Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Animal Science
versão On-line ISSN 2221-4062
versão impressa ISSN 0375-1589
S. Afr. j. anim. sci. vol.53 no.4 Pretoria 2023
http://dx.doi.org/10.4314/sajas.v53i4.07
The impact of multi-species bacteria as a probiotic on productive performance and egg quality of laying Japanese quail
Okasha M. HamadaI, #; Gafaar M. El-GendiI; K M. EidII; Waleed AbdelmoezIII
IAnimal Production Department, Faculty of Agriculture, Benha University, Moshtohor 13736, Egypt
IIAnimal Production Research Institute, Agriculture Research Centre, Giza,12618 Egypt
IIIAnimal Production Department, Faculty of Agriculture, Al-Azhar University, Nasr City 11751 Egypt
ABSTRACT
This study investigated the effect of individual and/ or a mixture multi-species bacteria as a probiotic (Pediococcus acidilactici (T1), Enterococcus faecium (m74) + Pediococcus acidilactici (T2), and Bacillus licheniformis + B. subtilis (T3)) supplementation at different levels on productive performance and egg quality characteristics in laying Japanese quail. At 42 d, 350 chicks were chosen with nearly similar live body weight and divided into ten groups of 35 chicks/group. Chicks were fed the basal diet as a control group (L0) or basal diet supplemented with three levels L1 (1.25), L2 (1.75), and L3 (2.25) g/kg diet, respectively. The bacteria-supplemented diets (T1 and T2) had an average feed intake that was the lowest and similar to control group. Better feed conversion was obtained in T1 birds in the first and third month and in the second month in T2. Age at sexual maturity was gradually decreased by dietary supplementation of T3 and Li. Probiotics increased egg production (EP) and egg mass (EM). EP, egg weight (EW), and EM increased gradually due to T3 and L2 supplementation. Bacteria-supplemented diets markedly increased absolute albumen and yolk weight as well as absolute and relative eggshell weight. In conclusion, the mixture of E. faecium (m74) + P. acidilactici and B. licheniformis + B. subtilis improved feed conversion ratio (FCR), egg production, and egg quality traits of laying Japanese quail.
Keywords: Bacillus licheniformis, Bacillus subtilis, Enterococcus faecium (m74), egg quality, performance, quail
Introduction
Due to the ban on in-feed antibiotics in the chicken sector, safe replacement-feed components are required. The use of antibiotics has made a substantial contribution to the development of animal husbandry because of the extraordinary improvement in animal production performance and animal health. However, the drawbacks of antibiotic feed additions have gradually been acknowledged, despite the considerable economic gains they provide (Abdel-Moneim et al., 2020). It is vital to create a non-toxic, non-residual antibiotic replacement in feed additives to mitigate the hazards of antibiotic abuse in human health, while also maintaining efficiency in animal production (Yu et al., 2020). In this context, feed additives that stimulate growth are continually being produced, with probiotics being one of the most effective supplements for poultry diets that have the capacity to enhance the immunological response, gastrointestinal health, conformation, and microbiota of poultry (Abd El-Moneim& Sabic 2019; Ebeid et al., 2019; Fathi 2018). Probiotics (Rezaeipour et al., 2015), prebiotics (Hutsko et al., 2016), antioxidant vitamins (Ghazi et al., 2015), minerals (Oliveira et al., 2014), and herbal essential oils (Peng et al., 2016) have been shown to have therapeutic effects. Probiotics are live organisms or microbial feed supplements aimed at improving intestinal microbial balance (Salehimanesh et al., 2016). Many studies have looked into the use of Pediococcus acidilactici, Enterococcus faecium (m74), Bacillus licheniformis, and Bacillus subtilis individually or as a mixture of two types in poultry production.
Utilizing a probiotic blend of types of bacteria may have beneficial, cumulative impacts on the growth, health, and welfare of birds. For example, it can improve poultry performance and growth promoters (Yang et al., 2012; Ribeiro Jr et al., 2014; Fathi 2018); product quality (Lei et al., 2013; Youssef et al., 2013; Zhou et al., 2015; Abou-Kassem et al., 2021); nutrient digestibility (Li et al., 2014; Hossain et al., 2015); intestinal morphology, microbial aspects, and blood metabolites (Hazrati et al., 2020; Nour et al., 2021); and immune function (Liao et al., 2015). Furthermore, they have the potential to benefit the environment or minimize pollution (Zhang & Kim, 2013; 2014). Using a probiotic combination with multiple strains may have a synergistic effect on bird production. Jazi et al. (2018) discovered synergistic and complimentary impacts; the gains experienced were probably the result of the many beneficial pathways each supplement possesses. Probiotics have garnered a lot of attention as feed supplements for poultry diets, but little is known about their positive, synergistic, and cumulative effects in the diet of laying Japanese quail, particularly in egg production and incubation attributes. The present work aimed to evaluate the effect of dietary supplementation with different types of bacteria (P. acidilactici, E. faecium (m74) + P. acidilactici, and B. licheniformis + B. subtilis) on egg production performance, egg quality traits, and chemical composition of eggs in laying Japanese quail.
Materials and Methods
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Al-Azhar University. A total number of 600, one-day-old Japanese quail were randomly divided into three dietary groups with nearly similar live body weight (7.45 ± 0.0077g). Chicks were kept under similar, standard hygienic and environmental conditions. Brooding cages with gas heaters were used for brooding chicks. The female to male ratio was 2:1 during the laying period. Chicks were divided into three groups of 180 chicks per group. Birds of the first, second, and third groups were fed a basal diet supplemented with either P. acidilactici (T1), E. faecium (m74) + P. acidilactici (T2), or B. licheniformis + B. subtilis (T3) as feed additives, respectively. Chicks of each group were subdivided into three subgroups each of 60 chicks receiving 1.25, 1.75, and 2.25 g/ kg of either T1, T2, or T3, respectively. The tenth group (60 chicks) was fed the basal diet only and was considered to be the control group. At 42 d, 350 chicks with nearly similar live body weight were randomly taken and divided into ten groups of 35 chicks/group. Birds were housed in cages constructed from 7-mm square welded wire mesh to provide secure footing, prevent leg injuries, and prevent chicks escaping through side walls.
Feed and clean fresh water were provided at all times and offered ad libitum. Birds were fed the basal diet, which was formulated according to the recommendations of the NRC (1994). The basic dietary composition is shown in Table 1.
Age at sexual maturity was determined as age in days when egg production for quail hens of each experimental group reached 10%. Egg production (number and weight) was recorded daily for three months after sexual maturity, and hen-day egg production was calculated. The monthly egg production average for three months after sexual maturity in each experimental group was calculated. Egg mass was calculated as the product of average egg weight and number of eggs laid.
Eggs were broken and yolk was separated from the albumen. The percentage of yolk, albumen, and eggshell (with its membranes) was expressed relative to EW. Eggshell thickness was measured from the sharp, blunt, and equatorial parts without a membrane with a 0.01-mm sensitive, electronic, digital micrometre. Eggshell percentage (ESP) was calculated using the equation of Aygun &Yetisir (2010):
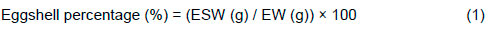
Only sound, clean eggs from each treatment were stored till incubation at 12.5 °C and 75-80% relative humidity for one week then incubated. Eggs were sprayed with Virkon disinfectant (15 g powder/litre of water) before being placed into the incubator. Eggs were allowed to warm overnight to room temperature prior to setting in order to reduce sweating of eggs. Incubation of eggs was carried out in a special automatic incubator (quail-egg-type incubator).
The temperature and relative humidity in the incubator were maintained at 37.5-37.7 °C and 60%, respectively, for the first 15 d. The eggs were automatically turned through 45° every hour. On day 16, the eggs were transferred to the hatchery trays, at temperature and relative humidity averages of 37.0 ± 0.5 °C and 75%, respectively (Petek et al., 2005). Hatching usually started on day 16 and was completed by the end of day 18. The quail chicks were removed and counted. The number of unhatched eggs was recorded for estimating the hatchability percentage. Hatchability percentage was calculated as the number of hatched chicks produced from fertile eggs.
Because the quail eggshell is coloured, tinted, and blotched, an accurate assessment of the development stage of the embryo by candling was not possible. Instead of candling, the unhatched eggs were broken for examination on day 18 and the number of eggs containing dead embryos and those free of embryos were counted and the percentage fertility was determined according to the method of Ozcelik et al. (2006). Fertility (%) was calculated as the number of hatched chicks plus number of eggs containing embryos from total incubated eggs set. Embryonic mortality was estimated for three intervals in the incubation period: from 1-6 d, from 7-14 d, and 15-17 d to determine the early, mid, and late embryonic mortality. This is known to depend on the cessation of embryonic development due to various factors that manifest in these periods.
The quail eggs were collected several times daily and stored at 15 °C for a period of not more than one week. Five eggs were taken from each experimental subgroup (total 50 eggs) to determine the following parameters: moisture content of egg yolks was determined using the AOAC 920.116 analytical method (AOAC, 2000). The moisture content of the samples was evaluated by drying them in a hot-air oven at 100 ± 5 °C until they reached a consistent weight. Fat content was determined using Soxhlet's method (AOAC, 2000), and egg protein content was estimated by applying Kjeldahl's method (William, 2000). Egg ash content was determined by using five grams of sample placed in a dry, clean, weighed crucible. The sample and crucible were placed in muffle furnace at 550-600 °C for 6-8 h with a gradual increase in temperature, then cooled in the desiccator and weighed. Ash content was calculated according to the following equation:
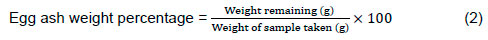
Egg pH value was determined according to the method of Pearson et al. (1976). The pH value was determined using an electrical pH meter.
Analysis of variance was carried out using the SAS procedure guide (SAS, 2004) for the traits of age of sexual maturity, feed consumption, feed conversion, fertility, hatchability, and chemical analysis of quail eggs according to the following linear model:

where: Xijk = the kth observation; j = overall mean; Ti = effect of the ith treatment; Lj = effect of the jth level applied; TLy = the interaction between the ith treatment and jth level applied; and eyk = the experimental error.
Responses in egg production, egg weight, egg mass, and egg quality were determined according to the following linear model:
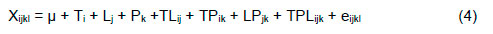
where: TPik = the interaction between the ith treatment and kth period; LPjk = the interaction between jth level applied and Kth period; TLPjk = the interaction between ith treatment and kth level applied as well as Kth period; and eijkl = the experimental error.
Results and Discussion
Average amount of feed consumed in gram per bird per day was estimated for three months (Table 2). Analysis of variance indicated an effect of treatments and levels of added probiotics, as well as an interaction between the kind and level of probiotic on the average feed consumption in all periods (P <0.001). The effect of the probiotic supplementation was statistically significant on feed conversion during the first month (P <0.01) and second month (P <0.05). The best feed conversion averages during the first month were obtained in I aying pullets fed a mixture of Entrococcus and Pediococcus. Average feed conversion was affected by the level of probiotic supplemented (P <0.001). The average feed conversion generally improved as the level of probiotic supplementation increased but without any characteristic trend. Supplementation with 1.75 and 2.25 kg probiotic per kg diet produced the best feed conversion during the 2nd and 3rd months, respectively. An interaction effect was found during the first month (P <0.05) and during the second and third months (P <0.001).
These results are scientifically feasible because the feed requirement and demand are known to increase with age to fulfil the increase in all biological reactions within the bird. The results concur with Abou-Kassem et al. (2021), who found marked increases in daily FI in the grower phases (1-21 d and 22-42 d) because of probiotic treatment. Moreover, Manafi et al. (2018) found substantial changes in overall FI. Variation in average feed consumption due to treatments applied and the time of estimation may be attributed to variation in the metabolic pathways within various experimental groups. Nutritive requirements differ according to the mode and rate of metabolism. Generally, applying probiotics at a level not exceeding 1.75 g/kg resulted in increasing feed consumption, compared to other two levels applied (Table 2). Changes occurred in average feed conversion according to bird's age and/or dietary additives applied and may be attributed to the state of metabolic activity in general and the changes in the balance that may occur between anabolic and catabolic processes. Premavalli et al. (2018) clarified that the improvement in FCR of quails fed diets supplemented with multispecies probiotics could be attributed to the probiotic's overall effects, which include changes in bacterial metabolism in the intestine, the maintenance of a favourable microbial population, and good feed digestion and absorption.
Data of age at sexual maturity (ASM) for birds in the experimental groups are listed in Table 2. It was found that ASM was affected by the kind of probiotic added to the diet as well as the level of supplementation (P <0.05). Supplementing birds with probiotic preparations decreased ASM compared to birds fed the basal diet only (control group) and this decreased according to the kind of probiotic applied. Average ASM decreased with the 1.25, 1.75, and 2.25 g/kg treatments for birds fed the basal diet supplemented with T1, T2 and T3, respectively, when compared with control group.
Egg production (EP), egg weight (EW), egg mass (EM), and ANOVA results are detailed in Table 3. Treatments affected average egg production and egg mass (P <0.001). Supplement level influenced average egg weight and egg mass (P < 0.001). Feeding 1.75 g probiotic/kg diet produced the highest average egg weight and egg mass; 2.25 g probiotic/kg diet produced the highest average egg production. Experimental period influenced the average egg weight, egg production, and egg mass (P <0.001). Egg production and egg mass increased in older birds, with a maximum in the 2nd month of production, which decreased towards the end of the experiment. However, egg weight increased with age to reach a maximum at the end of the experiment (Table 3). All interaction effects on average egg production and egg mass were found to be significant (P <0.001).
Decreasing age at sexual maturity (ASM) may be attributed to the enhancement of growth rate and, concomitantly, the reproductive system, caused probiotic supplementation at an early age (a period of higher growth rate). Increasing levels of probiotics slightly increased ASM. As the level increased to 1.75 g/kg, average ASM increased from 51.67 d to 52.33 d. However average ASM was constant up to 2.25 g/kg of probiotics. Probiotics should therefore be applied at no more than 1.75 g/kg. This concurs with Ayasan et al. (2006), who found that ASM increased in birds fed supplementary probiotics (Protexin) at 0.5 and 1 g/kg diet, producing an ASM of 51-53 d and 51-57 d, respectively, compared to the control (47-57 d). Various external and internal variables influence early sexual maturity. Lukanov & Pavlova (2020) confirmed that domestic Japanese quail reached sexual maturity at 4-5 w of age, and females begin laying eggs regularly at ~6 w of age when kept in ideal conditions and given a proper lighting schedule. The results o f t h e c u r r e n t s t u d y may support the concept that ASM is a trait of high heritability and trying to improve it by improving environmental conditions (including nutrition) has limited success (El-Deen et al., 2008; Kaye et al., 2016).
The interaction between kind and level of probiotics was of statistically significant (P <0.01). This indicates the importance of level for the kinds of probiotics applied. Probiotic application efficiency may be influenced by parameters such as microbial species composition (single or multi-strain), liveability, supplementary administration dose, application method and frequency, and food composition (Mikulski et al., 2012). Zamanizadeh et al. (2021) reported variation in egg production of Japanese quail due to dietary supplementation of two probiotics, Aspergillus oryzae and Saccharomyces cerevisiae (P<0.001). The highest egg productions were recorded in birds receiving S. cerevisiae at 200 mg/kg diet and/or a mixture of two probiotics at 100 and 200 mg/kg diet. Zamanizadeh et al. (2021) suggests that the improvement in the metabolic processes of nutrient absorption and utilization may be linked to increased egg production and egg weight as a result of the probiotics. Probiotics have been shown to change gut flora by lowering pH, boosting intestinal enzyme activity, and increasing food digestibility (Yang et al., 2005). Shell formation and deposition are controlled hormonally and depend on the functional efficiency of the biological action of thyroid hormones and parathyroid hormones (calcitonin and parathormone), as well as the activity of the kidney in the conversion of vitamin D3 to its metabolic form (1,25(OH)2-D3). All of these regulators depend on genetic capacity rather than the nutritional regime (Molino et al., 2015).
The effect of treatments on average egg mass can therefore be attributed to their effect on egg number rather than on average egg weight. The highest average egg production and egg mass were found in groups of birds receiving T1 and T3, rather than T2. Absolute and proportional weights of egg components and shell thickness (EST) were estimated for three months after ASM to determine the effect of treatments applied and/or level of probiotics supplemented in laying quail (Table 4). T3 increased the absolute and relative weights of albumen and EST when compared to the other treatments. The concentration of probiotics added to the diet affected the absolute and relative weights of the eggshell and absolute albumen and yolk (P <0.001). Feeding quail a diet supplemented with 1.75 g probiotic/kg ration had the highest average absolute weights of albumen and yolk and EST. In addition, variations were found in all estimated parameters due to bird age.
All averages increased up to the second month of the egg production period, with different magnitudes of increase, then tended to remain constant up to the third month (Figure 1). Different results were obtained in EST, which decreased over time, being higher until the second month. The findings concur with those of Zamanizadeh et al. (2021), who reported no changes between probiotic treatments in albumen %, all yolk parameters (yolk weight, yolk %, and yolk colour), or shell weight (P >0.05). Decreased EST with age may be due to the increase in egg weight with an increase in surface area of the egg.
Mikulski et al. (2020) noticed that in comparison to birds without probiotic supplementation, Hy-Line Brown hens fed probiotic-supplemented diets had thicker eggshells (P <0.002) and heavier eggshells (P <0.008). Without affecting yolk%, the increases in eggshell thickness and weight were accompanied by a drop in albumen percentage (P = 0.043).
Eggs laid by birds fed the basal diet alone or with probiotics at different levels were incubated when egg production reached more that 50% to elucidate the effect of probiotic treatments on hatching parameters (fertility-Fr, hatchability-H, and embryonic mortality; Table 5). There was no effect of the kind of probiotic or the level applied on either Fr (%), H (%) or embryonic mortality (early, mid, and late) during incubation. Regardless of the probiotic applied, feeding quail 1.75 and 1.25 g probiotic/kg diet produced higher fertility and hatchability. Feeding quail 1.25 g probiotic/kg ration produced the lowest mid- and late-embryonic mortality (Table 5). Dietary probiotics had no effect on hatchability, supporting the contention that nutritional factors have no effect on fertility, hatchability, or embryonic development. Many studies have found that the fertility and hatchability of viable Japanese quail eggs were 48-94% and 40.00-70.34%, respectively, from 8-52 w of age (Farooq et al., 2001; Khurshid et al., 2004; El-Hindawy et al., 2021). The results concur with Güçlü (2011), who reported that probiotics and prebiotics raised the percentage of fertilized eggs and hatchability, but not statistically. Hajiaghapour & Rezaeipour (2018) found that the dietary treatments including probiotics had no significant effect on hatchability percentage.
In contrast, El-Hindawy et al. (2021) observed that fertility and hatchability percentages (Fr% and H%) increased with increasing probiotics administration (P <0.01 and P <0.05, respectively). Mojgani et al. (2020) found that quail administered probiotics (108 CFU/ml B. megaterium) had an improvement in H% and a 10% reduction in embryonic mortality compared to the control (P <0.05).
Moisture, ash, protein, and fat percentage, as well as pH value in egg contents were affected by adding probiotics to layer diets at different concentrations (P <0.001). A higher moisture percentage was found in eggs laid by birds on T1 , compared to the control. Eggs laid by birds on T3 had the highest average protein percentage and lower average fat percentage. However, birds that received T2 or T3 laid eggs with a similar ash% (2.06 and 2.08%, respectively), which was higher that the control and T1 (1.58%). No variation was found in average pH value due to treatments applied and ranged from 7.33 in the control to 7.50 on T2. The content of the probiotics didn't affect the protein, fat, and ash percentages, but moisture percentage (P <0.05) and pH (P <0.001) were different.
Moisture percentage ranged from 73.38% in eggs laid by birds treated with 1.75 g probiotic/kg and 73.87% in eggs laid by birds fed 1.25 g/kg. The pH was 7.50 and 7.54 in birds fed diets supplemented with 1.25 g/kg diet and 1.75 g/kg, respectively (Table 6). The chemical composition of quail eggs is comparable to that of chicken eggs, with ~88% water, 10% protein, and 1% ash (Tolik et al., 2014). Genchev (2012) reported that quail eggs have a greater crude protein content (14.1-14.6%) in the albumen and a lower ash content (0.76-0.78%) in the albumen and yolk (1.1-1.3 percent) than was reported by Dudusola (2010). Sinanoglou et al. (2011) found that quail egg yolks had a lower fat and higher ash content (27.45% and 2.63%, respectively) than chicken egg yolks. However, results of hatchability must be re-evaluated in terms of the variability in chemical parameters of incubated eggs if increasing hatchability is being targeted.
Conclusion
It is recommended that adding Pediococcus acidilactici bacteria as a probiotic (T1) to layer quail diets at 1.25 mg/kg will improve productive performance and egg quality characteristics.
Acknowledgments
Faculty of Agriculture, Benha University, and Al-Azhar University, Egypt.
Author's contributions
OHM, WAM, and KME collected data. OHM, GME, and WAM conducted the statistical analysis. OHM, GME, KME, and WAM collaborated in interpreting the results, wrote the initial draft of this manuscript, and finalized the manuscript. OHM and GME developed the original hypothesis and designed the experiment. The authors have read and approved the manuscript.
Conflict of interest statement
There is absolutely no conflict of interest with any individual or organization regarding the writing, materials discussed in the manuscript or publishing.
References
Abd El-Moneim, A.E. & Sabic, E.M. 2019. Beneficial effect of feeding olive pulp and Aspergillus awamori on productive performance, egg quality, serum/yolk cholesterol and oxidative status in laying Japanese quails. J. Anim. Feed Sci. 28(1):52-61. [ Links ]
Abdel-Moneim, A.E., Selim D.A., Basuony, H.A., Sabic, E.M., Saleh, A.A., Ebeid, T.A. 2020. Effect of dietary supplementation of Bacillus subtilis spores on growth performance, oxidative status, and digestive enzyme activities in Japanese quail birds. Trop. Anim. Health Prod. 52(2):671-680. [ Links ]
Abou-Kassem, D.E., Elsadek, M.F.M Abdel-Moneim, A.E., Mahgoub, S.A., Elaraby, G.M., Taha, A.E., Elshafie, M.M., Alkhawtani, D.M., Abd El-Hack, M.E., Ashour, E.A. 2021. Growth, carcass characteristics, meat quality, and microbial aspects of growing quail fed diets enriched with two different types of probiotics (Bacillus toyonensis and Bifidobacterium bifidum). Poult. Sci. 100(1):84-93. [ Links ]
AOAC. 2000. Association of Official Analytical Chemists. Official Methods of Analysis. AOAC Arlington, VA. [ Links ]
Ayasan, T., Ozcan, B.D., Baylan, M., Canogullari, S. 2006. The effects of dietary inclusion of probiotic protexin on egg yield parameters of Japanese quails (Coturnix coturnix Japonica). Int. J. Poult. Sci. 5(8):776-779. [ Links ]
Aygun, A., Yetisir, R. 2010. The relationships among egg quality characteristic of different hybrid layers to forced molting programs with and without feed withdrawal. J. Anim. Vet. Adv. 9(4):710-715. [ Links ]
Dudusola, I.O. 2010. Comparative evaluation of internal and external qualities of eggs from quail and guinea fowl. Int. Res. J. Plant Sci. 1(5):112-115. [ Links ]
Ebeid, T.A., Fathi, M.M., Al-Homidan, I., Ibrahim, Z.H., Al-Sagan, A.A. 2019. Effect of dietary probiotics and stocking density on carcass traits, meat quality, microbial populations and ileal histomorphology in broilers under hot climate conditions. Anim. Prod. Sci. 59(9):1711-1719. [ Links ]
El-Deen, M., Bahie, W.S., El-Tahawy, Y.A., Meky, M.A. 2008. Inheritance of age at sexual maturity and its relationships with some production traits of Japanese quail. Egyptian Poult. Sci. 28:1217-1232. [ Links ]
El-Hindawy, M.M., Ashour, E.A., Abd El-Hack, M.E., Mahgoub, S., Aboelenin, S.M., Soliman, M.M., El-Tarabily, K.A., Abdel-Moneim, A.E. 2021. Productive performance, fertility and hatchability, blood indices and gut microbial load in laying quails as affected by two types of probiotic bacteria. Saudi J. Biol. Sci. 28(11):6544-6555. [ Links ]
Farooq, M., Aneela, K., Durrani, F.R., Muqarrab, A.K., Chand, N., Khurshid, A. 2001. Egg and shell weight, hatching and production performance of Japanese broiler quails. Sarhad J. Agric. (Pakistan). [ Links ]
Fathi, M.A. 2018. Effect of dietary supplementation of bacteria as growth promoters on performance of broiler chickens. Egyptian Poult. Sci. Journal 38(2):391-408. [ Links ]
Genchev, A. 2012. Quality and composition of Japanese quail eggs (Coturnix coturnix Japonica). Trakia Journal of Sciences 10(2):91-101. [ Links ]
Ghazi, S., Amjadian, T., Norouzi, S. 2015. Single and combined effects of vitamin C and oregano essential oil in diet, on growth performance, and blood parameters of broiler chicks reared under heat stress condition. Int. J. Biometeorol. 59(8):1019-1024. [ Links ]
Güçlü, B.K. 2011. Effects of probiotic and prebiotic (mannanoligosaccharide) supplementation on performance, egg quality and hatchability in quail breeders. Ankara Üniversitesi Veteriner Fakültesi Dergisi 58 (1):27-32. [ Links ]
Hajiaghapour, M., Rezaeipour, V. 2018. Comparison of two herbal essential oils, probiotic, and mannan- oligosaccharides on egg production, hatchability, serum metabolites, intestinal morphology, and microbiota activity of quail breeders. Livest. Sci. 210:93-98. [ Links ]
Hazrati, S., Rezaeipour, V., Asadzadeh, S. 2020. Effects of phytogenic feed additives, probiotic and mannan-oligosaccharides on performance, blood metabolites, meat quality, intestinal morphology, and microbial population of Japanese quail. Br. Poult. Sci. 61(2):132-139. [ Links ]
Hossain, M.M., Begum, M., Kim, I.H. 2015. Effect of Bacillus subtilis, Clostridium butyricum and Lactobacillus acidophilus endospores on growth performance, nutrient digestibility, meat quality, relative organ weight, microbial shedding, and excreta noxious gas emission in broilers. Vet Med (Praha) 60(2):77-86. [ Links ]
Hutsko, S.L., Meizlisch, K., Wick, M., Lilburn, M.S. 2016. Early intestinal development and mucin transcription in the young poult with probiotic and mannan oligosaccharide prebiotic supplementation. Poult. Sci. 95(5):1173-1178. [ Links ]
Jazi, V., Foroozandeh, A.D., Toghyani, M., Dastar, B., Koochaksaraie, R.R. 2018. Effects of Pediococcus acidilactici, mannan-oligosaccharide, butyric acid and their combination on growth performance and intestinal health in young broiler chickens challenged with Salmonella typhimurium. Poult. Sci. 97(6):2034-2043. [ Links ]
Kaye, J., Akpa, G.N., Alphonsus, C., Kabir, M., Zahraddeen, D., Shehu, D.M. 2016. Response to genetic improvement and heritability of egg production and egg quality traits in Japanese quail (Coturnix coturnix japonica). ASRJETS 16(1):277-292. [ Links ]
Khurshid, A., Farooq, M., Durrani, F.R., Sarbiland, K., Manzoor, A. 2004. Hatching performance of Japanese quail. Livest. Res. Rural. Dev. 16(1):2. [ Links ]
Lei, K., Li, Y.L., Yu, D.Y., Rajput, I.R., Li, W.F. 2013. Influence of dietary inclusion of Bacillus licheniformis on laying performance, egg quality, antioxidant enzyme activities, and intestinal barrier function of laying hens. Poult. Sci. 92(9):2389-2395. [ Links ]
Li, Y-B., Xu, Q-Q., Yang, C-J., Yang, X., Lv, L.E., Yin, C-H., Liu, X-L., Yan, H. 2014. Effects of probiotics on the growth performance and intestinal microflora of broiler chickens. Pak. J. Pharm. Sci. 27. [ Links ]
Liao, X.D., Ma, G., Cai, J., Fu, Y., Yan, X.Y., Wei, X.B., Zhang, R.J. 2015. Effects of Clostridium butyricum on growth performance, antioxidation, and immune function of broilers. Poult. Sci. 94(4):662-667. [ Links ]
Lukanov, H., Pavlova, I. 2020. Domestication changes in Japanese quail (Coturnix coturnix japonica): A review. World's Poult. Sci. J. 76(4):787-801. [ Links ]
Manafi, M., Hedayati, M., Mirzaie, S. 2018. Probiotic Bacillus species and Saccharomyces boulardii improve performance, gut histology, and immunity in broiler chickens. S. Afr. J. Anim. Sci. 48(2):379-389. [ Links ]
Mikulski, D., Jankowski, J., Naczmanski, J., Mikulska, M., Demey, V. 2012. Effects of dietary probiotic (Pediococcus acidilactici) supplementation on performance, nutrient digestibility, egg traits, egg yolk cholesterol, and fatty acid profile in laying hens. Poult. Sci. 91(10):2691-2700. [ Links ]
Mikulski, D., Jankowski, J., Mikulska, M., Demey, V. 2020. Effects of dietary probiotic (Pediococcus acidilactici) supplementation on productive performance, egg quality, and body composition in laying hens fed diets varying in energy density. Poult. Sci. 99(4):2275-2285. [ Links ]
Mojgani, N., Razmgah, N., Torshizi, M.A.K., Sanjabi, M.R. 2020. Effects of three Bacillus species on hatchability, growth performance and serum biochemistry in Japanese quails fed diet contaminated with Aflatoxin b1. Acta Sci. - Anim. Sci. 42. [ Links ]
Molino, A.B., Garcia, E.A., Santos, G.C., Vieira Filho, J.A., Baldo, G.A.A., Almeida Paz, I.C.L. 2015. Photostimulation of Japanese quail. Poult. Sci. 94(2):156-161. [ Links ]
Nour, M.A., El-Hindawy, M.A., Qattan, S.Y.A., Abou-Kassem, D.E., Ashour, E.A., Aboelenin, S.M., Soliman, M.M. & Abdel-Moneim, A-M.E. 2021. Effect of graded levels of dietary Bacillus toyonensis and Bifidobacterium bifidum supplementation on growth, carcass traits and ileal histomorphometry and microbiota of growing quails. Saudi J. Biol. Sci. 28(8):4532-4541. [ Links ]
Oliveira, T.F.B., Rivera, D.F.R., Mesquita, F.R., Braga, H., Ramos, E.M., Bertechini, A.G. 2014. Effect of different sources and levels of selenium on performance, meat quality, and tissue characteristics of broilers. J Appl Poult Res. 23(1):15-22. [ Links ]
Ozcelik, M., Cerit, H., Ekmen, F., Dogan, I. 2006. Effect of the hatching month as an environmental factor on the hatching features of bronze turkeys. Turkish J. Vet. Anim. Sci. 30(2). [ Links ]
Pearson, D., Harold, E., Ronald. S.K., Ronald, S. 1976. Chemical Analysis of Food. Churchill Livingstone. Edinburg. UK 575. [ Links ]
Peng, Q.Y., Li, J.D., Li, Z., Duan, Z.Y., Wu, Y.P. 2016. Effects of dietary supplementation with oregano essential oil on growth performance, carcass traits and jejunal morphology in broiler chickens. Anim. Feed Sci. Technol. 214:148-153. [ Links ]
Petek, M., Baçpinar, H., Mustafa Ogan, M., Balci, F. 2005. Effects of egg weight and length of storage period on hatchability and subsequent laying performance of quail. Turkish J. Vet. Anim. Sci. 29(2):537-542. [ Links ]
Premavalli, K., Sangilimadan, K., Omprakash, A. 2018. Effect of supplementation of multi-species probiotic on production performance of Japanese quail. Inter. J. Chem. Stud 6:2164-2166. [ Links ]
Rezaeipour, V., Valizadeh, A., Abdullahpour, R., Sadeghi, A.R. 2015. Effects of dietary threonine and a multi-strain probiotic (Primalac) supplementation on growth performance, blood metabolites and carcass characteristics in Japanese quails. Poult. Sci. J. 3(2):135-141. [ Links ]
Ribeiro Jr, V., Albino, L.F.T., Rostagno, H.S., Barreto, S.L.T., Hannas, M.I., Harrington, D., De Araujo, F.A., Ferreira Jr, J.C., Ferreira, M.A. 2014. Effects of the dietary supplementation of Bacillus subtilis levels on performance, egg quality and excreta moisture of layers. Anim. Feed Sci. Technol. 195:142-146. [ Links ]
Salehimanesh, A, Mohammadi, M. Roostaei-Ali Mehr, M. 2016. Effect of dietary probiotic, prebiotic and synbiotic supplementation on performance, immune responses, intestinal morphology, and bacterial populations in broilers. J. Anim. Physiol. Anim. Nutr. 100 (4):694-700. [ Links ]
Sinanoglou, V.J., Strati, I.F., Miniadis-Meimaroglou, S. 2011. Lipid, fatty acid, and carotenoid content of edible egg yolks from avian species: A comparative study. Food Chem. 124(3):971-977. [ Links ]
Tolik, D., Poawska, E., Charuta, A., Nowaczewski, S., Cooper, R. 2014. Characteristics of egg parts, chemical composition, and nutritive value of Japanese quail eggs-a review. Folia Biologica (Kraków) 62(4):287-292. [ Links ]
William, H. 2000. Official Methods of Analysis of AOAC International. AOAC Official Method 985.29. [ Links ]
Yang, C.M., Cao, G.T., Ferket, P.R., Liu, T.T., Zhou, L., Zhang, L., Xiao, Y.P., Chen, A.G. 2012. Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens. Poult. Sci. 91(9):2121-2129. [ Links ]
Yang, S-C., Chen, J-Y., Shang, H-F., Cheng, T-Y., Tsou, S-C., Chen, J-R. 2005. Effect of synbiotics on intestinal microflora and digestive enzyme activities in rats. World J. Gastroenterol. 11 (47):7413. [ Links ]
Youssef, A.W., Hassan, H.M.A., Ali, H.M., Mohamed, M.A. 2013. Effect of probiotics, prebiotics and organic acids on layer performance and egg quality. Asian J. Poult. Sci. 7(2):65-74. [ Links ]
Yu, W., Hao, X., Zhiyue, W., Haiming, Y., Lei, X. 2020. Evaluation of the effect of Bacillus subtilis and Pediococcus acidilactici mix on serum biochemistry, growth promotion of body and visceral organs in Lohmann Brown chicks. Braz. J. Poult. Sci. 22. [ Links ]
Zamanizadeh, A., Mirakzehi, M.T., Agah, M.J., Saleh, H., Baranzehi, T. 2021. A comparison of two probiotics Aspergillus oryzae and Saccharomyces cerevisiae on productive performance, egg quality, small intestinal morphology, and gene expression in laying Japanese quail. Italian J. Anim. Sci. 20(1):232-242. [ Links ]
Zhang, Z.F., Kim, I.H. 2013. Effects of probiotic supplementation in different energy and nutrient density diets on performance, egg quality, excreta microflora, excreta noxious gas emission, and serum cholesterol concentrations in laying hens. J. Anim. Sci. 91(10):4781-4787. [ Links ]
Zhang, Z.F., Kim, I.H. 2014. Effects of multistrain probiotics on growth performance, apparent ileal nutrient digestibility, blood characteristics, cecal microbial shedding, and excreta odor contents in broilers. Poult. Sci. 93(2):364-370. [ Links ]
Zhou, X., Jin, E., Li, S., Wang, C., Qiao, E., Wu, G. 2015. Effects of dietary supplementation of probiotics (Bacillus subtilis, Bacillus licheniformis, and Bacillus natto) on broiler muscle development and meat quality. Turkish J. Vet. Anim. Sci. 39(2):203-210. [ Links ]
Submitted 24 July 2022
Accepted 3 December 2022
Published 26 August 2023
# Corresponding author: hamada.okasha@fagr.bu.edu.eg














