Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Animal Science
versión On-line ISSN 2221-4062
versión impresa ISSN 0375-1589
S. Afr. j. anim. sci. vol.53 no.4 Pretoria 2023
http://dx.doi.org/10.4314/sajas.v53i4.03
Effect of a probiotic blend in broiler chicken diets and its effect on growth performance, carcass traits, and haematological profile
M. MousapoorI; C. LosaccoII; A. SeidaviI, #; M. NosratiI; W.A.G. de AraújoIII; L.F.T. AlbinoIV; V. LaudadioII; V. TufarelliII, #; D. De MarzoII
IDepartment of Animal Science, Rasht Branch, Islamic Azad University, Rasht 41857-43999, Iran
IISection of Veterinary Science and Animal Production, Department of Precision and Regenerative Medicine and Jonian Area, University of Bari 'Aldo Moro', 70010 Valenzano, Bari, Italy
IIIFederal Institute of Education, Science and Technology of Northern Minas Gerais, Januária-MG 39480-000, Brazil
IVDepartment of Animal Science, Federal University of Viçosa, Viçosa-MG 36570-000, Brazil
ABSTRACT
The study aimed to assess the effect of different dietary levels of a probiotic blend (Probio Enzyme®) during the first 14 days of age (DOA) or up to 42 DOA, on growth performance, carcass and digestive tract traits, and haematological profiles of broiler chicks. A total of 540, one-day-old broiler chicks were randomized assigned to nine treatments: four dietary probiotic blend levels (250, 500, 750, and 1,000 g/ton) within two feeding periods (0-14 and 0-42 d, respectively), whereas the ninth treatment was a control diet without any dietary probiotic supplementation. The feed intake (FI) was found to be higher in broilers fed 1,000 g/ton of probiotic blend when fed during the first 14 d; however, BW gain and feed efficiency were not influenced by the treatments. Carcasses (deplumed and full) from broilers fed 250 g/ton of probiotic blend (0-14 DOA) were heavier than the other groups; the same was observed for leg portions. Broiler duodenum, jejunum and ileum weights, and ileum percentage were greater when fed diets without any supplementation. The haematological profile of broilers was not affected by the dietary treatments.
Keywords: broiler, diet, feed additive, growth, probiotics
Introduction
Increases in the incidence of human infections from antibiotic-resistant bacteria have been hypothesized to be directly related to the overuse of antibiotics required for human medical prophylaxis and to therapeutics in food animal production (Hume, 2011; Chand et al., 2022). Considering the severe restriction or total ban on using antibiotics as growth promoters in poultry production, probiotics and enzymes have been suggested as an alternative (Cimrin et al., 2020; Khan et al., 2021). Khaksefidi and Ghoorchi (2006) reported some improved immunocompetence in broiler chicks against Newcastle disease virus when Bacillus subtillis was used as a probiotic additive in diet. Ashyerizadeh et al. (2009) concluded that a probiotic blend containing Lactobacillus acidophilus, L. casei, Enterococcus faecium, and Bifidobacterium bifidium inoculations could be used as a substitute for flavomycin growth promoters. In another study, probiotics displayed a growth-promoting effect comparable to the antibiotic, avilamycin (Mountzouris et al., 2007). However, some researchers have reported that probiotics are not capable of preventing pathogenic bacteria in intestinal microbial flora (Lin et al., 2009). Timmerman et al. (2006) point out that, according to the examination of 13 published studies, the high productivity rates of broiler chicks reduce the effect of probiotics. Exogenous enzymes may be added to broiler chick diets containing these by-products as an aid for fibre digestion or to solubilize phytic phosphorus (phytase), thereby reducing their negative effects on broiler chick production parameters (Choct, 2006). Some of these indigestible fibre compounds, especially the soluble fraction, are capable of improving the proliferation of undesirable microorganisms in the intestinal wall and lumen. Supplementation with enzymes markedly decrease the viscosity and increase the dry matter of digesta in the intestinal lumen (Malayoglu et al., 2010). These implications underline the idea that the addition of enzymes can help to reduce the multiplication of pathogenic microorganisms on diets without any antibiotics (Bhogoju & Nahashon, 2022).
Probiotic supplements for the development and stabilization of intestinal flora are used particularly before stressful changes such as new housing and environments, while enzymes are used to help with the breakdown of a wide variety of feed components that would normally be left unused and would maybe act as a substrate for the growth of bacterial pathogens (e.g., E. coíi and Salmonella spp.). The combination of both additives could bring a significant number of advantages to animal production and management, such as a more economic feed efficiency, a better availability of key nutrients to animals, less manure with a lower level of phosphorus and nitrogen, as well as cleaner animals.
Therefore, the aim of this study was to determine the effect of different dietary levels of a probiotic blend as a natural growth promoter, supplemented during different rearing periods, on growth performance, carcass traits, and haematological profiles of broiler chickens.
Materials and Methods
Experimental protocols were approved by the Animal Care Committee of the Islamic Azad University (process #17-33-5-9013; 93-12-7) and were performed in accordance with recommendations of the Iranian Council for Control of Animal Experimentation.
A total of 540 one-day-old Ross 308 (Aviagen, Newbridge, Scotland, UK) male broiler chickens were purchased from a commercial hatchery. Birds were placed in cages with dimensions of 1.0x1.0x0.6 m, providing a floor area of 0.1 m2 per bird, in a thermostatically-controlled, curtain-sidewall poultry barn. The cage floor was covered with paper roll litter. The feeding trial lasted up to 42 days of age. Each cage of 10 chicks (initial BW of 41.37 ± 2.1 g) was assigned to a specific dietary treatment. The ambient temperature inside the poultry barn was maintained with supplementary heat generated by thermostatically-controlled gasoline rocket heaters, and humidity was added to the barn atmosphere via a water spray to maintain a relative humidity of 55-65%. The ambient temperature was controlled at 33 °C at the time of placement and was decreased periodically to reach 23 °C when the chicks were three weeks old. This temperature was maintained until the end of the trial. Constant light was provided on day 1, but on day 2, the light was 21 h per day until the end of the study. A two-phase feeding program was used on this study and consisted of a starter diet from 1 -21 d and a grower diet from 22-42 d (Table 1).
Diets met or exceeded Ross 308 catalogue recommendations according to the producer instructions. The treatments were randomized into nine dietary groups as follows: four dietary probiotic blend levels (250, 500, 750, and 1,000 g/ton) within two feeding periods (0-14 and 0-42 d, respectively), whereas the ninth treatment was a control diet without any dietary supplementation. The probiotic blend (Probio Enzyme®, Xvet, Germany) was a commercial probiotic supplement containing enzymes and included: B. íicheniformis, B. subtiíis, Enterococcus faecium, L. acidophilus, p-glucanase 3.2.1.4, p-glucanase 3.2.1.6, p-xylanase 3.2.1.8, a-amylase 3.2.1.1.9 (under the EU regulation No 234/2011), protease, and cellulase.
To determine the broilers' growth performance, the feed intake (FI) and weight gain (WG) were recorded at the end of the two periods. Feed efficiency (FE = WG/ FI), energy intake (EI = kcal consumed/day), energy efficiency (EE = kcal/g of WG), protein intake (PI = g of protein consumed/day), and protein efficiency (PE = g of protein consumed/g of WG) were assessed. Mortality rate was recorded to allow the correction of performance data. The birds and feed were weighed at 1, 21, and 42 d in order to calculate the WG, FI, FCR, viability, and production efficiency index (PEI) of 42-d-old birds, according to the following equations:
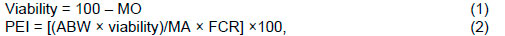
where: MO = mortality, ABW = average body weight at slaughter, MA = market age, and FCR = feed conversion ratio (FCR = 1/FE).
At the age of 42 d, before the blood collection, the feed was removed from all the birds for a period of four hours in an attempt to facilitate the stabilization of plasma constituents; all blood sampling was done in the morning to further reduce the variability of the plasma constituents. Then, a 5 ml sample of venous blood was collected from the ulnaris vein of the wing, sampled from each replicate (Hasan et al., 2022). The whole blood sample was transferred from the syringe into a tube coated with 10 mg of the anticoagulant, EDTA; blood samples were centrifuged at 3000 rpm * 20 min. Plasma was collected and stored at -20 °C until analyses following standard protocols. Serum TG (triglycerides), CHOL (total cholesterol), VLDL (very-low density lipoproteins), LDL (low-density lipoproteins), HDL (high-density lipoproteins), plasma glucose, uric acid, total protein, albumin, and globulin were analysed using commercial enzymatic kits (Wako Pure Chemicals Industries, Ltd., Richmond, VA).
After blood collections, three birds from each replicate were selected and weighed; the averages from these birds for each parameter were calculated and used as one experimental unit to assess carcass traits. Birds were fully defeathered via the dry method. Feet were separated from the carcass at the tibio-tarsal joint. Neck, wing tips, digestive tract, and liver were removed, and the carcass was weighed (cold carcass weight, after chilling). Economically relevant cuts of carcass and offal were separated. Breast muscle, including the skin and sternum, were dissected free from the carcass. Legs (thighs and drumsticks) were dissected by the ex-articulation at the hip joint and by dissecting tissue from the iliac bone. All abdominal fat, including that around the rectum, gizzard and proventriculus, was collected. Collected cuts including breast, wings, thighs, and drumsticks (legs), heart, neck, gizzard, digestive tract, and abdominal fat were weighed. The total weight of dissected parts was related to the totally eviscerated carcass. Relative portion percentages were calculated according to the following equation:
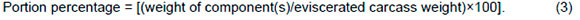
Data were subjected to analysis of variance (ANOVA) using a two-way procedure based on the following model:

Where: μ = general average, Ai = treatment effect, and eijkl = incidental residual effect of observation. After statistical difference confirmation, the General Linear Model (PROC GLM) was applied, and the differences among means (P <0.05) were assessed using Duncan's multiple range test (SAS, 2012). Relative percentage was used as a descriptive statistic to determine the ratio between carcass components and eviscerated carcass total weight.
Results
The performance traits of broilers fed diets including different levels of probiotic blend during the first 14 days of age or over the entire rearing period (1-42 DOA) are reported in Table 2. Feeding the probiotic at 1000 g/ton during the starter period led to higher FI (P = 0.027), energy intake (P = 0.026), and protein intake (P = 0.033) compared to the other treatments. Furthermore, broilers on the control diet resulted in higher final BW (P = 0.009) than on the experimental diets. Feeding birds the control diet resulted in the best deplumed and full carcass weights (P = 0.004), as well as leg cut weight (P = 0.042) (Table 3). The intestinal measurements of broilers under the different dietary treatments are provided in Table 4. Most of the examined traits differed markedly among groups; the weight of the duodenum, jejunum, and ileum resulted in heavier in birds on the control diet (P <0.05). Moreover, broilers fed the control diet had a higher jejunum and ileum yield (P <0.0001) and ileum width (P <0.0001). The colon tract was not influenced by treatments. The haematological profile of broilers fed diets containing different levels of probiotic blend over different supplementation periods was not influenced by treatments.
Discussion
Broiler chicks fed during the probiotic blend over the initial 14 d presented higher total feed and nutrient intakes. However, these results did not produce an improvement in final weight at 42 DOA. High feed and nutrient intakes without any performance gain are uneconomical and undesirable by the poultry industry.
Timmerman et al. (2007) gathered information based on 13 published studies and suggested that with the higher productivity rates of the broiler chicks, the effect of probiotics becomes smaller. Araújo et al. (2014) reported that the inclusion of an enzyme blend did not affect the feed intake of broiler chicks. Additionally, Abdelrahman & Saleh (2007) did not find any influence of the inclusion of glucanase on diets. Sarica et al. (2005) observed no significant differences in BWG, FI, and FCR of the broilers that were fed with enzyme blend treatment. Other authors gathered different information about probiotic and enzyme nutritional and immunological modulatory effects in broiler chickens.
Mountzouris et al. (2007), when studying the efficacy of a multi-species probiotic in broiler chick nutrition and comparing it to avilamycin antibiotics, verified a modulated composition and the activities of the cecal microflora resulted in a significant probiotic effect, but they did not report any performance results. In contradiction to the results presented in the current study, Mountzouris et al. (2010) showed that the use of probiotics on the diets was similar to the use of an antibiotic growth promoter (avilamycin) and superior to the use of control diets without any antibiotics. Regarding enzyme supplementation, Cao et al. (2010) reported that supplementing xylanase and phytase increased the weight gain of broiler chicks from 1 -21 d. These authors inferred that this higher performance was explained by the improvement of the apparent metabolisability of energy and nitrogen. However, none of these studies worked with the association of enzymes and probiotics in a unique Probio Enzyme® dietary additive.
The efficiency of probiotics and enzymes depends on several factors such as the period of use (as shown in this study's results), the bird's age, the environment, the bacterial challenger strains, the nutrient contents in feed, the solubility of dietary fibre, and other factors (Choct, 2006; Timmerman et al., 2006). Due to these facts, the results among the experiments can be very different in relation to performance results. Other undesirable results could be witnessed with the use of Probio Enzyme® additives in broiler chicks' feeds. No statistical difference was observed in carcass traits, resulting in equal or inferior parameters to those on the control diet without any additives. Yang et al. (2009), in an extensive review, focused on gathering information about alternatives to in-feed antibiotics that are capable of dietary modulation of the digestive tract microflora in broiler chickens. In most sources of the review, probiotics did not appear to be effective as a substitute for these antibiotic growth promoters without impacting broiler performance or carcass parameters. Ahmad (2006), when also aiming to review the impact of probiotics on broiler chick performance, gathered some contradictory observations among the trials. One of the reasons for the incongruity in the data identified by the author is the probiotic dosage. Another factor is the viability of these microorganisms in the digestive tract wall and lumen and their capacity for colonization and adhesion.
Different strains of probiotic bacteria may exert different effects based on specific capabilities and enzymatic activities, even within one species (Ahmad, 2006). The proposed mechanisms of pathogen inhibition by the intestinal microbiota include nutrient competition, production of toxic conditions and compounds (volatile fatty acids, low pH, and bacteriocins), and the contest of binding sites on the intestinal epithelium (Yang et al., 2009). The probiotic blend mechanism inoculated five different strains of probiotic bacteria (6. licheniformis, B. subtilis, E. faecium, and L. acidophilus), which may be due to the exclusion of competitive mechanisms as one or more of these bacteria strains have antagonistic functions on the development of others, thereby resulting in a low effectiveness of probiotic functions. Lin et al. (2009) clarified this phenomenon: they reported that the multiple probiotic supplements had no significant effect on preventing bacterial infections. The researchers might have attributed it to the antagonism among the different strains of probiotics in the multi-strain supplement.
Regarding the enzyme component of the probiotic blend that was used, other authors did not demonstrate effects when promoting the increased broiler carcass traits. Araújo et al. (2014) did not verify an influence on carcass parameters of broiler chicks when they were fed with dietary enzymes during the entire husbandry period. The use of a combination of endo-1,4-p xylanase (equivalent to 1,400 xylanase units g-1) and endo-1,3-p glucanase (200 glucanase units g-1) in feed did not affect the breast but it decreased the thigh and drumstick weights in broiler chickens. Choct (2006) gathered information in a review paper concerning the enzymes commonly used in poultry industry. Results were shown in studies around the world reporting the effectiveness of these feed additives, or a lack thereof. The most significant reason for the non-effectiveness of enzymes is because they are substrate-dependent. In diets where the substrate is low, the effectiveness of these enzymes is impaired.
Some desirable results were observed in the carcass trait in the present study. Similar to the results that were reported in the current study, Mutus et al. (2006) did not observe an impact on the live performance of the birds throughout the 6-week feeding trial when they were fed with diets inoculated with B. licheniformis and B. subtilis (containing 2.3 x 108 CFU/g of spores for each strain). However, they reported that the thickness of the medial and lateral wall of the tibia, tibio-tarsal index, ash, and P content were substantially improved by the probiotic. These facts can explain the high thigh and drumstick percentages without affecting the performance parameters that were witnessed.
However, other researchers have gathered contradictory results; for instance, Malayoglu et al. (2010) did not verify the influence of enzyme supplementation on internal organ weights. Engberg et al. (2004) verified that the addition of xylanase increased chymotrypsin and lipase activities. Under these facts, the argument that physiology would be "lazy" and reduce the endogenous production of enzymes is fallacious. Another hypothesis is the influence of probiotic organisms on the size of the gastrointestinal tract. Probiotics induce beneficial effects on the host by improving the properties of the indigenous microflora and digestive tract size and weight (Ghadban, 2002). Thus, it is often implied that a more robust digestive tract will make a healthier animal, which, in turn, digests and uses nutrients more efficiently (Willis et al., 2011), but this was not evidenced in this paper. Yan et al. (2007) reported that p75 and p40 were the first probiotic bacterial proteins that were demonstrated to promote intestinal epithelial homeostasis through specific signalling pathways, promoting antiapoptotic and proliferation responses. According to Smirnov et al. (2005), the dietary probiotic enlarged the goblet cell "cup" area throughout the small intestine, increased the presence of mucin glycoprotein in the jejunum, and the expression of mucin mRNA in the probiotic-fed chicks. Due to the lack of scientific publication of these results, there is still much controversy over the effects of Probio Enzyme® in the gastrointestinal tract of birds, and, consequently, there is an opening for further research on this issue in particular.
Classically, the first investigations in the area reported a high correlation between both total serum protein or albumin levels and the protein content in body composition (lean meat) (Thomas and Combs, 1967). Low serum total protein and albumin levels had a positive correlation with a marked decrease in weight gain and feed intake (Ologhobo, 1992). The results in the current study differ consistently from these authors, with a negative correlation between WG and carcass traits and serum albumin levels in Probio Enzyme®-supplemented birds. On the other hand, some more recent trials report that serum albumin levels are positively correlated with high stress levels and low performance. It was shown that preslaughter treatment (catching, crating, and transportation) during the summer increases blood albumin, which is a reliable indicator of stress in broilers (Yalçinet al., 2004). Ak§it et al. (2006) also reported that plasma albumin content was increased by high ambient temperature when heat-stressed broiler chickens were crated at 34 °C. Hernández et al. (2012), working with low-protein diets, found an increase of 3% in the feed conversion ratio when the plasma albumin levels were reduced. Thus, the positive correlation of serum albumin with high performance is very questionable. High serum albumin values in treatments where the birds showed low performance are therefore understandable.
Conclusions
The results of this research demonstrate that there are very few benefits in using this probiotic blend in the broiler diet. Some growth traits improved, but not sufficiently to justify the use of the product in terms of a cost-benefit analysis, particularly if the aim of using the probiotic blend is as an antibiotic growth promoter substitute. Thus, the search for an effective substitute for antibiotics in poultry diets must consider the factors that influence the efficacy of natural feed additives in order to increase digestibility, to balance the desirable gut microorganisms, and to promote poultry performance.
Acknowledgements
Financial support by Rasht Branch, Islamic Azad University (Grant Number 4.5830) is gratefully acknowledged. We are grateful to the Research Support Foundation of the State of Minas Gerais (FAPEMIG), Brazil for their support.
Author contributions
All the authors approved the final version of the manuscript. MM, CL, AS, MN, WAGA, LFTA, VL, VT, and DDM: conceptualization, formal analysis, methodology, validation, writing-review & editing, writing-original draft.
Data availability
Data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflict of Interest Declaration
The authors declare no conflicts of interest.
References
Abdelrahman, M.M., Saleh, F.H. 2007. Performance of broiler chickens fed on corn- sunflower meal diets with IS- glucanase enzyme. JJAS. 3(3): 272-280. [ Links ]
Ahmad, I. 2006. Effect of probiotics on broilers performance. Int. J. Poult. Sci. 5 (6): 593-597. [ Links ]
Akçit, M., Yalçin, S., Özkan, S., Metin, K., Özdemir, D. 2006. Effects of temperature during rearing and crating on stress parameters and meat quality of broilers. Poult. Sci. 85: 1867-1874. [ Links ]
Araújo, W.A.G., Albino, L.F.T., Rostagno, H.S., Hannas, M.I., Pessoa, G.B.S., Messias, R.K.G., Lelis, G.R., Ribeiro, Jr V. 2014. Sunflower meal and enzyme supplementation of the diet of 21- to 42-d-old broilers. Braz. J. Poult. Sci. 16: 17-24. [ Links ]
Ashyerizadeh, A., Dabiri, N., Ashyerizadeh, O., Mirzadeh, K.H., Roshanfekr, H., Mamooee, M. 2009. Effect of dietary antibiotic, probiotic and prebiotic as growth promoters, on growth performance, carcass characteristics and haematological indices of broilers chickens. Pak. J. Biol. Sci. 12 (1): 52-57. [ Links ]
Bhogoju, S., & Nahashon, S. 2022. Recent advances in probiotic application in animal health and nutrition: A review. Agriculture, 12(2), 304. [ Links ]
Cao, P.H., Li, F.D., Li, Y.F., Ru, Y.J., Péron, A., Schulze H., Bento, H. 2010. Effect of essential oils and feed enzymes on performance and nutrient utilization in broilers fed a corn/soy-based diet. Int. J. Poult. Sci. 9(8): 749-755. [ Links ]
Chand, N., Ali, P., Alhidary, I.A., Abelrahman, M.M., Albadani, H., Khan, M.A., Seidavi, A., Laudadio, V., Tufarelli, V. & Khan, R.U. 2021. Protective effect of grape (Vitis vinifera) seed powder and zinc-glycine complex on growth traits and gut health of broilers following Eimeria tenella challenge. Antibiotics 10, 86. https://doi.org/10.3390/antibiotics10020186 [ Links ]
Choct, M. 2006. Enzymes for the feed industry: Past, present and future. Worlds Poult. Sci. J. 62(1): 5-16. [ Links ]
Cimrin, T., Tunca, R.I., Avsaroglu, M.D., Ayasan, T., Kücükersan, S. 2020. Effects of an antibiotic and two phytogenic substances (cinnamaldehyde and 1,8-cineole) on yolk fatty acid profile and storage period-associated egg lipid peroxidation level. Rev. Bras. Zootec. 49:e20190270. [ Links ]
Engberg, R.M., Hedemann, M.S., Steenfeldt, S., Jensen, B.B. 2004. Influence of whole wheat and xylanase on broiler performance and microbial composition and activity in the digestive tract. Poult. Sci. 83: 925-938. [ Links ]
Ghadban, G.S. Probiotics in broiler production - a review. Arch Für Geflügelkunde. 66: 49-58. [ Links ]
Hasan, M.N., Chand, N., Naz, S., Khan, R. U., Ayaçan, T., Laudadio, V., & Tufarelli, V. 2022. Mitigating heat stress in broilers by dietary dried tamarind (Tamarindus indica L.) pulp: Effect on growth and blood traits, oxidative status, and immune response. Livest. Sci. 264, 105075. [ Links ]
Hernandez, F., López, M., Martinez, S., Megías, M.D., Catalá, P., Madrid, J. 2012. Effect of low-protein diets and single sex on production performance, plasma metabolites, digestibility, and nitrogen excretion in 1- to 48-day-old broilers. Poult. Sci. 91(3): 683-692. [ Links ]
Hume, M.E. 2011. Historic perspective: Prebiotics, probiotics, and other alternatives to antibiotics. Poult. Sci. 90: 2663-2669. [ Links ]
Józefiak, D., Rutkowski, A., Jensen, B.B., Engberg, R.M. 2007. Effects of dietary inclusion of triticale, rye and wheat and xylanase supplementation on growth performance of broiler chickens and fermentation in the gastrointestinal tract. Anim. Feed Sci. Technol. 132 (1-2): 79-93. [ Links ]
Khaksefidi, A., Ghoorchi, T. 2006. Effect of probiotic on performance and immunocompetence in broiler chicks. J. Poult. Sci. 43: 296-300. [ Links ]
Khan, K., Aziz, K., Khan, N., Khan, S., & Ayasan, T. (2021). Effect of enzyme and yeast-based feed additives on growth, nutrient digestibility, meat quality and intestinal morphology of fattening rabbits. J. Hell. Vet. Med. Soc. 72(4), 3511-3518. doi: https://doi.org/10.12681/jhvms.29404 [ Links ]
Lázaro, R., Latorre, M.A., Medel, P., Gracia, M., Mateos, G.G. 2004. Feeding regimen and enzyme supplementation to rye-based diets for broilers. Poult. Sci. 83(2): 152-160. [ Links ]
Lin, J., Chiub, Y., Linc, N., Chua, C., Huang, K., Liao, K., Peng, K. 2009. Different effects of probiotic species/strains on infections in preschool children: A double-blind, randomized, controlled study. Vaccine 27: 1073-1079. [ Links ]
Malayoglu, H.B., Baysal, S., Misirlioglu, Z., Polat, M., Yilmaz, H., Turan, N. 2010. Effects of oregano essential oil with or without feed enzymes on growth performance, digestive enzyme, nutrient digestibility, lipid metabolism and immune response of broilers fed on wheat-soybean meal diets. Br. Poult. Sci. 51(1); 67-80. [ Links ]
Mountzouris, K.C., Tsirtsikos, P., Kalamara, E., Nitsch, S., Schatzmayr, G., Fegeros, K. 2007. Evaluation of the efficacy of a probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poult. Sci. 86: 309-317. [ Links ]
Mountzouris, K.C., Tsitrsikos, P., Palamidi, I., Arvaniti, A., Mohnl, M., Schatzmayr, G., Fegeros, K. 2010. Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poult. Sci. 89(1):58-67. [ Links ]
Mushtaq, T., Sarwar, M., Ahmad, G., Mirza, M.A., Nawaz, H., Mushtaq, M.M.H., Noreen, U. 2007. Influence of canola meal-based diets supplemented with exogenous enzyme and digestible lysine on performance, digestibility, carcass, and immunity responses of broiler chickens. Poult. Sci. 86: 21442151. [ Links ]
Mutuç, R., Kocabagli, N., Alp, M., Acar, N., Eren, M., Gezen, Ç.Ç. 2006. The effect of dietary probiotic supplementation on tibial bone characteristics and strength in broilers. Poult. Sci. 85:1621-1625. [ Links ]
Ologhobo, A.D. 1992. Nutritive values of some tropical (West African) legumes for poultry. J. Appl. Anim. Res. 2(2): 93-104. [ Links ]
Sarica, S., Ciftci, A., Demir, E., Kilinc, K., Yildirim, Y. 2005. Use of an antibiotic growth promoter and two herbal natural feed additives with and without exogenous enzymes in wheat based broiler diets. South Afr. J. Anim. Sci. 35 (2): 61-72. [ Links ]
SAS. 2012. User's Guide Survival Analysis (book excerpt), version 12.1. SAS Institute Incorporated. Cary, North Carolina, USA. [ Links ]
Smirnov, A., Perez, R., Amitromach, E., Sklan, D., Uni, Z. 2005. Mucin dynamics and microbial populations in chicken small intestine are changed by dietary probiotic and antibiotic growth promoter supplementation. J. Nutr. 135(2): 187-192. [ Links ]
Thomas, O.P., Combs, G.F. 1967. Relationship between serum protein level and body composition in the chick. J. Nutr. 91 (4): 468-472. [ Links ]
Timmerman, H.M., Veldman, A., van den Elsen, E., Rombouts, F.M., Beynen, A.C. 2006. Mortality and growth performance of broilers given drinking water supplemented with chicken-specific probiotics. Poult. Sci. 85, 1383-1388. [ Links ]
Wang, Z.R., Qiao, S.Y., Lu, W.Q., Li, D.F. 2005. Effects of enzyme supplementation on performance, nutrient digestibility, gastrointestinal morphology, and volatile fatty acid profiles in the hindgut of broilers fed wheat-based diets. Poult. Sci. 84(6): 875-881. [ Links ]
Willis, W.L., Isikhuemhen, O.S., Hurley, S., Ohimain, E.I. 2011. Effect of phase feeding of fungus myceliated grain on oocyst excretion and performance of boiler chicken. Int. J. Poult. Sci. 10 (1): 1-3. [ Links ]
Yalçin, S., Özkan, S., Oktay, G., Çabuk, M., Erbayraktar, Z., Bilgili, S.F. 2004. Age-related effects of catching, crating, and transportation at different seasons on core body temperature and physiological blood parameters in broilers. J. Appl. Poult. Res. 13: 549-560. [ Links ]
Yan, F., Cao, H., Cover, T.L., Whitehead, R., Washington, M.K., Polk, D.B. 2007. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 132: 562575. [ Links ]
Yang, Y., Iji, P.A., Choct, M. 2009. Dietary modulation of gut microflora in broiler chickens: a review of the role of six kinds of alternatives to in-feed antibiotics. Worlds Poult. Sci. J. 65: 97-114. [ Links ]
Submitted 28 March 2023
Accepted 10 May 2023
Published 26 August 2023
# Corresponding authors: alirezaseidavi@iaurasht.ac.ir; vincenzo.tufarelli@uniba.it














