Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Animal Science
On-line version ISSN 2221-4062
Print version ISSN 0375-1589
S. Afr. j. anim. sci. vol.53 n.1 Pretoria 2023
http://dx.doi.org/10.4314/sajas.v53i1.10
The effect of formaldehyde treatment of canola oilcake meal and sweet lupins on the in situ dry matter and crude protein digestibility
T.S. BrandI, II, #; J.H.C van ZylI; O.DreyerI
IDirectorate: Animal Sciences, Department of Agriculture, Western Cape Government, Private Bag X1, Elsenburg, 7607, South Africa
IIDepartment of Animal Sciences, University of Stellenbosch, Private Bag X1, Matieland, 7602, South Africa
ABSTRACT
The value of feed protein sources in ruminant nutrition is measured by how effectively the protein is degraded in the rumen and converted into microbial protein. High-producing ruminants acquire high nutritional requirements to sustain their metabolic demands for production and performance. However, protein sources occasionally fall short in supplying the required amount of rumen undegradable protein and amino acids. Chemical treatment (formaldehyde) could be used to increase the efficiency of protein sources, which are highly degradable in the rumen. Canola oilcake meal (CM) and sweet lupin seed (SL) were treated with formaldehyde (40% w/v) at concentrations of 10 g/kg (F10) and 15 g/kg CP (F15). In this study, six Dohne Merino wethers fitted with rumen cannulas were used to determine the effect of formaldehyde treatment on the in situ dry matter and crude protein digestibility. The treatments entailed CM control (CMF0), CM treated with 10 g/kg CP formaldehyde (CMF10), CM treated with 15 g/kg CP formaldehyde (CMF15), SL control (SLF0) SL treated with 10 g/kg CP formaldehyde (SLF10) and SL treated with 15 g/kg CP formaldehyde (SLF15). Treatments were incubated in the rumen at time intervals of 0, 2, 4, 12, 36, 48, 72, and 96 hours. Overall, formaldehyde treatment significantly decreased rumen degradation at all outflow rates of both CM and SL. Therefore, formaldehyde treatment could be used to increase the rumen undegradable protein fraction. Potential improvement in animal performance in terms of live weight gain, average daily gain, and feed conversion efficiency has to be evaluated in production studies.
Keywords: bypass protein, chemical treatment, degradation, increase, nutrition, ruminant
Introduction
Recently, emphasis has been placed on increasing high-producing ruminant productivity by optimizing the animals' use of protein sources. Degradation models have identified that amino acids absorbed by the animal originate mainly from the feed protein escaping rumen degradation and microbial protein (MP) (Poos-Floyd et al., 1985). Das et al. (2015) stated that a variety of technical methods could be used to increase livestock productivity. Physical treatment (extrusion, grinding, and roasting) and chemical treatment (formaldehyde and alcohol application) could be used to increase the efficiency of certain protein sources that are highly degradable in the rumen.
The formaldehyde reaction consists of two steps entailing the rapid formation of a methylol compound, followed by a slow condensation reaction (Barry, 1976). Formaldehyde (HCHO) treatment reduces the activity of proteolytic bacteria on feed protein entering the rumen, by the formation of methylene cross-linkages between HCHO and the protein under ruminal pH conditions (Kumar et al., 2014). The correct concentration of formaldehyde must be applied to prevent overprotection. Overprotection results in the methylene cross-linkages being irreversible as the protein enters the small intestines (SI). Sequentially, the amino acids will be unavailable to the animal, reducing the protein availability directed towards tissue growth and production. A variety of studies proved that 1% (10 g/kg CP) formaldehyde treatment of feedstuff is sufficient for the majority of feedstuffs (Malik et al., 1981; Pratihar & Walli, 1995; Kondusamy, 2010). However, HCHO treatment differs between feedstuffs, being dependent on the protein solubility of the protein source (Barry, 1976).
Gulati et al. (2005) stated that HCHO treatment of a feedstuff has no harmful effect on the animal when ingested and no residue remains in the tissue of the animal (Wales et al., 2009). However, human safety precautions must be in place regarding HCHO application, due to sensitivity of the human sensory system. Nonetheless, formaldehyde treatment is allowed as a feed processing method in the EU (Wales et al., 2009). Previous research studies have indicated that HCHO treatment decreases both in situ and in vitro effective ruminal degradation (Rodehutscord et al., 1999) and protein degradation (Kumar et al., 2014). Formaldehyde (HCHO) treatment along with methionine supplementation resulted in (i) increased wool growth (Rodehutscord et al., 1999), (ii) increased protein digestion in the SI (Eghbali et al., 2011), (iii) improved body weight gain (BWG), feed conversion ratio (FCR), and average daily gain (ADG), (iv) decreased in vitro ammonia concentration of fishmeal (Kondusamy, 2010), and a (v) overall increase in amino acid availability for further absorption in the SI (Barry, 1976; Bhatt & Sahoo, 2019). In addition, formaldehyde could be used as fumigant gas (Wales et al., 2009) to reduce or prevent antimicrobial infestations during feed storage, ultimately extending animal feed storage life.
Shannak et al. (2000) stated that a research gap pertaining to trustworthy data based on the undegradable protein values (UDP) of concentrate ingredients existed. The aim of this study was thus to determine the effect of formaldehyde treatment at levels of 0 g/kg CP, 10 g/kg CP, and 15 g/kg CP on the in situ dry matter and crude protein digestibility of the locally-produced, plant protein sources, canola oilcake meal and sweet lupin seed. The rate of degradation was determined using the technique described by Orskov & McDonald (1979).
Materials and Methods
Ethical clearance for this study was granted by the Animal Care and Use Research Ethics Committee of Stellenbosch University (#21726) and DAEC (AP/NP/S/TB103) (Departmental Evaluation Committee) of the Agricultural Department of the Western Cape Government at Elsenburg. Six Dohne Merino wethers at a live weight -95 kg, already fitted with rumen cannulas, were housed at Kromme Rhee Experimental Farm of the Agricultural Department of the Western Cape Government. Each animal was placed in an individual paddock (2.1 m x 2.0 m). Feeding commenced twice a day, once in the morning (08:00) and the afternoon (16:00), along with fresh water being provided. They were fed an ad libitum basal diet consisting of 50:50 wheat straw and lucerne hay.
The trial consisted of six treatments being tested in situ, based on Orskov & Mcdonald's (1979) research on rumen protein degradability. The treatments entailed canola oilcake meal control (CMF0), canola oilcake meal treated with 10 g/kg CP formalin (40% w/v) (CMF10), canola oilcake meal treated with 15 g/kg CP formalin (40% w/v) (CMF15), sweet lupin seed control (SLF0), sweet lupin seed treated with 10 g/kg CP formalin (40 % w/v) (SLF10), and sweet lupin seed treated with 15 g/kg CP formalin (40% w/v) (SLF15).
Both plant protein sources (canola oilcake meal and sweet lupin seed) were purchased prior to the trial at our local cooperative (Kaap Agri). The canola oilcake meal and sweet lupin seed (Lupinus angustifolius) were separately ground to 2 mm size, using a hammer mill (serial no 372), after which the ground samples were filtered using a Retch AS200 apparatus, to get rid of any powder that could potentially influence the dry matter (DM) disappearance. Afterward, both canola oilcake meal and sweet lupin were separately placed in large zip lock bags at a 4 mm thickness level and sprayed with formalin (40 % w/v) (Das et al., 2015). The bag was then vigorously shaken for 5 minutes before storage (Subuh & Rowan, 1994). The sprayed samples were left at room temperature for 24 hours for the reaction to occur (Antoniewicz et al., 1992) and to prevent condensation of the formalin. After 24 hours, the samples were placed in tinfoil cups and retained in a force draught oven at 60 °C for 48 hours.
The dry matter (DM) and crude protein (CP) degradability of canola oilcake meal and sweet lupin seed were determined using the in situ technique described by Orskov & Mcdonald (1979). Both plant proteins were dried in a force draught oven for a minimum of 48 hours at 60 °C. Afterward, 5 g samples were weighed off in dracon bags (Brand & Jordaan, 2020) and tied off using a constrictor knot with a nylon string, along with being colour coded using a cable tie, relative to which treatment it contained. For easy retrieval from the rumen, a washer was connected at the end of the string. The bags were incubated in the rumen for seven different time intervals. The time intervals were 0, 2, 4, 12, 36, 48, 72, and 96 hours, with the 0-hour bag representing the control. The control bag was prepared identically to that of the other time intervals, except for not being placed in the rumen. It was also rinsed with tap water and placed in a force draught oven. The incubation period started with all seven bags (2, 4, 12, 36, 48, 72, 96 hours) being placed in the rumen every Monday morning at 07:00. The first bag retrieval was at 09:00 on a Monday morning, representing the 2-hour time interval, with the last bag being retrieved at 07:00 on Friday morning representing the 96-hour bag. Treatments were randomly assigned to the six wethers making use of a cross-over design. Thus, each sheep received all six treatments, with each treatment being replicated six times (a total of 288 observations).
After retrieval from the rumen, the bags were rinsed under cold running tap water, to prevent further degradation, until the colour of the water draining from the dracon bag was clear. The bags were then placed in the oven at 60 °C for a minimum of 48 hours. Afterward, the dried bags were weighed to determine the dry matter residue (Jordaan & Brand, 2020). The % nitrogen content of the residue was determined using a LECO TruMac N Nitrogen Determinator (LECO Corporation, Michigan, USA). The CP content of the dry matter was determined by multiplying the percentage N by a factor of 6.25.
The DM and CP disappearances were stated as percentages of the amount of residue remaining after rumen incubation. The Mitscherlich function was used to fit the percentage of material degraded in the rumen by means of SAS 9.4 software (SAS Institute Inc., 2016) to determine the DM and CP degradability parameters:
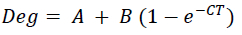
Deg represents the potential degradability at time, t (%), with A representing the rapidly soluble fraction (represents 0-hour disappearance (%)), B the fraction degraded over time (potentially degradable fraction (%)), and C the rate of degradation of the B fraction (%/h) (Jordaan & Brand, 2020).
With ruminal retention time affecting the degree of degradation, the fractional outflow rate of undegraded protein (UP) from the rumen (k) was considered in determining the percentage of effective degradation. The following k values were used: 0.02 (low intake level), 0.04, 0.06, and 0.08/h (high intake level) (Brand & Jordaan, 2020). The percentage of effective degradation (Degeff)was determined by using a Latin square factorial design, with two protein sources (canola oilcake meal and sweet lupin seed) and three treatments (control/ no formaldehyde application) (F0), 10g/kg CP formaldehyde (F10) and 15g/kg CP formaldehyde (F15) application). The percentage of effective degradation was determined using the equation:
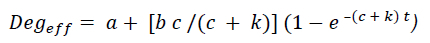
Results and Discussion
The in situ results of the DM disappearance parameters for the effect of formaldehyde treatment on plant protein sources canola oilcake meal (CM) and sweet lupin seed (SL) are summarized in Table 1. No interaction was present for the dry matter soluble fraction (A), dry matter potential degradable fraction (ß), and the rate of degradation of B (C), for protein source (CM & SL) and processing (formaldehyde treatment).
Significant differences were present for the DM soluble fraction (P = 0.036) and the rate of degradation of the potential degradable fraction estimates for the DM degradability (P <0.0001), for processing as the main factor. Significant differences was also present for all the DM non-linear parameters (P 0<.0001) for protein source as the main factor. Sweet lupin seed obtained a 23.6% (86.4% versus 66%) greater DM potential degradable fraction in the rumen of the sheep, relative to canola oilcake meal. These results was supported by Heuzé et al. (2020 & 2022), where the average potential degradable fraction of Lupinus angustifolius was 5% greater compared to canola oilcake meal (60% versus 55%).
The dry matter effective degradability (Degeff) for all treatments at various outflow rates is presented in Table 2. Significant differences were present for processing at all outflow rates. Formaldehyde treatment at both concentrations of 10 g/kg CP and 15 g/kg CP effectively decreased the DM effective degradation at each fractional outflow rate, with the largest effect seen at 0.08/h (from 57.6/h to 37.9/h). These results are supported by Eghbali et al. (2011), where canola oilcake meal treated with 12 g/kg CP HCHO effectively decreased the Degeff by 21.2% at an 0.08/h outflow rate. This suggests that formaldehyde application enhances the apparent digestibility of protein (Eghbali et al., 2011). Thus, the F10 treatment decreased the DM effective degradation by 10.1%, 14.1%, 15.6%, and 16.1% at outflow rates of 0.02, 0.04, 0.06, and 0.08, respectively. Additionally, the F15 treatment decreased DM effective degradation by 13.2%, 17.8%, 19.4%, and 20.0% at outflow rates of 0.02, 0.04, 0.06, and 0.08, respectively. Differences were also present at each fractional outflow rate for both protein sources, canola oilcake meal and sweet lupin seed (P <0.0001).
Figures 1 and 2 illustrate that formaldehyde treatment at concentrations of 10 g/kg and 15 g/kg CP effectively decreases the DM disappearance of both canola oilcake meal and sweet lupin seed at different rumen incubation time intervals (0, 2, 4, 12, 36, 48, 72, and 96 hours).
The in situ results of the CP disappearance parameters for the effect of formaldehyde treatment on plant protein sources, canola oilcake meal (CM) and sweet lupin seed (SL), are summarized in Table 3. An interaction was observed for the soluble fraction, potential degradable fraction, and the rate of degradation of the potential degradable fraction (P <0.0001). Therefore, the main effects could not be interpreted. Formaldehyde treatment at both concentrations (F10 and F15) decreased the soluble fraction of canola oilcake meal but increased the soluble fraction in sweet lupin seed. Formaldehyde treatment increased the potential degradable fraction of canola oilcake meal by 11.8% (F10) and 32.1% (F15). These results are similar to results presented by Subuh et al. (1994), where canola oilcake meal treated with 8 g/kg CP (30% formalin) decreased the ruminal degradation by 11.2 %. Additionally, Eghbali et al. (2011) suggested that canola oilcake meal treated with 12 g/kg CP HCHO led to a 30.7% decrease in the soluble fraction, 11.9% increase in potential degradable fraction, and a 43.5% decrease in the rate of degradation of the potential degradable fraction. Furthermore, formaldehyde treatment decreased the rate of degradation of the CP potential degradable fraction of both canola oilcake meal and sweet lupin seed by 70% (CMF10), 80% (CMF15), 80% (SLF10), and 90% (SLF15), respectively.
However, formaldehyde treatment decreased the potential soluble fraction of sweet lupin seed by 3.3% (F10) and 4.2% (F15). The nitrogen digestibility of Lupinus angustifolius is 2.8 % greater than canola oilcake meal (80% versus 77.2%) (Heuzé et al., 2020 & 2022). Thus, the above result can potentially be explained by the protein solubility influencing the potential degradable protein fraction. It is, therefore, probable that 10 g/kg CP and 15 g/kg CP HCHO treatment of the sweet lupin seed exceeded the optimal level of HCHO treatment. Thus, overprotection resulted in a slight decrease in the potential degradable fraction due to complexes formed between HCHO and the protein group, rendering the sweet lupin seed less digestible and decreasing the protein digestibility (Gulati et al., 2005; Das et al., 2015).
The crude protein effective degradability (Degeff) for all treatments at various outflow rates is presented in Table 4. Significant differences were present for processing at all outflow rates of 0.02, 0.04, 0.06, and 0.08/h. Formaldehyde treatment at both concentrations of 10 g/kg CP and 15 g/kg CP formaldehyde (HCHO) effectively decreased the CP effective degradation at each fractional outflow rate, with the largest effect seen at 0.08/h (from 71.7% to 38.1%). Thus, F10 concentration treatment decreased CP effective degradation by 16.5%, 23.6%, 26.5%, and 28.2%, at outflow rates of 0.02, 0.04, 0.06, and 0.08, respectively. Additionally, the F15 concentration treatment decreased CP effective degradation by 21.2%, 29.7%, 32.6%, and 33.6%, at outflow rates of 0.02, 0.04, 0.06, and 0.08, respectively. Differences were also present at each fractional outflow rate for both protein sources, canola oilcake meal and sweet lupin seed (P <0.0001). This is supported by results obtained by Rodehutscord et al. (1999), where lupins treated with 4 g/kg CP HCHO markedly decreased the fractional outflow rate of nitrogen disappearance at a 0.03/h outflow rate.
Figures 3 and 4 illustrate that formaldehyde treatment at both concentrations of 10 g/kg CP and 15 g/kg CP HCHO gradually decreases the CP degradability of both canola oilcake meal and sweet lupin seeds at different rumen incubation time intervals (0, 2, 4, 12, 36, 48, 72, and 96 hours). Canola oilcake meal was used due to the decreased rate of nitrogen digestibility in ruminants being 3.5% lower compared to soybean meal and 20.8% lower than high protein fishmeal (Canola oilcake meal N digestibility (77.2%) versus soybean meal (80%) & fishmeal (97.5%)) (Heuzé et al., 2015 & 2020b).
A variety of previous studies have suggested that formaldehyde (HCHO) treatment of different protein sources supports increased live weight gain (LWG) and improved feed conversion efficiency (Peter et al., 1971; Spears et al., 1980; Bhatt & Sahoo, 2019) of ruminants. Gupta & Gupta (2012) and Chopra et al. (2013) also found similar results obtained at a 10 g/kg CP HCHO application, compared to a 20 g/kg CP HCHO application that drastically decreased LWG and impaired FCE. Additionally, Kondusamy (2010) indicated that 10 g/kg CP HCHO application markedly decreased the nitrogen solubility and in vitro ammonia levels of sardine fishmeal. With formaldehyde being a product of intermediate metabolism in mammals (Gulati et al., 2005), it is an effective strategy and feasible technology (Kumar et al., 2014) to increase the rumen undegradable protein or bypass protein of protein sources which is highly degradable in the rumen. Nonetheless, HCHO application is crucial to optimize the quantity and quality (Bhatt & Sahoo, 2019) of the protein available in the small intestines of the ruminant.
Conclusion
Processing (formaldehyde treatment) at both 10 g/kg and 15 g/kg CP concentrations, increased the rapidly soluble fraction and lowered the rate of degradation of the DM potential degradable fraction (P <0.0001), with plant protein sources CM and SL obtaining potential degradable fractions of 66% and 86.4%, respectively. Formaldehyde treatment at both concentrations substantially decreased effective DM degradation (Degeff) at all outflow rates with the largest effect seen at 0.08/h. Formaldehyde 10 g/kg CP treatment decreased the Degeff by 16.1 % and formaldehyde 15 g/kg CP treatment decreased Degeff by 19.7% (at an outflow rate of 0.08/h).
Formaldehyde treatment at both concentrations substantially increased the crude protein (CP) potential degradable fraction of CM by 11.8% (F10) and 32.1% (F15), respectively. In contrast with the above, F10 and F15 formaldehyde application decreased the potential degradable fraction of SL by 3.3% and 4.2%, respectively. Nonetheless, HCHO treatment decreased (P <0.0001) Degeff at all outflow rates, with the largest effect seen at 0.08/h. Formaldehyde 10g/kg CP treatment decreased the CP Degeff by 27.9% and formaldehyde 15g/kg CP treatment decreased Degeff by 33.6%.
To conclude, formaldehyde (HCHO) treatment effectively decreased both DM and CP rumen degradation at all outflow rates for both CM and SL. Therefore, HCHO application can be used to increase the rumen undegradable protein fraction of highly degradable protein sources. Potential improvement of animal performance in terms of live weight gain, average daily gain, and feed conversion efficiency should be tested in practice.
References
Antoniewicz, A. M., Vuuren, A. M. van, Koelen, C. J. van der, & Kosmala, I. 1992. Intestinal digestibility of rumen undegraded protein of formaldehyde-treated feedstuffs measured by mobile bag and in vitro technique. Anim. Feed Sci. Technol. 39, 111-124 https://doi.org/https://doi.org/10.1016/0377-8401(92)90035-5. [ Links ]
Barry, T. N. 1976. The effectiveness of formaldehyde treatment in protecting dietary protein from rumen microbial degradation. Grassl. Res. Institute, Hurl. Maidenhead, Berk. SL6 5LR 35, 221-229https://doi.org/https://doi.org/10.1079/PNS1970035. [ Links ]
Bhatt, R. S., & Sahoo, A. 2019. Effect of adding formaldehyde treated protein alone and with Saccharomyces cerevisiae in diet on plane of nutrition, growth performance, rumen fermentation, and microbial protein synthesis of finisher lambs. Small Rumin. Res. 171, 42-48 https://doi.org/10.1016/j.smallrumres.2018.12.005. [ Links ]
Brand, T. S., & Jordaan, L. 2020. Effect of extrusion on the rumen undegradable protein fraction of lupins. S. Afr. J. Anim. Sci. 50, 779-785 https://doi.org/10.4314/sajas.v50i6.2. [ Links ]
Chopra, A., Malik, N. S., & Makkar, G. S. 2013. Effect of feeding formaldehyde-treated groundnut meal with or without urea on the nutrient utilization in buffalo calves. J. Res. - Punjab Agric. Univ. 17, 326-328 https://doi.org/ISSN:0048-6019. [ Links ]
Das, L. K., Kundu, S. S., Datt, C., Kumar, D., & Tariq, H. 2015. In situ ruminal degradation kinetics of dry matter, crude protein, and neutral detergent fiber of tropical ruminant feedstuffs. Indian J. Anim. Nutr. 32, 45-51. [ Links ]
Eghbali, M., Kafilzadeh, F., Hozhabri, F., Afshar, S., & Kazemi-bonchenari, M. 2011a. Treating canola meal changes in situ degradation, nutrient apparent digestibility, and protein fractions in sheep. Small Rumin.Res. 96, 136-139 https://doi.org/10.1016/j.smallrumres.2011.01.005. [ Links ]
Gulati, S. K., Garg, M. R., & Scott, T. W. 2005. Rumen protected protein and fat produced from oilseeds and/or meals by formaldehyde treatment; their role in ruminant production and product quality: A review. Aust. J. Exp. Agric. 45, 1189-1203 https://doi.org/10.1071/ea04131. [ Links ]
Gupta, N., & Gupta, B. 2012. Effect of feeding formaldehyde treated groundnut-cake on the growth and nutrient utilization in Karan Swiss calves. Indian J. Anim. Sci. 54, 1065-1068 https://doi.org/ISSN:0367-8318. [ Links ]
Heuzé, V., Thiollet, H., Tran, G., Lessire, M., & Lebas, F. 2022. Blue lupin (Lupinus angustifolius) seeds. Feed. a Program. by Inst. Natl. la Rech. Agron. (INRAE), French Agric. Res. Cent. Int. Dev. Modélisation Systémique Appliquée aux Ruminants Food Agric. Organ., https://www.feedipedia.org/node/23099. [ Links ]
Heuzé, V., Thiollet, H., Tran, G., Nozière, & Lebas, F. 2020a. Rapeseed meal. Feed. a Program. by Inst. Natl. la Rech. Agron. (INRAE), French Agric. Res. Cent. Int. Dev. Modélisation Systémique Appliquée aux Ruminants Food Agric. Organ., https://www.feedipedia.org/node/52. [ Links ]
Heuzé, V., Tran, G., & Kaushik, S. 2015. Fish Meal. Feed. a Program. by Inst. Natl. la Rech. Agron. (INRAE), French Agric. Res. Cent. Int. Dev. Modélisation Systémique Appliquée aux Ruminants Food Agric. Organ., https://www.feedipedia.org/node/208. [ Links ]
Heuzé, V., Tran, G., & Kaushik, S. 2020b. Soybean Meal. Feed. a Program. by Inst. Natl. la Rech. Agron. (INRAE), French Agric. Res. Cent. Int. Dev. Modélisation Systémique Appliquée aux Ruminants Food Agric. Organ., https://www.feedipedia.org/node/674. [ Links ]
Jordaan, L., & Brand, T. S. 2020. The effect of extrusion with molasses and addition of chitosan or tannins on the rumen undegradable protein fraction of plant protein sources. Stellenbosch Univ. Dep. Anim. Sci. Fac. AgriSciences. [ Links ]
Kondusamy, A. 2010. Effect of varying levels of heat and formaldehyde treatment of sardine fishmeal on nitrogen solubility, in vitro ammonia release, and protein fractions. Indian J. Anim. Sci. 80, 1003-1007. [ Links ]
Kumar, S., Kumari, R., Kumar, K., & Walli, T. K. 2014. Roasting and formaldehyde method to make bypass protein for ruminants and its importance: A review. Dir. Knowl. Manag. Agric. 85, 223-230 https://doi.org/ISSN0367-8318. [ Links ]
Malik, N. S., Makkar, G. S., Kansal, J. S., & Ichhponani, J. S. 1981. Growth, metabolic and rumen studies on rations containing formaldehyde treated groundnut meal with urea based rations. Indian J. Anim. Sci. 51, 611. [ Links ]
Orskov, E. R., & McDonald, I. 1979. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 92, 499-503 https://doi.org/10.1017/S0021859600063048. [ Links ]
Peter, A., Hatfield, E., Owens, F., & Garrigus, U. 1971. Effects of aldehyde treatments of soybean meal on in vitro ammonia release, solubility and lamb performance. J. Nutr. 101, 605-611 https://doi.org/10.1093/jn/101.5.605. [ Links ]
Poos-Floyd, M., Klopfenstein, T., & Britton, R. A. 1985. Evaluation of laboratory techniques for predicting ruminal protein degradation. J. Dairy Sci. 68, 829-839 https://doi.org/10.3168/jds.S0022-0302(85)80900-0. [ Links ]
Pratihar, S. K., & Walli, T. K. 1995. Comparative effect of formaldehyde treatment of groundnut cake and mustard cake on growth performance of kids. Page 46 in Proceedings of VIIIth Animal Nutrition Research Workers' Conference. 7-9 Dec 1995, Mumbai, Compendium II. [ Links ]
Rodehutscord, M., Young, P., Phillips, N., & White, C. L. 1999. Wool growth in Merino wethers fed lupins untreated or treated with heat or formaldehyde, with and without a supplementation of rumen protected methionine. Anim. Feed Sci. Technol. 82, 213-226 https://doi.org/https://doi.org/10.1016/S0377-8401(99)00108-X. [ Links ]
SAS Institute Inc. 2016. SAS/SHARE® 9.4: User's Guide, Second Edition. Cary, United States of America. [ Links ]
Shannak, S., Südekum, K. H., & Susenbeth, A. 2000. Estimating ruminal crude protein degradation with in situ and chemical fractionation procedures. Anim. Feed Sci. Technol. 85, 195-214 https://doi.org/10.1016/S0377-8401(00)00146-2. [ Links ]
Spears, J., Hatfield, E., & Clark, J. 1980. Influence of formaldehyde treatment of soybean meal on performance of growing steers and protein availability in the chick. J. Anim. Sci. 50 https://doi.org/10.2527/jas1980.504750x. [ Links ]
Subuh, A. M. H., Rowan, T. G., & Lawrence, T. L. 1994. Effect of heat or formaldehyde treatment and differences in basal diet on the rumen degradability of protein in soyabean meal and in rapeseed meals of different glucosinolate content. Anim. Feed Sci. Technol. 49, 297-310. [ Links ]
Wales, A., Allen, V., & Davies, R. 2009. Chemical treatment of animal feed and water for the control of Salmonella. Foodborne Pathog. Dis. 7, 3-15 https://doi.org/10.1089/fpd.2009.0373. [ Links ]
Submitted 11 October 2022
Accepted 25 January 2022
Published 11 April 2023
# Corresponding author: Tertius.Brand@westerncape.gov.za














