Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Animal Science
versão On-line ISSN 2221-4062
versão impressa ISSN 0375-1589
S. Afr. j. anim. sci. vol.53 no.1 Pretoria 2023
http://dx.doi.org/10.4314/sajas.v53i1.06
Evaluation of Phyllanthus niruri L. powder on growth performance, haematology, and intestinal morphology of broilers
T. PasaribuI, #; M. SukirmanII; Y. SaniIII; B. BakrieI; S. RusdianaI
IResearch Center for Animal Husbandry, National Research and Innovation Agency Cibinong Science Center, Bogor District 16915, West Java, Indonesia
IIRespati University Indonesia, Jl. Bambu Apus I No.3, East Jakarta, Jakarta, Indonesia, 13890
IIICenter for Biomedical Research, National Research and Innovation Agency Cibinong Science Center, Bogor District 16915, West Java, Indonesia
ABSTRACT
The objective of this study was to evaluate the dietary supplementation of Phyllanthus niruri L. powder (meniran powder, MeP) on the productivity, haematology profiles, and intestinal morphology of broilers. A total of 200 female, one-day-old chickens were allocated to five treatments randomly, with four replications (10 birds per replicate): control, without antibiotic and MeP; T2, Zn-bacitracin 0.05%; T3, MeP 0.02%; T4, MeP 0.03%; and T5, MeP 0.05%. A completely randomized design was employed. The results showed that MeP supplementation at 0.02%, 0.03%, and 0.05% did not affect the body weight, feed intake, feed conversion ratio, haemoglobin, packed cell volume; and lymphocyte, red blood cell, heterophil, monocyte, eosinophil, and basophil counts, but at 0.03% and 0.05%, appeared to reduce white blood cells (WBCs) compared to those in the control and Zn-bacitracin groups. Microscopically, MeP supplementation in the diet increased the height and cellular growth of the mucosal villi of the duodenum, jejunum, and ileum, but the villi appeared fragile as mucosal epithelial cell necrosis was noted in the taller villi. Thus, it is concluded that MeP did not affect broiler performance and haematology profiles, except that the number of WBCs at 0.03% and 0.05% was less than that in the control and Zn-bacitracin groups.
Keywords: broiler, haematology profiles, intestinal morphology, Phyllanthus niruri L. powder.
Introduction
Antibiotic growth promoters (AGPs), such as Zn-bacitracin, are generally mixed into animal feed at small doses (sub-therapeutic levels) to suppress stress, enhance immunity, and/or prevent diseases and optimize nutrient absorption in the intestinal wall, thus improving feed efficiency and product quality (meat, eggs, milk) so that they have a positive impact on poultry performance. The intestine must be a defence to prevent the entry of pathogenic microbes into the intestinal mucus layer, using beneficial microbiota and immunoglobulin A (IgA) produced by the host to maintain optimal nutrient absorption in the intestine (Broom, 2018). AGPs play a role in reducing pathogenic bacteria in the intestine by increasing the population of beneficial microbiota (such as Lactobacillus spp.) (Kulkarni et al., 2022), which has a positive impact on bird performance. The negative effects of AGPs include the presence of residual effects in animal products, such as meat and eggs, and the induction of antibiotic resistance against bacteria (Manyi-Loh et al., 2018).Therefore, the use of AGPs has been banned in many countries.
Since January 2018, the use of AGPs in poultry diets has been effectively prohibited by the government of Indonesia. The regulation of the Minister of Agriculture of the Republic of Indonesia (number 14/PERMENTAN/PK.350/5/2017) concerning the classification of animal drugs as referred to in Article 16 (1a, b and 2) states that every person is prohibited from using feed ingredients mixed with certain hormones or antibiotics as feed additives. Therefore, it is necessary to develop an alternative approach using other materials with functions similar to AGPs that is safe and healthy for the livestock and consumers.
Many bioactive plants have been developed to replace antibiotics. Phyllanthus niruri L. is reported to contain flavonoids, lignans, tannins, coumarins, terpenes, phenylpropanoids, and saponins and has been used as a medicinal herb (Bagalkotkar et al., 2006; Rusmana et al., 2017). The most dominant bioactive compounds in Phyllanthus sp. are hypophyllanthin, catechin, epicatechin, rutin, and quercetin, and chlorogenic, ellagic, caffeic acid, malic, and gallic acids (Mediani et al., 2017). These compounds have been reported to have antibacterial properties (Mujeeb et al., 2014). An in vitro study showed that the combination of P. niruri L. extract, Anacardium occidentale shell liquid smoke, and Syzygium aromaticum leaf extract could inhibit the growth of E. coli and Salmonella sp. (Pasaribu et al., 2018). Phyllanthus niruri L. extract inhibited the growth of E. coli, which was indicated by a clear zone at 19.3 mm (Lestariningsih et al., 2015a). Phyllanthus niruri L. powder at a level of 1.2% can minimize E. coli in the broiler intestine (Lestariningsih et al., 2015b). It was also reported that 65% P. niruri L. extract at a dose of 1 ml/kg body weight (BW), administered orally for 7 days reduced white blood cells (WBCs) in chickens infected with Mycoplasma gallisepticum (Hidanah et al., 2018). Further, 1% of P. niruri L. crude powder could improve the BW of broilers (Jagadeeswaran & Selvasubramanian, 2014). Sundaresan et al. (2007) reported that 1% of P. niruri L. powder added to chicken rations containing aflatoxins B1 (100 ppb) showed better performance than chickens without the 1% P. niruri L. powder. Phyllanthus niruri L. extract at a concentration of 65% at a dose of 1 ml/kg BW decreased the total number of WBCs in broilers infected with M. gallisepticum bacteria (Hidanah et al., 2018). The administration of 10 g/kg Phyllanthus amarus powder to chickens not infected with bacteria decreased the number of leucocytes (WBCs) but increased the red blood cell (RBC) count (Unigwe et al., 2020). Thanabal et al. (2020) reported that the addition of 1% P. niruri L. powder to guinea fowl did not affect the number of WBCs and RBCs.
Information on the effect of P. niruri L. powder on the intestinal morphology of chickens is still limited, but the treatment of Wistar rats with P. amarus at a dose of 400-800 mg/kg BW caused some distortion and signs of inflammation in gastric and duodenal epithelial cells (Adjene et al., 2011). However, the administration of P. niruri L. powder up to a dose of 0.05% of the ration did not affect the weight and length of the intestine (Pasaribu et al., 2021). Therefore, the current study was conducted to evaluate the effect of P. niruri L. powder supplementation on the productivity, haematological profile, and changes in intestinal morphology in broilers.
Materials and Methods
The use of animals in this study was approved by the Indonesian Center for Animal Research and Developmental Experimental Animal Welfare Commission, Indonesian Agency for Agricultural Research and Development: Animal Welfare and Experiments (No. 1806.107.001.052/L-2/APBN 2016). The P. niruri L. plants were collected from North Bogor, West Java, Indonesia. The plants were first dried in an oven at 60 °C for 4-5 days; then, the dried plants were ground and filtered using a 60-mesh sieve to obtain 250 μηι of P. niruri L. powder (meniran powder, MeP), with a moisture content of 3.94%.
This biological study was carried out for 28 days and was a completely randomized design. A total of 200 female, one-day-old broiler chickens of Ross MB 202 RSX (yellowish white feathers, single comb, and yellow legs) were allocated to five treatments randomly, with four replications (10 birds per replicate). The five treatment groups consisted of T1: control/without antibiotic and MeP; T2: Zn-bacitracin 0.05%; T3: MeP 0.02%; T4: MeP 0.03%; and T5: MeP 0.05%. Zn-bacitracin treatment was administered by mixing it in the ration of the chickens. The method was that Zn-bacitracin was mixed into 500 g of feed ingredients and stirred until homogeneous; this mixture was named X. After it was homogeneous, X was then mixed into 1 kg of ration and stirred again until homogeneous; this was named Y. Finally, Y was mixed into the basal diets until homogeneous, and ready to be used as T2 treatment. In this way, animal welfare was maintained even in different phases of growth. The animals were reared in a deep-litter poultry house. The animal house was cleaned and disinfected before use. Drinking water and feed were provided ad libitum throughout the experimental period. Parameters of BW gain and feed consumption were recorded weekly. Animals that were found dead were recorded and were necropsied immediately to observe pathological changes and to determine the cause of death.
The composition and chemical analyses of the basal diet are presented in Table 1, and the chemical composition of MeP is presented in Table 2.
Blood was collected randomly at the end of the trial from two birds in each replication with an equal BW (eight birds per treatment) for haematological analyses, including RBC count, packed cell volume (PCV), and WBC count, following the method described by Dutta et al. (2013); and haemoglobin concentration, using the Sahli method (Patil, 2013). Differentiation of WBCs was also conducted for lymphocytes, monocytes, heterophils, eosinophils, and basophils (Gandasoebrata, 2010). The blood was collected from the auricularis vein using 1 ml syringes and 26G χ 1/2" needles. The blood was immediately transferred into a test tube containing EDTA for further analysis.
Necropsies of birds were conducted at the end of the trial after they were sacrificed by exsanguination at both large blood vessels for pathological examination. Intestinal tracts were collected for microscopic examinations and fixed in 10% buffered neutral formalin. The tissues of the duodenum, jejunum, and ileum were stained using hematoxylin and eosin and examined under a light microscope following the method described by Henwood (2017).
All data were analysed using analysis of variance (ANOVA) in a completely randomized design with SAS (version 9.1. SAS Institute Inc., Cary). When the ANOVA was significant, the means were separated using Duncan's multiple range test at α = 0.05.
Results and Discussion
MeP supplementation at a level of 0.02-0.05% in broiler chickens did not affect (P >0.05) their BW compared to the control and Zn-bacitracin groups (Table 3). The greatest weight gain was seen in the Zn-bacitracin group, whereas the highest feed intake and feed conversion ratio (FCR) were in the MeP 0.02% group (T3). It is speculated that the bioactive compounds hypophyllanthin, catechin, epicatechin, rutin, quercetin, and chlorogenic, ellagic, caffeic, malic, and gallic acids in 0.05% MeP improved performance. This means that the provision of up to 0.05% MeP to broilers has no negative effect. Jagadeeswaran & Selvasubramanian (2014) reported that P. niruri at a dose of 1% could improve the broiler's performance. Phyllanthus amarus powder (0.25-1.5%) did not substantially affect growth performance (Phuong & Thie, 2012). The mixture of P. niruri and garlic encapsulated at a dose of 0.8% improved the performance of broilers (Natsir et al., 2013). Phyllanthus niruri extract singly inhibited the growth of E. coli, with an inhibition zone of a diameter of 19.3 mm in vitro (Lestariningsih et al., 2015a). The combination of P. niruri extract, A. occidentale shell liquid smoke, and S. aromaticum leaf extract inhibited the growth of E. coli and Salmonella sp. in vitro (Pasaribu et al., 2018). The effect of P. niruri at a level of 1.2% inhibited the growth of E. coli in the broiler intestine (Lestariningsih et al., 2015b). Escherichia coli and the host compete to absorb nutrients in the intestine, thus, the E. coli population must be eliminated so that the absorption of nutrients can be maximized by the host. The decline in the population of pathogenic bacteria in the gastrointestinal tract provides opportunities for gut walls to absorb nutrients, conferring a more positive impact on the performance of chickens. This indicates that a dose of up to 0.05% MeP is safe to use as a natural feed additive for broilers. Another study reported that the supplementation of pine needles and vitamin E powder in feed improved the performance of female Japanese quail (Khan et al., 2019) but Pinus ponderosa leaf supplementation in Japanese quail feed did not affect performance (Shah et al., 2019). This indicates that not all plant bioactive substances can improve performance. Supplementation of glutamine and glucose in chickens under heat stress markedly improved the pectoralis major muscle of broilers, which had a positive impact on body weight gain (Hu et al., 2016). The provision of vitamin E at 100 mg/kg of feed did not affect the performance of Japanese quails reared under low ambient temperatures but was still better than a dose of 150 mg/kg (Shah et al., 2016).
The feed intake of broilers was similar (P >0.05) among the control, Zn-bacitracin, and MeP (0.020.05%) supplementation groups (Table 3). This indicated that the addition of up to 0.05% MeP did not affect the feed intake, as well as the palatability. Palatability in cattle depends on the response to the sense of taste and the nervous system of the brain. In poultry, the number of taste receptors is less than that in mammals (Lamichhane et al., 2018), and feed rejection occurs only when spoilage or damage occurs. The addition of the 0.05% MeP dose did not affect the palatability of chickens.
There were no differences in MeP supplementation at doses of 0.02%, 0.03%, and 0.05% compared to the control and Zn-bacitracin supplementation in the FCR of broilers (P >0.05) (Table 3). Although not statistically different (P >0.05), a dose of 0.02% MeP (T3) showed an improvement in feed conversion of up to 4% compared to the control. When compared to the Zn-bacitracin-supplemented broilers (T2), the FCR in the birds in the T3 group improved feed conversion by 14%, in T4 by ~4.7%, and T5 by ~1.2%. Zn-bacitracin is included as an AGP in small amounts to improve feed efficiency, growth, and animal health (Danzeisen et al., 2011). The 0.02% MeP supplementation supported a better feed efficiency than the control and the Zn-bacitracin groups. As a feed additive for broiler growth, the MeP dose was less (0.02%) than the Zn-bacitracin supplementation (0.05%). Reduced pathogenic microbes in the intestine enable the host to increase nutrient absorption in the intestine, resulting in increased growth. MeP at a dose of 0.02% resulted in a better effect on feed conversion than the supplementation of the antibiotic, Zn-bacitracin, indicating that MeP can replace Zn-bacitracin
supplementation. Feeding chickens with 1% of P. niruri L. for four weeks showed a better FCR than the negative control ration (Jagadeeswaran & Selvasubramanian, 2014).
Although MeP supplementation at doses of 0.02-0.05% did not affect (P >0.05) the BW and FCR compared to the control and Zn-bacitracin groups, its use at those doses is still beneficial to poultry farmers because it could be used as a natural feed additive to prevent the growth of Eimeria tenella parasites. Intestinal histopathological test results proved that coccidia was found only in the control and Zn-bacitracin treatment groups, but not in groups supplemented with MeP at doses of 0.02%, 0.03%, and 0.05%. Khan et al. (2022) reported that the dosage of fennel seed in broiler feed was variable. Similarly, supplementation with MeP still needs additional research because of the variable dosage. Improved growth performance and feed conversion ratio can be obtained by supplementing sodium diacetate with up to 0.03-0.05% in the diet (Wen et al., 2017). However, referring to the current study and others (Sundaresan et al., 2007; Jagadeeswaran & Selvasubramanian, 2014), to increase BW and feed efficiency, not more than 1 % of MeP supplementation is recommended. Histopathological pictures of the intestine, liver, and kidneys should be reviewed for animal welfare purposes.
Five of the 200 broilers died during this study (2.5%). Mortality occurred in the first 7 days, but from day 8 to the end of the study, no mortality occurred. The mortality was still below the standard (3%).
The erythrocyte, eosinophil, heterophil, lymphocyte, monocyte, and haematocrit values did not differ (P > 0.05) among the MeP doses of 0.02%, 0.03%, and 0.05% compared to the control and Zn-bacitracin supplementation (Table 4). Basophils were not detected because there were very few in the blood. The number of erythrocytes was 2.8863-3.0663 χ 106/mm3 in this study at MeP doses of 0.20.5%. The MeP treatments at 0.02% and 0.03% tended to increase the number of erythrocytes; it is assumed that at these doses, the content of the MeP bioactive substances supports the formation of erythrocytes. The erythrocyte count decreased slightly at 0.05% MeP, which indicates that a high MeP dose does not support erythrocyte formation. Although it decreased slightly, the numbers were still within the normal limits of erythrocytes in broilers, at 2.3-3.5 χ 106/mm3 (Wakenell, 2010). Phyllanthin, as the dominant bioactive substance in P. niruri L., does not affect the number of erythrocytes. This indicates that MeP up to a dose of 0.05% in the rations does not have a negative effect on erythrocyte production. Several factors that affect the RBC count in chickens include age, sex, and nutritional status (Elagib et al., 2011). Another factor that affects the number of erythrocytes is active compounds, such as saponins, tannins, and flavonoids, at high concentrations (Nijveldt et al., 2001).
Haemoglobin and haematocrit (PCV) values were not affected by MeP supplementation up to a dose of 0.05% in chicken rations. At this MeP dose, the amount of haemoglobin was between 11.38% and 11.71% and the PCV was between 29.94% and 30.30%. Normal haemoglobin levels in chickens are 7.0-13.0% (Wakenell, 2010), and the normal PCV range in chickens is between 24% and 43% (Samour, 2006). Thus, the haemoglobin and haematocrit values (PCV) were within the normal ranges.
The number of WBCs in the 0.03% and 0.05% MeP treatment groups was lower than the numbers in the other treatments (P <0.05). This indicates that a dose of MeP of 0.03% and 0.05% affects the number of WBCs. The difference (P <0.05) between the MeP (0.03% and 0.05%) and Zn-bacitracin treatments indicates that the higher the dose of bioactive substances, the more the leukocyte count is influenced. Asare et al. (2011) reported that the administration of an aqueous extract of meniran at a dose of 2000 mg/kg in rats through drinking water also reduced the number of WBCs compared to controls. The normal levels of WBCs in rats range from 6-40 χ 103/mm3 (Smith & Mangkoewidjo, 1988). Although the WBC numbers in the 0.03% and 0.05% MeP treatment groups were low, they were still within the normal limits. The number of WBCs is influenced by sex, environment, drugs, presence of disease or inflammation, environment, age, and nutritional content of the feed (Suriansyah et al., 2016). Phyllanthus buxifolius leaf powder supplementation in the rations of quail reduced WBCs (Wardah et al., 2017). Phyllanthus niruri extract lowered the number of WBCs in chickens infected with M. gallisepticum (Hidanah et al., 2018). Bioactive substances of Sapindus rarak also reduced the number of WBCs (Pasaribu et al., 2015). The bioactive substance in rosemary, rosmarinic acid, which was supplemented in broiler diets, could interact and modulate the humoral immunity of broilers (Rostami et al., 2017). Elnaggar et al. (2016) reported that rosemary markedly increased the white blood cell count. This indicates that bioactive substances from plants affect the number of WBCs in chicken blood.
Leukocyte differentiation was not different between the control, Zn-bacitracin, and MeP treatment groups, but overall levels were increased compared to the normal levels (P >0.05) (Table 4). Differences in the leukocyte values are due to several factors, including sex, environment, drugs, presence of disease or inflammation, environment, age, and nutritional content of the feed (Samour, 2006; Suriansyah et al., 2016). Agranulocyte lymphocytes form the highest component of WBCs in poultry blood (Sturkie & Grimminger, 1976; Samour, 2006). Lymphocytes, heterophils, monocytes, eosinophils, and basophils play a role in recognizing the microbes in immune reactions and help the inflammation and healing processes (Guyton & Hall, 2013). The main function of lymphocytes is to form antibodies in response to the presence of antigens (foreign objects). The lymphocytes produced in the thymus increase rapidly when foreign antigens enter the body (Tizard, 2017). MeP extract at a concentration of 30% reduced the number of lymphocytes in the blood of broiler chickens infected with the enterotoxin, E. coli (Wahjuni et al., 2017).
In the current study, MeP up to a dose of 0.05% and Zn-bacitracin treatment did not impact the number of lymphocytes and monocytes (P >0.05). This showed that MeP did not affect lymphocyte production, so it is safe for use in chickens. Monocytes obtained the same result: MeP did not affect the action of monoblasts for monocyte production. Arabinogalactan obtained from processed P. niruri L. tea had immunological properties in rat peritoneal macrophages (Mellinger et al., 2008). Phyllanthus niruri L. has immunomodulatory, antiviral, antibacterial, antihyperglycemic, and hepatoprotective properties (Lee et al., 2016). MeP at low doses has the ability to stimulate the formation of antibodies for adjuvant activity (Francis et al., 2002). The normal percentage of lymphocytes in chickens is ~34% (Merck Veterinary Manual, 2011). The number of lymphocytes in the 0.05% MeP treatment was higher than that in the 0.02% MeP treatment, whereas the 0.02% MeP treatment showed the same lymphocyte number as the Zn-bacitracin treatment. Thus, the higher the MeP dose, the higher the lymphocyte number.
The number of heterophils in the MeP treatment was similar to the Zn-bacitracin treatment (P >0.05). The higher the MeP dose, the lower the heterophil number. Provision of up to 0.05% MeP showed that the number of heterophils was 32.75-48.50%. The normal number of heterophils is 2530% of WBCs (Swenson, 1984; Hodges, 1997). This indicates that the bioactive substances in MeP increase heterophil production in the broiler. The functions of heterophils include finding, digesting, and eliminating foreign materials, and they are the first line of defence (Guyton & Hall, 2013). The eosinophil number increases during parasitic infection; basophils are responsible for inflammation. If there is inflammation, the basophil count increases. MeP at doses of 0.02%, 0.03%, and 0.05% did not induce differences in monocytes and eosinophils from the control and Zn-bacitracin treatments (P >0.05).
Microscopic changes in the duodenum indicated an increase in the height of the epithelial cells of the mucosal villi, followed by necrosis, haemorrhage, infiltration of mononuclear cells (mainly lymphocytes and macrophages), and an increased number of goblet cells. The height of the mucosal villi was increased in treatment T1 but not in treatments T2, T3, T4, and T5. Necrosis, haemorrhage, and mononuclear cell infiltration (lymphocytes and macrophages) of mucosal villi were found in all treatments, whereas coccidia were found only in T1 and T2 (Figures 1 and 2; Table 5), but not in T3, T4, and T5 (Figures 3-5; Table 5). Numerous goblet cells were found in T2, T3, and T5.
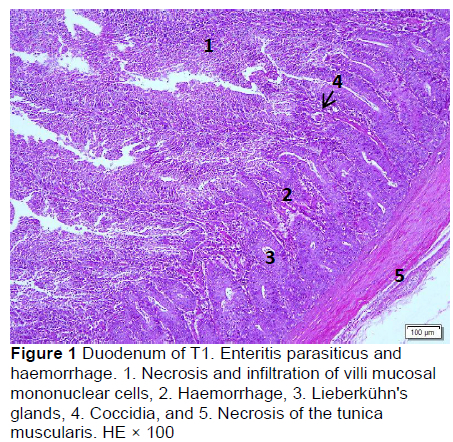
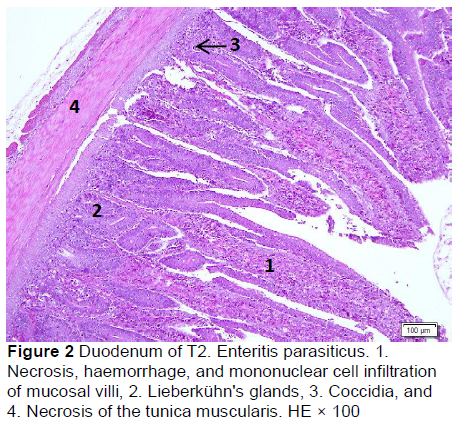
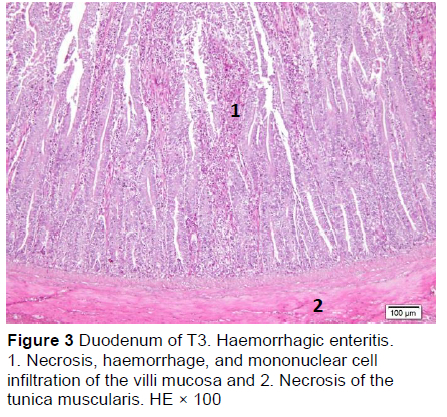
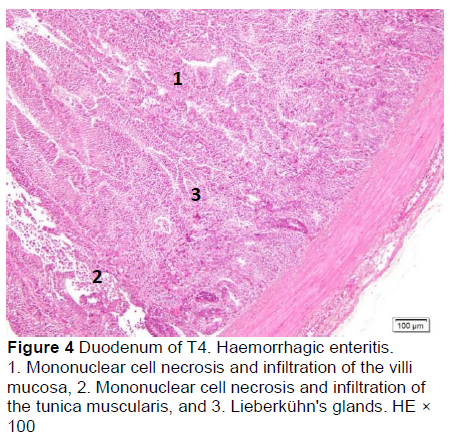
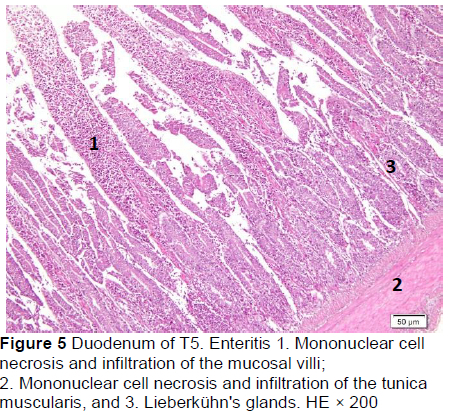
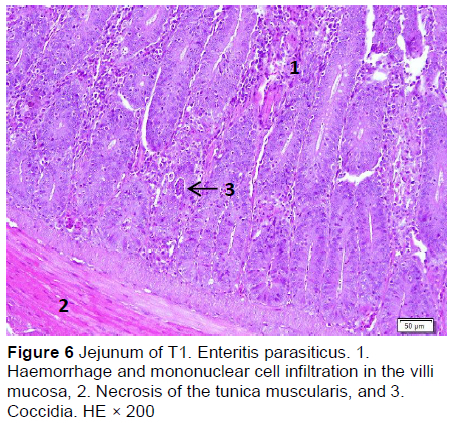
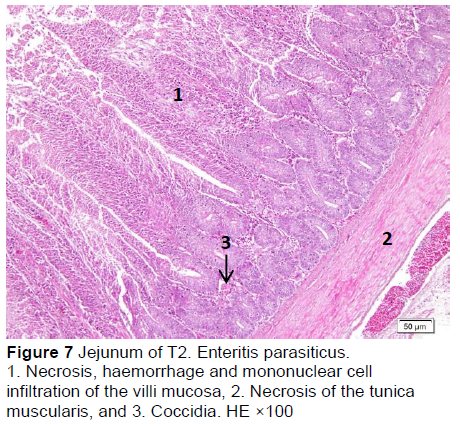
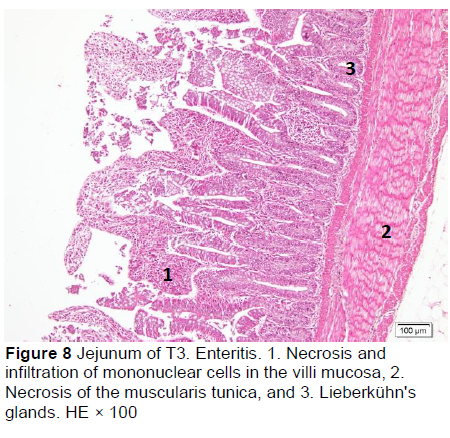
Similar microscopic lesions were found in the jejunum, such as necrosis of the villi mucosa and muscular layers, haemorrhage, infiltration of mononuclear cells (lymphocytes and macrophages), and goblet cells in all treatments (Figures 6-10; Table 5). Coccidial infection in the jejunum was found in T1 and T2.
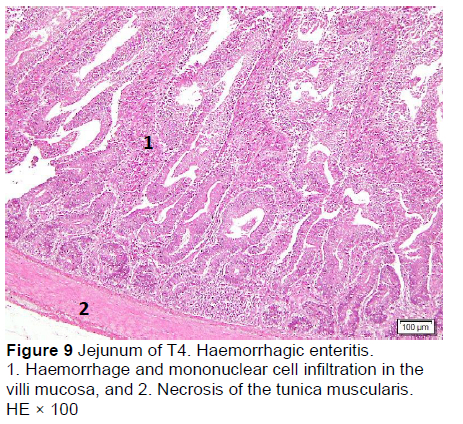
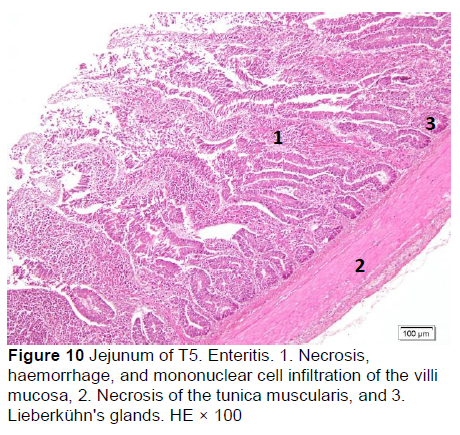
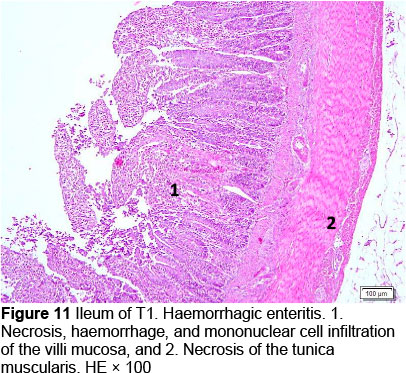
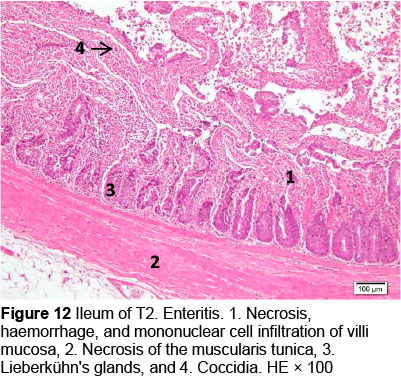
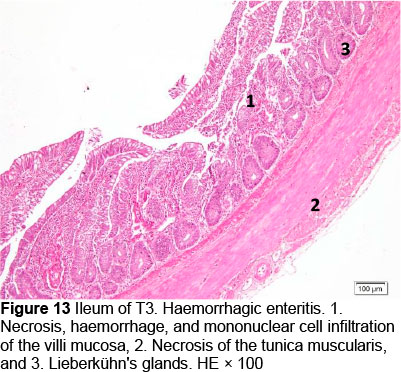
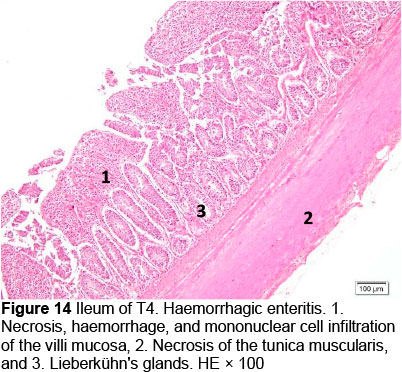
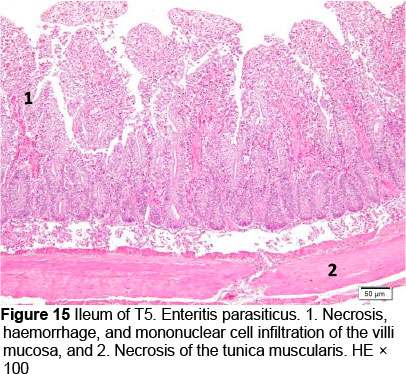
Microscopic changes noted in the ileum consisted of necrosis of the villi mucosa and tunica muscularis, haemorrhage, and infiltration of mononuclear cells (lymphocytes and macrophages) in all treatments. The presence of necrosis, haemorrhage, and infiltration of mononuclear cells, particularly lymphocytes and macrophages, may be due to an excessive growth of the mucosal villi; the epithelial cells become vulnerable to digestive activities (Cooper et al., 2013). Goblet cells were found in T2, T3, T4, and T5, whereas infiltration of neutrophils was seen only in T1. It appeared that the MeP supplementation up to 0.05% may not only stimulate the increase in mucosal villi height but also causes the epithelial cells of the villi mucosa to become vulnerable to digestive activities, particularly when the height of the mucosal villi is over a certain limit. Therefore, necrosis, haemorrhage, and infiltration of mononuclear cells were present in all treated animals. However, these microscopic changes should be investigated further.
The presence of coccidia in T1 and T2 (Figures 1 and 2; Table 5) can be explained; the broiler pens used in this study were used previously for an Eimeria sp. study. The residues of coccidia may have been present in the pen as they can survive outside the host. However, the coccidia was not found in T3, T4, and T5 (Figures 3-5; Table 5). Other infections, such as necrotic enteritis and coccidiosis, may also be involved in these pathological changes as secondary infections. Coccidia were not detected in chickens treated with MeP, indicating that the bioactive substance of phyllanthin in the MeP diet may prevent and inhibit the development of coccidia in the intestinal tract.
Conclusion
Meniran (P. niruri L.) powder did not affect the performance and haematology profiles of the broilers, except that the number of WBCs at 0.03% and 0.05% MeP was less than that in the control and Zn-bacitracin groups. Microscopically, we showed that coccidial growth may be inhibited in the duodenum, jejunum, and ileum.
Acknowledgment
My gratitude to the State Budget (APBN) of Indonesia, which supported this research. Thanks also to Tetty Hastuti and Nur Rochman Wibowo who supported funding, and Endang Wahyu who helped technically in the experimental laboratory.
Author's contributions
TP, YS, and MS conducted the experiments. TP, MS, and SR performed statistical analyses of the data in the experiments. TP conceived, wrote, and revised the manuscript. BB and YS revised the manuscript and all the authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
References
Adjene, J.O., Abudu, I.E. & Nwose, E.U., 2011. Histological effects of chronic administration of aqueous extract of Phyííanthus amarus on the stomach and the duodenum of Wistar rats. Electron J. Biomed. 1, 9-13. https://biomed.uninet.edu/2011/n1/adjene.html [ Links ]
Asare, G.A., Addo, P., Bugyei, K., Gyan, B., Adjei, S., Otu-Nyarko, L.S., Wiredu, E.K. & Nyarko, A., 2011. Acute toxicity studies of aqueous leaf extract of Phyííanthus niruri. Interdiscip. Toxicol. 4, 206-210. doi: 10.2478/v10102-011-0031-9 [ Links ]
Bagalkotkar, G., Sagineedu, S.R., Saad, M.S. & Stanslas, J., 2006. Phytochemicals from Phyííanthus niruri Linn. and their pharmacological properties: A review. J. Pharm, Pharm. 58,1559-1570. DOI 10.1211/jpp.58.12.0001 [ Links ]
Broom, L.J., 2018. Gut barrier function: Effects of (antibiotic) growth promoters on key barrier components and associations with growth performance. Poult. Sci. 97, 1572-1578. http://dx.doi.org/10.3382/ps/pey021 [ Links ]
Cooper, K.K., Songer, J.G. & Uzal, F.A., 2013. Diagnosing clostridial enteric disease in poultry. J. Vet. Diagn. Invest. 25, 314-327. DOI: 10.1177/1040638713483468 [ Links ]
Danzeisen, J.L., Kim, H.B., Isaacson, R.E., Tu, Z.J. & Johnson, T.J., 2011. Modulations of the chicken cecal microbiome and metagenome in response to anticoccidial and growth promoter treatment. PLoS One. 6(11), e27949. doi: 10.1371/journal. pone.0027949 [ Links ]
Dutta, R.K., Islam, M.I. & Md. Ashraful Kabir, M.A., 2013. Haematological and biochemical profiles of gallus indigenous, exotic and hybrid chicken breeds (Gaííus domesticus L.) from Rajshahi, Bangladesh. Bangladesh J. Zool. 41, 135144. DOI: 10.3329/bjz.v41i2.23314 [ Links ]
Elagib, H.A.E. & Ahmed A.D.A., 2011. Comparative study on haematological values of blood of indigenous chickens in Sudan. Asian J. Poul. Sci. 5, 41-45. DOI: 10.3923/ajpsaj.2011.41.45 [ Links ]
Elnaggar, A.S., Abdel-Latif, M.A., El-Kelawy, M.I. & ELHamid, H.S.A., 2016. Productive, physiological, and immunological effect of rosemary leaf meal (Rosmarinus officinaíis) supplementation in broiler diets. Egypt. Poult. Sci. 36, 859-873. [ Links ]
Francis, G., Kerem, Z., Makkar, H.P.S. & Becker, K., 2002. The biological action of saponins in animal systems: A review. British J. Nut., 88, 587-605. DOI: 10.1079/BJN2002725 [ Links ]
Gandasoebrata, R., 2010. Clinical Laboratory Guide. Jakarta. Dian Rakyat. p 25-30. [ Links ]
Guyton, A.C. & Hall, J, E., 2013. Textbook of Medical Physiology. Jakarta (ID): Medical Book Publisher. 12th Edition. EGC. Jakarta. Pp 1172. [ Links ]
Hidanah, S., Sabdoningrum, E.K., Wahjuni, R.S. & Chusniati, S., 2018. Effects of meniran (Phyííanthus niruri L.) administration on leukocyte profile of broiler chickens infected with Mycopíasma gaííisepticum. Vet. World., 11, 834839. doi: 10.14202/vetworld.2018.834-839 [ Links ]
Henwood, A.F., 2017. Hematoxylin and eosin staining of mucins of the gastrointestinal tract. J. Histotechnol. 40, 21-24. doi.org/10.1080/01478885.2017.1264556 [ Links ]
Hodges, R.D., 1997. Normal Avian (Poultry) Haematology. Comparative Clinical Haematology. Oxford: Blackwell Scientific Publications. Pp. 737. [ Links ]
Hu, H., Bai, X., Wen, A., Shah, A. A., Dai, S., Ren, Q., Wang, S., S. He, S. & Wang, L., 2016. Assessment of interactions between glutamine and glucose on meat quality, AMPK, and glutamine concentrations in pectoralis major meat of broilers under acute heat stress. J. Appl. Poult. Res. 25, 370-378. http://dx.doi.org/10.3382/japr/pfw021 [ Links ]
Jagadeeswaran, A. & Selvasubramanian, S., 2014. Growth promoting potentials of indigenous drugs in broiler chicken. Int. J. Adv. Vet. Sci. and Technol. 3, 93-98. http://www.notoare.com/13427505 [ Links ]
Khan, I.U., Shah, A.A., Sahibzada, F.A., Azam Hayyat, A., Nazar, M., Mobashar, M., Tariq, A. & Nighat Sultana, N., 2019. Carcass characteristics and serum biochemical profile of Japanese quail by the supplementation of pine needles and vitamin E powder. Biologia. 1-8. https://doi.org/10.2478/s11756-019-00225-y [ Links ]
Khan, R.U., Fatima, A., Naz, S., Ragni, M., Tarricone, S., & Tufarelli, V., 2022. Perspective, opportunities, and challenges in using fennel (Foeniculum vulgare) in poultry health and production as an eco-friendly alternative to antibiotics: A review. Antibiotics. 11, 278. https://doi.org/10.3390/antibiotics11020278 [ Links ]
Kulkarni, R.R., Gaghan, C., Gorrell, K., Sharif, S. & Taha-Abdelaziz K., 2022. Probiotics as alternatives to antibiotics for the prevention and control of Necrotic enteritis in chickens. Pathogens. 11, 692:1-20. doi: 10.3390/pathogens11060692 [ Links ]
Lamichhane, U., Saroj Regmi, U.S. & Sah, R., 2018. Changes in palatability of poultry feed using garlic, ginger, and their combination. Acta Sci. Agric. 2, 68-72. [ Links ]
Lee, N.Y., Khoo, W.K., Adnan, M.A., Mahalingam, T.P., Fernandez, A.R. & Jeevaratnam, K., 2016. The pharmacological potential of Phyllanthus niruri. J. Pharma. and Pharma. 68, 953-969. DOI: 10.1111/jphp.12565 [ Links ]
Lestariningsih, Sjofjan, O. & Sudjarwo, E., 2015a. Pengaruh tepung tanaman meniran terhadap aktivitas antimikroba bakteri asam laktat dan Escherichia coli. J. Ilmu-Ilmu Pet. 25, 55-60. [ Links ]
Lestariningsih, Sjofjan, O., & Sudjarwo, E., 2015b. Pengaruh tepung tanaman meniran (Phyllanthus niruri Linn) sebagai pakan tambahan terhadap mikroflora usus halus ayam pedaging. J. Agripet. 15, 85-91. [ Links ]
Manyi-Loh, C., Mamphweli, S., Meyer, E. & Okoh, A., 2018. Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Review. Molecules. 23, 1-48. doi: 10.3390/molecules23040795 [ Links ]
Mediani, A., Abas, F., Maulidiani, M., Khatib, A., Tan, C.P., Ismail, I.S., Shaari, K. & Ismail, A., 2017. Characterization of metabolite profile in Phyllanthus niruri and correlation with bioactivity elucidated by nuclear magnetic resonance based metabolomics. Molecules. 22, 1-14. doi:10.3390/molecules22060902 [ Links ]
Mellinger, C.G., Cipriani, T.R., Noleto, G.R., Carbonero, E.R., Oliveira, M.B.M., Gorin, P.A.J. & Iacomini, M., 2008. Chemical and immunological modifications of an arabinogalactan present in tea preparations of Phyllanthus niruri after treatment with gastric fluid. Int. J. Biol. Macromol. 43, 115-120. DOI: 10.1016/j.ijbiomac.2008.04.001 [ Links ]
Mujeeb, F., Bajpai, P. & Pathak N., 2014. Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos. BioMed. Res. Int. 2014, 1-14. doi.org/10.1155/2014/497606 [ Links ]
Merck Veterinary Manual, 2011. Haematological and serum biochemical reference guides. In Merck Veterinary Manual. 10th ed. Fielder, S.E. (ed). Online version. Merck Sharp & Dohme Carp. A subsidiary of Merck & Co., Inc. Whitehouse Station. NJ. USA. [ Links ]
Natsir, M.H., Hartutik, Sjofjan, O. & Widodo, E., 2013. Effect of either powder or encapsulated form of garlic and Phyllanthus niruri L. mixture on broiler performances, intestinal characteristics, and intestinal microflora. Int. J. Poult. Sci. 12, 676-680. DOI: 10.3923/ijps.2013.676.680 [ Links ]
Nijveldt, R.J., Nood, E.V., Hoorn, D.E.V., Boelens, P.G., Norren, K.V. & Leeuwen, P.A.V., 2001. Flavonoids. A Review. Probable mechanism of action and potential application. The American J.Clinic Nut. 74, 418-425. doi: 10.1093/ajcn/74.4.418 [ Links ]
Pasaribu, T., Wina, E., Sumiati, Setiyono, A. & Astuti. D. A., 2015. The effect of S. rarak microparticles on blood profile and productivity of broiler chickens raised on litter system inoculated with E. tenella The 5th International Conference on Sustainable Animal Agriculture for Developing Countries. 445-447. [ Links ]
Pasaribu, T., Sinurat, A.P., Wina, E., Purwadaria, T., Haryati, T. & Susana, I.W.R., 2018. Effectiveness of bioactive combinations of several plant substances to inhibit the growth of Escherichia coli and Salmonella sp. JITV., 23(3): 112-122. doi.org/10.14334/jitv.v23i3.1851 [ Links ]
Pasaribu, T., Sukirman, M., Wibowo, N.R. & Kostaman, T., 2021. The influence of Phyllanthus niruri L. powder and Zn-bacitracin antibiotics on the relative weight of carcasses and intestines of broiler. IOP Conf. Series: Earth and Environmental Science 788 (2021) 012046,1 -8. doi: 10.1088/1755-1315/788/1/012046 [ Links ]
Patil, P.J., 2013. Variability and accuracy of Sahli's method in estimation of haemoglobin concentration. NJIRM. 4, 38-44. [ Links ]
Phuong, N.H. & Thieu, N.Q., 2012. Effect of feeding different Phyllanthus amarus powder concentrations in chicken diets on their growth performance and health. Proceedings of the International Conference Livestock - Based Farming Systems, Renewable Resources, and the Environment. 6-9 June 2012, Dalat, Vietnam. [ Links ]
Rostami, H., Seidavi, A., Dadashbeiki, M., Asadpour, Y., Simões, J., Shah, A.A., Laudadio, V., Losacco, C., Perillo, A. & Tufarelli V., 2018. Supplementing dietary rosemary (Rosmarinus officinalis L.) powder and vitamin E in broiler chickens: Evaluation of humoral immune response, lymphoid organs, and blood proteins. Environ. Sci. Pollut. Res. 1 -7. https://doi.org/10.1007/s11356-018-1209-x [ Links ]
Rusmana, D., Wahyudianingsih, R., Elisabeth, M., Balqis, B., Maesaroh, M. & Widowati, W., 2017. Antioxidant activity of Phyllanthus niruri extract, rutin, and quercetin. Indones. Biomed. J. 9, 84-90. DOI: 10.18585/inabj.v9i2.281 [ Links ]
Samour, J.H., 2006. Diagnostic value of haematology. In: Harrison, G.J., and Lightfoot, T.L. (eds). Clinical Avian Medicine Volume II. Palm Beach, Florida: Spix Publishing Inc., 587-610. [ Links ]
Shah, A.A., Khan, M.S., Khan, S., Ahmad, N., Alhidary, I.A., Khan, R.U. & Shao, T., 2016. Effect of different levels of alpha tocopherol on performance traits, serum antioxidant enzymes, and trace elements in Japanese quail (Coturnix coturnix japonica) under low ambient temperature. R. Bras. Zootec. 45, 622-626. doi.org/10.1590/S1806-92902016001000007 [ Links ]
Shah, A.A., Khan, I.U., Fayaz Ahmed Sahibzada, F.A.,Tauseef, I., Kalsoom, U. & Sultana, N., 2019. Biological and biochemical characteristics of male reproductive system, serum metabolites, and carcass quality of Japanese quails by the supplementation of Pinus ponderosa leaves and α-tocopherol acetate. Reprod. Dom. Anim. 00, 1-9. doi: 10.1111/rda.13521 [ Links ]
Smith, J. B. & Mangkoewidjojo, S., 1988. Pemeliharaan, Pembiakan Dan Penggunaan Hewan Percobaan di Daerah Tropis. Universitas Indonesia. Jakarta. [ Links ]
Sturkie, P.D. & Grimminger, P., 1976. Blood: Physical Characteristic, Formed Elements, Haemoglobin, and Coagulation in Avian Physiology. 3rd ed. Springer-Verlag New York, Inc, (USA): Heidelberg Berlin Press. Pp. 65. [ Links ]
Sundaresan, N.R., Thirumurugan, R., Jayakumar, S. & Purushothaman, M.R., 2007. Protective effectiveness of Phyllanthus niruri against short-term experimental aflatoxicosis in broiler chicken. Indian J. Poult. Sci., 42, 153-156. [ Links ]
Suriansyah., Ardana, I.B.K., Anthara, M. S. & Anggreni, L.D., 2016. Leukosit ayam pedaging setelah diberikan paracetamol. Indonesia Med. Vet. 5, 165-174. [ Links ]
Swenson, M.J., 1984. Duke's Physiology of Domestic Animals. 10th Ed. Publishing Associates, a Division of Cornell University. Ithaca and London. [ Links ]
Thanabal, C., Ramamurthy, N., Churchil, R.R., Gnanaraj, T.P. & Arivazhagan, M., 2020. Ameliorative effects of Phyllanthusniruri on haematological and serum biochemical profile of guinea fowls raised with aflatoxin-contaminated feed. J. Entomolo and Zool. Studies. 8, 1016-1020. [ Links ]
Tizard, I., 2017. Veterinary immunology. An Introduction. 10th ed. WB Saunders Co. Department of Veterinary Pathobiology, Texas A&M University, College Station, Texas. Pp 552. [ Links ]
Unigwe, C.R., Enibe, F., Igwe, K.K., Igwe, I.R., Stephen, N.O., Koleosho, S.A., Balogun, F.A., Shobowale, O.M. & Okonkwo, C.J.B., 2020. Effects of Phyllanthus Amarus (stone-breaker) leaf meal supplementation on haematology and serum biochemistry of broiler chickens. Direct Res. J. Biol. Biotechnol. 6, 57-63. DOI: https://doi.org/10.26765/DRJBB20724428 [ Links ]
Wahjuni, R.S., Diyantoro, Sabdoningrum, E.K. & Hidanah, S., 2017. Immunomodulation effect of meniran (Phyllanthus niruri Linn.) on blood profile of broiler chickens infected with enterotoxin of antibiotic-resistant Escherichia coli. Advances in Social Science, Education, and Humanities Research (ASSEHR), 98 1st International Conference Postgraduate School Universitas Airlangga: Implementation of Climate Change Agreement to Meet Sustainable Development Goals (ICPSUAS 2017). [ Links ]
Wakenell, P.S., 2010. Haematology of chicken and turkeys. In DJ. Weiss and KJ. Wardrop. (eds). Veterinary Haematology. 6th ed. John Wiley & Sons. Ames. Iowa. USA. p.957-967. [ Links ]
Wardah, J., Rahmahani. & Sopandi, T., 2017. Effect of Phyllanthus buxifolius leaf as a feed supplement on liver function and haematological response of quail (Coturnix coturnix japonica) challenged with infectious Newcastle disease virus. Int. J. Poult. Sci., 16, 354-363. DOI: 10.3923/ijps.2017.354.363 [ Links ]
Wen, A., Bai, X., Dai, S., Shah, A.A. & Hu, H., 2017. Effect of dietary sodium diacetate on growth performance, carcass characteristics, meat quality, intestinal pH, and Escherichia coli of broilers. Anim. Prod. Sci. 1-6.http://dx.doi.org/10.1071/AN15884** [ Links ]
Submitted 28 March 2022
Accepted 29 December 2022
Published 5 April 2023
# Corresponding author: pasaributiurma@yahoo.com














