in the first and second experiments, mannanase activity and enzyme half-life tests were carried out; in the third experiment, complete tests were carried out for the best results, namely mannanase activity (U/mL), saccharification activity (U/mL), and enzyme half-life tests.
Mannanase E. javanicum BS4 was produced using a Tray Bioreactor, which had a capacity of 100 kg and a substrate thickness of 1 cm. The production stages include inoculum preparation, Mandels mineral production, and raw material preparation (coconut cake). Enzyme production began with the preparation of BS4 inoculum on Potato Dextrose Agar (PDA) and Potato Dextrose Broth (PDB) media. The inoculum was incubated at 28 °C for 5 d on PDA medium and propagated on PDB media using a shaking incubator at 28 °C with agitation of 125 rpm for 5 d. Mandels mineral preparation was made at a dose of 1.5 D and sterilized at 121 °C for 15 min. The head cake to be used was mixed with distilled water and sterilized at 121 °C for 15 min. Coconut cake was mixed with inoculum and Mandels minerals with conditioning at a moisture content of 60%. Enzyme extraction was carried out by dissolving coconut cake from fermented solid substrates in 0.5 M Na-acetate buffer at a ratio of 1:10. Extraction results were precipitated with ammonium sulphate (technical) at 515.8 g/L (75% concentration) and stored at 4 °C overnight. The result of the enzyme concentration was centrifuged at 10,000 rpm for 10 min with refrigerated centrifugation. The supernatant was discarded, and the pellet was dissolved using 0.5 M Na-acetate buffer at as much as 5% of the extraction volume. Filtration treatment in the second experiment was achieved using a 0.45 mm Whatman membrane and a vacuum pump.
Submerged fermentation: The inoculum was prepared by the addition of sterile water into a freshly grown PDA slant. Submerged fermentation was carried out by inoculating 2.5 mL of spore suspension into 250 ml. Each Erlenmeyer flask was supplemented with 50 mL of 1 dose Mandels medium solution and 1.5 g (3%) of Copra meal as a substrate. The samples were incubated for 5 d at room temperature in a shaker incubator. At the end of fermentation, the cell mass was separated by centrifugation for 15 min at 3000 rpm and 4 °C.
Preservation of enzyme results from submerged culture fermentation and solid state fermentation: This stage used glycerol, technical sorbitol, and mannitol to carry out the preservation of liquid enzymes fermented using submerged culture and fermented solid substrates. The negative control was an enzyme without the addition of a preservative and the treatment used was the preservative (glycerol, sorbitol, or mannitol) at a concentration of 20%. Enzymes were stored at a 4 °C and room temperature.
Changes in enzyme activity were described using relative enzyme activity curves over storage time at each temperature.
Enzyme preservation of solid state fermentation results using filtration and non-filtration treatments: The next stage of the first experiment used two treatments, with and without filtration. Enzymes were stored at 4 °C and room temperature. Changes in enzyme activity were described by curves of the relative activity of enzymes with storage time at each temperature treatment.
Enzyme preservation at 20% and 30% glycerol and sorbitol concentrations: Enzymes from the filtration treatment were used to determine the relationship between the concentration of the preservative and the stability of the enzyme. The best concentration of preservatives from the results of the second experiment (glycerol and sorbitol) were 20% and 30%. Negative control results (without the addition of a preservative) were compared with the results of the addition of a preservative. All samples were incubated at 4 °C and room temperature.
Determination of mannanase activity: One enzyme unit (U) is the amount of enzyme that catalyses a reaction at 1 mole of product per minute. Enzyme activity was determined by determining the reducing sugar content of the reaction between the enzyme and the substrate (0.5% locus bean gum). The sample was diluted with Na-acetate buffer at pH 5.8 to obtain a good absorbance value. Reducing sugar levels was determined using a DNS reagent. The activity was determined by reacting 0.5 mL of the substrate with 0.5 mL of an enzyme that had been heated for 30 min at 40 °C in a water bath. Controls were used to determine the content of reducing sugars in enzymes and substrates by comparing enzymes, DNS, and substrates without incubation. The results of the reaction were measured using a spectrophotometer at a wavelength of 540 nm. The mannose concentration was calculated using the standard mannose curve with a concentration series of 100-800 μg/ml and the mannanase activity was calculated using the following equation:
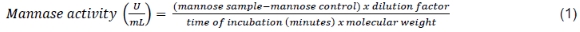
A saccharification assay was used to determine the saccharification ability of the enzymes analysed in palm kernel meal substrates. This assay was carried out using 2% PKC as a substrate in Na-acetate buffer (0.1 M, pH 5.8). Two ml of the substrate was mixed with 2 ml of an appropriately diluted enzyme. The sample should be incubated in the shaker at 120 rpm at 40 °C for 2 h, while the controls were heated at 100 °C for 5 min to stop the enzyme reaction, without incubation time. After stopping the sample's enzyme reaction by heating for 5 min at 100 °C, the filtrate was separated by centrifuging at 2000 rpm for 15 min. The mannose concentration was determined using the DNS method and a spectrophotometer at a wavelength of 540 nm. The results were converted using a standard glucose curve and saccharification activity was calculated using the Equation 2:
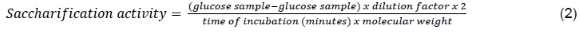
Mannanase E. javanicum BS4 half-life was calculated using the log curve of enzyme activity and incubation time (y = ax+b), followed by determination using Equation 3.

where t(50) is the temperature at which the enzyme activity drops to 50% and a is the slope of the log curve of enzyme activity
Results and Discussion
The relative activity of the enzymes after an incubation period of 7-9 d showed that the enzyme preservation in submerged cultures had decreased in activity by 75% (Fig. 1a, b), whereas the results using solid state fermentation (Fig. 1c, d) were good at room temperature and 4 °C. Enzyme half-life (Table 1) was determined using Equation 3. Calculation of the half-life proved that there was a decrease in enzyme activity resulting from submerged culture fermentation after 3-8 d. Enzymes produced by solid state fermentation for the control treatment and the addition of glycerol could not be determined due to increased activity.
The results of the mannitol and sorbitol treatment decreased activity by more than 50% after incubation for 5 d. Enzymes resulting from solid state fermentation were used as test materials in the second experiment. The filtration treatment yielded more stable results with the addition of sorbitol and mannitol (Fig. 2) compared to the non-filtered results. However, in both treatments, it was not possible to determine the half-life of the enzyme because there was an increase in enzyme activity that could not be explained. The results of the addition of mannitol and sorbitol tended to have similar results. However, mannitol had low water solubility, so could not be stored at concentrations above 22%. Accordingly, glycerol and sorbitol were used for the last experiment.
The last experiment used filtered enzymes. Figure 3 illustrates the results in the relative activity of enzymes based on storage temperature and concentration of the preservative. The results demonstrated that the relative activity of enzymes decreased gradually with storage time. Figures 3a and 3b indicate that the enzyme activity after storage at room temperature did not differ between the 20% and 30% glycerol and sorbitol treatments, but in the glycerol treatment at 4 °C, 20% was more volatile than 30% (Fig. 3c). Fluctuating data at 20% and 30% sorbitol were evident, but 30% sorbitol produced a higher activity than 20% sorbitol (Fig. 3d).
The determination of the best preservative was carried out by calculating the half-life of the enzyme. Enzyme half-life was determined after enzyme preservation for 32 d. Table 2 shows that the preservative can extend half-life and storage at 4 °C better than storage at room temperature. However, at 4 °C, there was an increase in enzyme activity in the control and 20% glycerol so the half-life of the enzyme could not be calculated.
Table 3 illustrates the comparison between mannanase and saccharification activity on days 0, 6, and 32. Saccharification activity on day 0 was not calculated so changes in activity were obtained from activity data on days 6 and 32. Mannanase activity at these three points experienced a decrease in activity at 4 °C and room temperature. There was an increase in saccharification activity in all treatments incubated at 4 °C, whereas at room temperature, there was an increase in activity only with the use of 30% sorbitol. Mannanase activity in the control at the beginning of storage was higher than activity with the addition of a preservative. In the preservative treatments, a comparison of the preservative with the enzyme was carried out at a ratio of 30:70. In the control, the enzyme was 100%, whereas with the treatment, only 70% of the enzyme was used.
Preservation results over storage time showed fluctuating results so it was difficult to discern the best results. The research data was expected to provide results in the form of a linear curve or a decrease in activity over storage time. Based on the results of the study, it was found that enzymes produced from solid state fermentation were more stable than enzymes from fermented submerged cultures over the same time and incubation conditions. Enzymes resulting from solid state fermentation had agents that could protect enzymes, such as proteins and minerals (Verduzco-Oliva & Gutierrez-Uribe, 2020). These proteins and minerals were extracted from the rest of the fermentation substrate. The same molecules may be formed during submerged culture fermentation, but limited substrate levels will reduce the level of these compounds. Based on these results, enzymes resulting from solid state fermentation were used for further experiments using filtration treatments. The results of the filtration treatment were better than those of the non-filtration treatment because the filtration process meant that some microorganisms were unable to pass through the filter membrane so that the results used were more sterile and produced more stable results (Ghunim et al., 2018). There is also the possibility that the filtration process reduced the proteases formed at the end of the fermentation and could decompose the active site of the mannanase.
Sorbitol and mannitol produced similar results; mannitol and sorbitol are isomers. Natural mannitol has low solubility in water. Mannitol can crystallize at concentrations above 22%. Therefore, in the final experiment, glycerol and sorbitol were used. Another factor to consider in the use of sorbitol is that it has better stability in the liquid phase, and is cheaper (Mohammadi et al., 2021). Concentrations of the preservatives were compared at 4 °C. At room temperature, enzymes exhibited similar activities. The control had a shorter half-life than with the addition of preservatives. Sorbitol at a concentration of 30% produced the best enzyme activity. Using a concentration of 20% of sorbitol or glycerol produced the same half-life at room temperature. Sorbitol is the best polyhydric alcohol for maintaining enzyme stability. Although glycerol has the same properties as a preservative, sorbitol has a faster binding reaction and can therefore inhibit hydrolysis (Zappaterra et al., 2021). Sorbitol and glycerol regulate osmotic pressure in enzymes and have hydrophilic properties; they can bind to water and reduce the water content around enzymes so as to extend storage time (Brochier et al., 2014).
The outcomes of saccharification over incubation time tended to be the same at room temperature and with the addition of glycerol, but not at 4 °C when the activity of saccharification increased. This allowed the preservation agent to be completely blended and counted during the saccharification activity study. A comparison of mannanase and saccharification activities revealed that when the active site was destroyed, enzyme activity decreased over the incubation period. Saccharification activity using a palm kernel cake substrate, which is more complex than locus bean gum, resulted in more stable outcomes. The results of saccharification can be used as a parameter in the field for determining the volume of enzyme addition to feed containing high palm kernel meal contents.
Acknowledgments
The authors thank the Indonesian Research Institute for Animal Production for the funding.
Authors' contributions
TH, TP, FP, AP, and FS contributed equally to this work.
Conflict of interest declaration
The authors have no conflicts of interest to declare.
References
Adrizal. A., Yusrizal, Y., Fakhri, S., Haris, W., Alim E. & Angel, C.R., 2011. Feeding native hens diets containing palm kernel meal with or without enzyme supplementation: 1. Feed conversion ratio and egg production. J. Appl. Poult. Res. 20(1):40-49. https://doi.org/10.3382/japr.2010-00196 [ Links ]
Alshelmani, M.I., Kaka, U., Abdalla, E.A., Humam, A.M. & Zamani, H.U., 2021. Effect of feeding fermented and non-fermented palm kernel cake on the performance of broiler chickens: A review. Worlds Poult. Sci. J. 77(2): 377-388. https://doi.org/10.1080/00439339.2021.1910472 [ Links ]
Brochier, B., Marczak, L.D., Noreña, C.P., 2014. Use of different kinds of solutes alternative to sucrose in osmotic dehydration of Yacon. Braz. Arch. Biol. Technol. 58(1): 34-40. https://doi.org/10.1590/s1516-8913201400035 [ Links ]
Ghuneim, L.A.J., Jones, D.L., Golyshin, P.N., Golyshina, O.V., 2018. Nano-sized and filterable bacteria and archaea: Biodiversity and function. Front. Microbiol. 9: 1971. https://doi.org/10.3389/fmicb.2018.01971 [ Links ]
Mohammadi, M., Shareghi, B., Farhadian, S., Saboury, A.A., 2021. The effect of sorbitol on the structure and activity of carboxypeptidase A: Insights from a spectroscopic and computational approach. J. Mol. Liq. 330: 115710. https://doi.org/10.1016/j.molliq.2021.115710 [ Links ]
Purwadaria, T., Haryati, T., Frederick, E. Tangendjaja, B., 2003. Optimization of ß-mannanase production on submerged culture of Eupenicillium javanicum as well as pH and temperature enzyme characterizations. JITV 8:46-54. [ Links ]
Rodríguez-Fernández, D.E., Parada, J.L., Medeiros, A.B.P., de Carvalho, J.C., Lacerda, L.G., Rodríguez-Leon, J.A, Soccol, C.R., 2013. Concentration by ultrafiltration and stabilization of phytase produced by solid-state fermentation. Process Biochem. 48(2): 374-379. https://doi.org/10.1016/j.procbio.2012.12.021 [ Links ]
Verduzco-Oliva, R., Gutierrez-Uribe, J.A., 2020. Beyond enzyme production: Solid state fermentation (SSF) as an alternative approach to produce antioxidant polysaccharides. Sustainability 12(2): 495. https://doi.org/10.3390/su12020495 [ Links ]
Zappaterra, F., Rodriguez, M.E., Summa, D., Semeraro, B., Costa, S., Tamburini, E., 2021. Biocatalytic approach for direct esterification of ibuprofen with sorbitol in biphasic media. Int. J. Mol. Sci. 22(6): 3066. https://doi.org/10.3390/ijms22063066 [ Links ]
Submitted 25 March 2023
Accepted 12 September 2023
Published 19 November 2023
# Corresponding author: tharyati2017@gmail.com














