Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Animal Science
On-line version ISSN 2221-4062
Print version ISSN 0375-1589
S. Afr. j. anim. sci. vol.53 n.1 Pretoria 2023
http://dx.doi.org/10.4314/sajas.v53i1.05
Serological and haemato-biochemical insights into bovine leukosis in dairy cattle in D.I. Khan, Pakistan
Habib UllahI; A. NasirII, #; M. KashifII; M. SajidIII; A. SikandarIV; M. Umer FarooqI; A. RahmanII; F. UllahV
IFaculty of Veterinary & Animal Sciences, Gomal University, Dera Ismail Khan, Pakistan
IIDepartment of Clinical Sciences, University of Veterinary & Animal Sciences, Lahore-Jhang campus, Pakistan
IIIDepartment of Pathobiology, University of Veterinary & Animal Sciences, Lahore-Jhang campus, Pakistan
IVDepartment of Basic Sciences, University of Veterinary & Animal Sciences, Lahore-Jhang campus, Pakistan
VUniversity of Agriculture, Dera Ismail Khan, Pakistan
ABSTRACT
Bovine leukosis is an economically important disease of dairy cattle caused by the bovine leukaemia virus (BLV). The study aimed to determine the seroprevalence, haemato-biochemical effects, and risk factors pertinent to the prevalence of bovine leukosis in Holstein-Friesian purebred dairy cattle in the D.I. Khan region of Pakistan. A total of 192 sera were assayed using an enzyme-linked immunosorbent assay. Overall, 31.3% (60/192) cattle were detected as seropositive. There was a marked increase in total leukocyte count (11.29 ± 0.48χ103/μl), lymphocytes (5.73 ± 0.42%), monocytes (0.81 ± 0.06%), haemoglobin (11.09 ± 0.46 g/dl), red blood cells (7.23 ± 0.37 χ106/μl), and packed cell volume (31.75 ± 1.48%) in seropositive cattle. Serum biochemical parameters in seropositive cattle showed a marked increase in the liver enzymes, alanine transaminase (24.25 ± 1.03 U/l) and aspartate aminotransferase (49.33 ± 3.31 U/l), with a marked decrease in glutathione peroxidase (1365.63 ± 12.03 (U/l) and superoxide dismutase (2.14 ± 0.13 U/ml) activity. A significant association of age, pregnancy, breeding method, milk yield, and health status of seropositive animals with bovine leukosis was also recorded. The prevalence was higher in animals which were older, pregnant, artificially inseminated, low milk producers, and had a history of ailments. The current study found that bovine leukosis virus could cause changes in internal homeostasis, oxidative stress, and liver dysfunction, all of which should be considered during a control regimen. It was concluded that bovine leukosis was moderately prevalent in the D.I. Khan region in Pakistan.
Keywords: Bovine leukaemia virus, enzyme-linked immunosorbent assay, haemato-biochemical evaluation, Holstein-Friesian cows
Introduction
Bovine leukosis is an important neoplastic disease of cattle and is caused by bovine leukaemia virus (BLV) of the genus, Deltaretrovirus, and family, Retroviridae (Ramiz et al., 2021). The disease is usually subclinical and may assume an asymptomatic path in which the infected animals act as a transmission agent without themselves showing any sign of disease. The virus infects lymphocytes and introduces its genome into the host genome, thereby instigating lifelong infection with persistent lymphocytosis and an increase in B-lymphocytes in the lymph nodes (Selim et al., 2020). BLV was first perceived in diseased cattle by Miller et al. (1969) in tissue culture (Khudair et al., 2021). The disease is transferred both horizontally and vertically. Horizontal transmission of the disease occurs through contact with infected animals, infected lymphocytes, blood transfusion, rectal palpation, use of common needles, and mechanical transmission by insects (Polat et al., 2015); vertical transfer occurs through ingestion of colostrum and also the affected placenta (Selim et al., 2020). The oncogenic properties of the virus may produce pathogenicity in farm workers drinking unpasteurized milk (Buehring et al., 2014). This illness is listed as a disease of economic importance to international trade by the World Organization for Animal Health (OIE).
The disease results in increased heifer replacement costs, decreased milk production, reduced reproductive performance, premature culling of animals, as well as international trade restrictions (Khan et al., 2019). It also enacts a restriction on the import of exotic semen and cattle (Nishimori et al., 2017). According to the OIE, bovine leukosis has a global spread, infecting large numbers of susceptible host animals. The reported global prevalence differs widely. Variable prevalence has been reported globally: in Canada, 26%; United States, 84% (Bartlett et al., 2013);Turkey, 48.3%; Iran, 64.7% (Rodriguez et al., 2011); China, 21% (Ma et al., 2016); Egypt, 20.8% (Selim et al., 2020); South Korea, 10.2% (Kim et al., 2017); Philippines, 4.8% (Polat et al., 2015); and Japan, 28.6% (Murakami et al., 2011). The occurrence of bovine leukosis has been reported to be highly associated with various risk factors including gender, age, pregnancy, breed, milk yield, herd size, breeding, health status, and country of import. The existence of the disease is higher in older, pregnant animals, exotic breeds, low milk-producing animals, herd sizes >200, artificially inseminated cows, and in imported animals (Norbyet al., 2016; Khan et al., 2019; Ramiz et al., 2021).
Bovine leukosis has been described as affecting the haemato-biochemical parameters of the host, including increased blood cell counts, leukocytosis, lymphocytosis, neutropenia, and monocytopenia (Nikolay et al., 2013). The serum aspartate aminotransferase concentrations were found to be decreased in BLV-infected cows (Akalin et al., 2015). Furthermore, increased creatinine levels, decreased superoxide dismutase and glutathione peroxidase activity (Souza et al., 2011), and a marked decrease in calcium level are also associated with this disease (Ali et al., 2019). The test of choice for the diagnosis of bovine leukosis is agar gel immuno-diffusion (AGID) but in recent years it is replaced by enzyme-linked immunosorbent assay (ELISA) because AGID is time-consuming and requires skilled technicians. ELISA has an excellent ability to detect specific antibodies against BLV ( mainly the glycoprotein, gp51) (Giuseppe et al., 2004). Polymerase chain reaction (PCR) is also used for the diagnosis of BLV because PCR enables detection of the proviral genome integrated in the host genome (Villalobos-Cortes et al., 2017).
Despite being highly prevalent around the world, no study has ever been conducted to determine the distribution of infection in the vast region of Dera Ismail Khan (D.I. Khan), a southern, livestock district of Khyber Pakhtunkhwa, Pakistan. Therefore, the present study was aimed to investigate the seroprevalence, haemato-biochemical effects, and risk factors of bovine leukosis in dairy cattle in this area for the first time. It is hypothesized that the disease exists in the Holstein-Friesian breed of dairy cattle in the context of imported dairy cattle entrepreneurs trending in the area.
Materials and Methods
The study was conducted in accordance with the principles of good practice and approved by the Ethical Committee of University of Veterinary and Animal Sciences Lahore, sub campus Jhang.
The study was conducted in different areas of D.I. Khan, Khyber Pakhtunkhwa, situated close to the River Indus in a north-east to south-west direction. The summer season (April to September) is mainly dry and hot while December, January, and February are cold (winter) months. The district is highly populated with livestock, serving as the main source of income in rural areas. Commercial dairy farming has experienced a rising trend of importing dairy cattle of high yielding milk breeds such as Holstein-Friesians and Jerseys in the study area. Dairy cattle (Holstein, Friesian pure breed) of both sexes and different age groups (>2, >4, >6 years) were randomly selected for this study. A total of one hundred and ninety-two (n = 192) blood (sera harvested) samples from Holstein-Friesian (n = 192) dairy cattle were collected. A total volume of 3 ml of blood was collected aseptically from the jugular or coccygeal veins in two types of vacutainers i.e., EDTA and Gel/Clot activator tubes. The samples were transferred to the laboratory (Medicine Lab, College of Veterinary and Animal Sciences, sub campus, Jhang) for further analyses.
All the serum samples were examined serologically using an ELISA kit (Anti-BLV gp51 antibody detection, ID Vet; Garbels, France) to detect the antibodies against BLV. The collected blood samples were transferred into gel and clot activator tubes and centrifuged at 2000 rpm for 10 min to separate serum. The sera were transferred into Eppendorf tubes and stored at -18 °C till further ELISA processing. At the time of assay procedure, all the reagents were allowed to come to room temperature (21 ± 5 °C) before use and then homogenized using a Vortex. As per the manufacturers instructions, 80μ! of dilution buffer was added to each well of a 96-well plate. Then, 20 μ! of positive (PC) and negative controls (NC) were added into duplicate wells; 20 μl of each sample was added to the remaining wells. The plates were covered with aluminium foil and incubated for 45 min at 21 °C (± 5 °C). After incubation, three washings were carried out with 300 μl of wash solution in each well. After washing, 100 μl of conjugate was added to each well and was incubated for 30 min at 21 °C (± 5 °C). After washing, 100 μl of substrate solution was added to each well of the plate and incubated for 15 min at 21 °C (± 5 °C). If the immune complex was present, the peroxidase transformed the substrate into a blue-coloured compound. Then, 100 μl of stop solution was added to each well, making it yellow after blocking. The optical density of the colour development was read with the help of an ELISA reader at 450 nm (Biobase-EL10A, ELISA reader). The measurement of the intensity of colour and the number of antibodies present in serum samples were calculated.
The test was validated when the mean value of the NC optical density (OD) was greater than 0.7 (ODnc > 0.7); the mean value of the PC optical density was <30% of the negative control optical density i.e.,
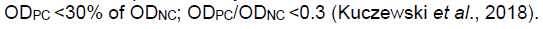
The results were interpreted according to the recommendations as: when the S/N % was equal to or less than 50%, the animal was regarded positive for BLV antibodies; when the S/N % of the sample was greater than 50% and less than 60%, the sample was declared doubtful; and when the S/N % was equal to or greater than 60%, the sample was graded as negative.
The formula used to calculate S/N (%) was:
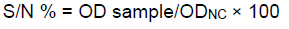
Haematological parameters (total leukocyte count, lymphocytes, monocytes, haemoglobin, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, red blood cells, mean corpuscular volume, packed cell volume, and platelets) in Holstein-Friesian cattle (n = 20 seropositive and n = 10 seronegative) were measured by using an automatic haematology analyser (Exigo-H400 Boul Medical, Sweden).
Transaminases, alanine transaminase (ALT), and aspartate aminotransferase (AST) in Holstein-Friesian dairy cattle (n = 16) were assayed by using commercially available kit (Randox, IFCC, RX Daytona Plus, UK). The oxidative stress levels of Holstein-Friesian dairy cattle (n = 16) were evaluated by determining the level of glutathione peroxidase enzyme activity and superoxide dismutase enzyme activity using standard kits (Ransod, Randox, UK).
The data obtained during the study were analysed using the SPSS software program (IBM SPSS Statistics, version 21). Pearson's chi-square test was used to determine the prevalence of bovine leukosis in cattle while a single sample t-test was used for haemato-biochemical parameters. The risk factors (sex, age, breed, pregnancy, breeding, milk yield, and health status) were also evaluated using a chi-square test. The outcomes were declared statistically significantly different at a probability of 5% (P <0.05).
Results and Discussion
The overall seroprevalence of bovine leukosis in the current study was 31.3% (60/192) in Holstein-Friesian dairy cattle. Mean values of haematological parameters of animals are shown in Table 1.
The values of total leukocytes, lymphocytes, monocytes, haemoglobin, red blood cells, and packed cell volume were higher in seropositive cattle than seronegative cattle (P <0.05). These imply the severity of viral infection in cattle. The elevated lymphocyte count is attributed to a 45-fold increase in infected CD 5+ and a 99-fold increase in infected CD 5- B-lymphocytes (Susan et al., 2016). The possible reason in this case in cattle is a shift to the left, in which the excessive demand of differentiation and maturation of the aforesaid cells occurs after the viral infection. The values of mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, mean corpuscular volume, and platelets were similar (P >0.05).There was no issue of malnutrition and or any injury in the cattle under study therefore the above values were found unaffected and indicate the volume/concentration in the cells/blood. The serum biochemical parameters of seropositive and seronegative Holstein-Friesian dairy cattle are shown in Table 2.
The mean values of ALT and aspartate aminotransferase were higher in seropositive cattle than seronegative cattle (P <0.05). The mean values of glutathione peroxidase and superoxide dismutase were lower in seropositive cattle. The seroprevalence of bovine leukosis was higher in older females that were pregnant or artificially inseminated, and in animals with prior disease history with reduced milk production (P <0.05) (Figure 1).
The seroprevalence (31.3%) observed in the current study is close to the prevalence (32.5%) reported in the UAE (25.7%; Hassan et al., 2020) and in Korea (35%; Suh et al., 2005). The current results do not concur with the findings of the research conducted in Japan (44.8%; Meas et al., 2000), Argentina (84%; Gutierrez et al., 2011), Canada (20.8%; Nekouei et al., 2015), Egypt (21.5%; Hamada et al., 2020), and China (21%; Ma et al., 2016). This difference in prevalence may be due to differences in housing systems, population density, animal raising practices, and geo-climatic variations.
Regarding the haematological parameters in Holstein-Friesian dairy cattle, the total leukocyte count was markedly higher in ELISA-positive cattle than in ELISA-negative cattle, as stated earlier (Yang et al., 2016).This increase in leukocyte count in all the positive cattle is a sign of mounting a competent immune response against the viral infection (Juliarena et al., 2007).The current outcome of total leukocyte count was not in accordance with Ali et al. (2019), who showed a non-significant association of total leukocyte count in seropositive and seronegative cattle. The mean value of lymphocytes was higher (P <0.05) in seropositive cattle; this was consistent with a study (Mekata et al., 2018) reporting higher level of lymphocytes in Japanese black (JB) adult cattle affected with bovine leukosis. The current outcome stands in disagreement with another study (Ali et al., 2019), in which a non-significant (P >0.05) association was present regarding lymphocytes.
This virus basically affects the genome of B-lymphocytes, which consequently stimulates further cell division, resulting in an increased number of peripheral blood lymphocytes (Ali et al., 2019). This effect in cattle may be due to breed differences, feed quality, and geo-biological variation. In the current study, the value of monocytes was markedly higher in seropositive cattle than in seronegative cattle, and was in agreement with a similar study (Notsu et al., 2018). The monocyte result was not in accordance with an earlier study (Yang et al., 2016), which showed a significant decrease in the level of monocytes in affected animals. In general, the monocytes are the components of the white blood cells that are generally elevated in the certain conditions of the body involving infections, chronic inflammation, and allergic reactions (Sikandar et al., 2012).
The mean value of haemoglobin was higher (P <0.05) in seropositive cattle, which is in accordance with the findings reported previously (Yang et al., 2016). An increased haemoglobin value reflects the abnormal morphology of red blood cells resulting from macrocytosis associated with viral infection. The severe stress mediated by the detrimental effects of the virus may even lead to death of the red blood cells. The outcome suggests that the cattle under investigation were experiencing a severe stage of viral infection. The mean value of red blood cells was higher in seropositive dairy cattle, which is not in accordance with the study of Notsu et al. (2018), who showed non-significant decreases in red blood cells; this difference may be due to the severity of infection in seropositive and seronegative cattle. The packed cell volume was markedly higher in seropositive cattle than in seronegative cattle; Yang et al. (2016) also reported significant increases in packed cell volume.
In terms of liver enzymes, seropositive cattle had higher ALT activity than seronegative animals (Table 2). The increased ALT indicates that the virus has invaded the liver via portal circulation and resides within the hepatocytes. Consequently, the disease has affected the liver parenchyma, leading to the higher enzyme concentration in the blood. The findings are consistent with those of Ali et al. (2019), who reported similar findings. Leukaemic lymphocytic cell infiltration in hepatic tissues may cause the liver function abnormalities that are manifested in the early stages of leukosis (Nikolay et al., 2013). Another study, which contradicted the findings of the current study, found that the level of ALT in seropositive animals remained unaltered (Akalin et al., 2015). The activity of another liver enzyme, AST, was considerably lower in BLV-infected animals than seronegative cattle. Another study (Nikolay et al., 2013) also reported decreased AST activity in seropositive cattle. The current findings differ from those of Ali et al. (2019), who found a substantial rise in AST enzyme activity in BLV-infected cattle. The possible metabolic cause of decreased AST levels in their study may be attributed to vitamin B6 deficiency, certain drugs, anorexia, and severe weight loss (Rossouw et al., 1978; Schwarz 1996).
Oxidative stress is a central issue in the transformation or death of living cells. In the current study, the activity of the antioxidant enzyme, GPx, was markedly lower in BLV-infected cattle than in seronegative cattle. It may be due to the viral infection, which can alter the oxidative status either by increasing the formation of nitric oxide or by inhibiting the synthesis of enzymes involved in the oxidative defence within the host cell. The result of reduced glutathione activity is not in agreement with the outcome of another study (Ali et al., 2019) who found non-significant changes in GPx activity. The current finding is consistent with the results of earlier investigations (Souza et al., 2011; Nikolay et al., 2013; Akalin et al., 2015) describing the oxidative status and the markers of oxidative stress in BLV-infected dairy cattle and showing a marked decrease in glutathione peroxidase activity. The activity of superoxide dismutase enzyme in the seropositive cattle was also lower in this study; reduced superoxide dismutase activity was different from another study (Souza et al., 2011), which showed nonsignificant (P >0.05) changes in superoxide dismutase activity in bovine leukosis.
In the current investigation, a relationship between the animal's sex and the prevalence of BLV infection was evidenced (P <0.05) (Figure 1). Another study found a substantial relationship between animal sex and disease incidence, with female animals manifesting higher prevalence than male animals (Khudhair et al., 2016). The most plausible reason for a higher incidence in females may be the importation of unscreened heifers (for fresh stock or replacement heifers) and a variety of stress-inducing cyclic physiological processes (such as pregnancy, lactation, breeding, oestrous) that females experience throughout their life span. Khan et al. (2019) found that the prevalence was higher in male animals than in females, which may be due to the fact that the referred study evaluated a larger sample size of male animals than the current study, which have led to the gender-based prevalence outcome. It could also be associated with the environmental variations in the study areas. The occurrence of BLV was also found to be linked to the age of animals. Seropositivity was higher in animals above the age of six years (P <0.05). This finding is consistent with several earlier studies (Murakami et al., 2011; Ramiz et al., 2021). Several reasons may contribute to the greater frequency of BLV in elderly animals. The longer an animal lives, the more chances it has of being exposed to and interacting with BLV-infected animals. Furthermore, elderly animals are more prone to infections (Khudhair et al., 2016). A negative relationship was observed between the ages of seropositive animals in another investigation (Erskine et al., 2012), implying that the animals might be infected at any age.
The current study revealed a link between pregnancy and BLV infection. Pregnant animals had a higher prevalence than non-pregnant animals. In buffalo, similar outcomes were obtained (Selim et al., 2020) and this could be due to pregnancy, which acts as a stressor on animals, lowering the immunological competency of the dam and raising the risk of BLV infection. This results differs from Selim et al. (2020), who reported non-significant (P >0.05) associations between seropositivity and pregnancy of animals. This could be due to the fact that the disease is mainly transmitted horizontally.
The current investigation found an association between BLV seropositivity and breeding strategy. In comparison to natural service, animals bred by artificial insemination (AI) showed higher seropositivity, in agreement with Ramiz et al. (2021). On farms, it is common practice to use a single glove for AI of multiple animals; the blood-contaminated glove increases the risk of spreading BLV-infected lymphocytes among individual animals in the herd.
In the present study, a marked decrease in milk production in dairy animals was associated with the incidence of BLV compared to seronegative animals (P <0.05). This could be attributed to BLV-infected animal frailty and decreased feed intake. Similar findings have also been observed in another study (Nekouei et al., 2015). This could be due to the animal's health declining over time, weight loss, or other factors, resulting in a steady decline in milk yield. Our findings differ from those of Jacobs et al. (1991) and Heald et al. (1992), who found no significant link between milk production and BLV. This could also be attributed to differences in geographical location, feeding techniques, management aspects, and knowledge of contemporary husbandry practices pivotal for disease control and prevention.
We found a significant correlation of animal health history with BLV in seropositive animals. The prevalence was higher in those animals that had any history of previous disease including abortions, repeat breeding, mastitis, or infectious diseases. This may be ascribed to the down-regulated immune status of the host as a result of previous diseases increasing susceptibility to BLV.
Conclusion
The results of the current study confirmed that the disease was prevalent in the Holstein-Friesian dairy cattle in different areas of the Dera Ismail Khan region of Pakistan. It has been observed that the disease affects the haematological and biochemical parameters of infected animals in the presence of varied pathological alterations, leading to physiological stress and liver function disorders in the host. A greater degree of attention is required to establish effective prevention and control measures subsequent to the screening of all animals at dairy farms and adopting good husbandry practices, segregation, and eliminating infected animals.
Acknowledgement
The authors would like to thank all the facilitators for their support in sample collection and processing, and the staff of the Department of Clinical Sciences for their technical assistance.
Authors' contribution
AN, MK, and MS conceived the idea and facilitated the execution of research activities. HU, AR, and MUF performed the sampling and testing. FU helped in the statistical analysis and AS equally contributed in the manuscript write-up.
Conflict of Interest
The authors declare that there is no conflict of interest related to this article.
References
Abdalla, E.A., Rosa, G.J., Weigel, K.A. & Byrem, T. 2013. Genetic analysis of leukosis incidence in United States Holstein and Jersey populations. Int. J. Dairy Sci. 96 (9): 6022-6029. doi.org/10.3168/jds.2013-6732. [ Links ]
Aiello, S.E., Moses, M.A. 2016. The Merck Veterinary Manual, 11th Edition, Wiley, New York City, United States. [ Links ]
Akalin, P.P., Ataseven, V.S., Firat, D., Ergun, Y., Baçpinar, N. & Ozcan, O. 2015. Selected biochemical and oxidative stress parameters and ceruloplasmin as acute phase protein associated with bovine leukaemia virus infection in dairy cows. Bull. Vet. Inst. Pulawy. 59: 327-330. doi: 10.1515/bvip-2015-0048. [ Links ]
Ali, A.F., Selim, A., Manaa, E.A., Abdelrahman, A. & Sakr, A. 2019. Oxidative state markers and clinicopathological findings associated with bovine leukemia virus infection in cattle. Microb. Pathog. 136:103662. doi.org/10.1016/j.micpath.2019.103662. [ Links ]
Bartlett, P.C., Norby, B., Byrem, T.M., Parmelee, A., Ledergerber, J.T. & Erskine, R.J. 2013. Bovine leukemia virus and cow longevity in Michigan dairy herds. J. Dairy Sci. 96 (3): 1591-1597. doi. org/ 10.3168/jds.2012-5930. [ Links ]
Buehring, G.C., Shen, H.M., Jensen, H.M., Choi, K.Y., Sun, D. & Nuovo, G. 2014. Bovine leukemia virus DNA in human breast tissue. Emerg. Infect. Dis. 20 (5): 772-782. doi: 10.3201/eid2005.131298 [ Links ]
. De Giuseppe,A., Feliziani, F., Rutili, D. & De Mia, G.M. 2004. Expression of the bovine leukemia virus envelope glycoprotein (gp51) by recombinant baculovirus and its use in an enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol.11(1):147-151. doi: 10.1128/cdli. 11.1.147-151.2004. [ Links ]
Erskine, R.J., Bartlett, P.C., Byrem, T.M., Render, C.L., Febvay, C. & Houseman, J.T. 2012. Using a herd profile to determine age-specific prevalence of bovine leukemia virus in Michigan dairy herds. Vet. Med. Int. 2012. doi.org/10.1155/2012/350374. [ Links ]
Hamada, R., Metwally, S., Polat, M., Borjigin, L., Ali, A.O., Abdel-Hady, A.A., Mohamed, A.E., Wada, S. & Aida, Y. 2020. Detection and molecular characterization of bovine leukemia virus in Egyptian dairy cattle. Front. Vet. Sci. 7: 608. doi.org/10.3389/fvets.2020.00608. [ Links ]
Hassan, N.A., Mohteshamuddin, K., Anthony, A., Al Aiyan, A., Mohamed, M.E., Alfaki, I.M. & Barigye, R. 2020. Serological evidence of enzootic bovine leukosis in the peri-urban dairy cattle production system of Al Ain, United Arab Emirates. Trop. Anim. Health. Prod. 52 (5): 2327-2332. Doi.org/10.1007/s11250-020-02262-1. [ Links ]
Heald, M.T., Waltner-Toews, D., Jacobs, R.M. & McNab, W.B. 1992. The prevalence of anti-bovine leukemia virus antibodies in dairy cows and associations with farm management practices, production, and culling in Ontario. Prev. Vet. Med. 14 (1-2): 45-55. doi.org/10.1016/0167-5877 (92)90083-R. [ Links ]
Jacobs, R.M., Heeney, J.L., Godkin, M.A., Leslie, K.E., Taylor, J.A., Davies, C. & Valli, V.E. 1991. Production and related variables in bovine leukaemia virus-infected cows. Vet. Res. Commun. 15 (6): 463-474. Doi.org/10.1007/bf00346546. [ Links ]
Juliarena, M.A., Gutierrez, S.E. & Ceriani, C. 2007. Determination of proviral load in bovine leukemia virus-infected cattle with and without lymphocytosis. Am. J. Vet. Res. 68(11):1220-5. doi: 10.2460/ajvr.68.11.1220. [ Links ]
Khan, M.F., Siddique, U., Shah, A.A., Khan, I., Anwar, F., Ahmad, I., Zeb, M.T., Hassan, M.F. & Ali, T. 2019. Seroprevalence of bovine leukemia virus (BLV) in cattle from the north-west of Pakistan. Pak. Vet. J. 40 (1):127-129. doi: 10.29261/pakvetj/2019.103. [ Links ]
Khudhair, Y.I., Hasso, S.A., Yaseen, N.Y. & Al-Shammari, A.M. 2016. Serological and molecular detection of bovine leukemia virus in cattle in Iraq. Emerg. Microbes & infect. 5 (1): 1-6. doi/full/10.1038/emi.2016.60. [ Links ]
Kim, H.U., Lee, E.Y., Lee, K.K., Kim, S.H., Moon, B.Y., So, B.J. & Kim, Y.H. 2017. Seroprevalence of the bovine leukemia virus among Korean native cattle in South Korea. Prev. Vet. Med. 41 (1): 52-55. doi:dx.10.13041/jpvm.2017.41.1.52. [ Links ]
Kuczewski, A., Orsel, K., Barkema, H.W., Kelton, D.F., Hutchins, W.A. & van der Meer, F.J. 2018. Evaluation of five different ELISAs for the detection of bovine leukemia virus antibodies. J. Dairy Sci.101 (3): 24332437. doi.org/10.3168/jds.2017-13626. [ Links ]
Ma, J.G., Zheng, W.B., Zhou, D.H., Qin, S.Y., Yin, M.Y., Zhu, X.Q. & Hu, G.X. 2016. First report of bovine leukemia virus infection in yaks (Sos mutus) in China. Biomed Res. Int. 2016. doi.org/10.1155/2016/9170167. [ Links ]
Mekata, H., Yamamoto, M., Kirino, Y., Sekiguchi, S., Konnai, S., Horii, Y., & Norimine, J. 2018. New hematological key for bovine leukemia virus-infected Japanese Black cattle. The J. Vet. Med. Sci. 80(2), 316-319. doi.org/10.1292/jvms. 17-0455. [ Links ]
Murakami, K., Kobayashi, S., Konishi, M., Kameyama, K.I., Yamamoto, T., & Tsutsui, T. 2011. The recent prevalence of bovine leukemia virus (BLV) infection among Japanese cattle. Vet. Microbiol. 148 (1): 8488. doi.org/10.1016/j.vetmic.2010.08.001. [ Links ]
Nekouei, O., van Leeuwen J., Sanchez, J., Kelton, D., Tiwari, A. & Keefe, G. 2015. Herd-level risk factors for infection with bovine leukemia virus in Canadian dairy herds. Prev. Vet. Med. 119 (3-4): 105-113. doi. org/10.1016/j.prevetmed.2015.02.025. [ Links ]
Nikolay, S., Dimitrinka, Z., Ivanka, S., Nikolina, R., Teodora, M. 2013. Investigation of some hematological and blood biochemical parameters in cattle spontaneously infected with bovine leukosis virus. Maced. Vet. Rev. 36 (2): 107-110. [ Links ]
Nishimori, A., Konnai, S., Okagawa, T., Maekawa, N., Goto, S., Ikebuchi, R., Nakahara, A., Chiba, Y., Ikeda, M., Murata, S. & Ohashi, K. 2017. Identification of an atypical enzootic bovine leukosis in Japan by using a novel classifica-tion of bovine leukemia based on immunophenotypic analysis. Clin. Vaccine Immunol. 24 (9): e00067-17. doi.org/10.1128/CVI.00067-17. [ Links ]
Norby, B., Bartlett, P.C., Byrem, T.M. & Erskine, R.J. 2016. Effect of infection with bovine leukemia virus on milk production in Michigan dairy cows. Int. J. Dairy Sci. 99 (3): 2043-2052. doi. org/10.3168/jds.2015-10089. [ Links ]
Notsu, K., Hashida, S., Mitoma, S., Kubo, M., Arikawa, G., Agah, M.A., El-khalat, H.M., Mai, T.N., Nguyen, T.H., Elhanafy, E. & El Daous, H. 2018. Assessment of hematological parameters and carcass weight in bovine leukemia virus infection in slaughtered beef cattle. J. Vet. Epidemiol. 22 (1): 43-48. doi.org/10.2743/jve.22.43. [ Links ]
Polat, M., Ohno, A., Takeshima, S.N., Kim, J., Kikuya, M., Matsumoto, Y., Mingala, C.N., Onuma, M. & Aida, Y. 2015. Detection and molecular characterization of bovine leukemia virus in Philippine cattle. Arch. Virol. 160 (1): 285-296. Doi 10.1007/s00705-014-2280-3. [ Links ]
Ramiz, R.M., Ahmad, A., Ghafoor, A., Avais, M. & Iqbal, M.Z. 2021. Genotype detection and sero-prevalence of bovine leukemia virus along with associated risk factors in exotic and local breeds of cattle in and around Lahore, Punjab. Pak. J. Zool. 53 (3) 1169-1172. doi.org/10.17582/journal.pjz/20190822070849. [ Links ]
Rodriguez, S.M., Florins, A., Gillet, N., De Brogniez, A., Sánchez-Alcaraz, M.T., Boxus, M., Boulanger, F., Gutiérrez, G., Trono, K., Alvarez, I. & Vagnoni, L. 2011. Preventive and therapeutic strategies for bovine leukemia virus: Lessons for HTLV. Viruses. 3(7): 1210-1248. doi: 10.3390/v3071210. [ Links ]
Selim, A., Megahed, A.A., Kandeel, S. & Abdelhady, A. 2020. Risk factor analysis of bovine leukemia virus infection in dairy cattle in Egypt. Comp. Immunol. Microbiol. Infect. Dis. 72: 101517. doi. org/10.1016/j.cimid.2020.101517. [ Links ]
Sikandar, A., Cheema, A.H., Younus, M., Aslam, A., Zaman, M.A. & Rehman, T. 2012. Histopathological and serological studies on paratuberculosis in cattle and buffaloes. Pak. Vet. J. 32(4): 547-551. [ Links ]
Souza, F.N., Monteiro, A.M., dos Santos, P.R., Sanchez, E.M., Blagitz, M.G., Latorre, A.O., Neto, A.M., Gidlund, M. & Della Libera, A.M. 2011. Antioxidant status and biomarkers of oxidative stress in bovine leukemia virus-infected dairy cows. Vet. Immunol. Immunopathol. 143 (1-2): 162-166. doi.org/10.1016/j.vetimm.2011.05.028. [ Links ]
Suh, G.H., Lee, J.C., Lee, C.Y., Hur, T.Y., Son, D.S., Ahn, B.S., Kim, N.C. & Lee, C.G. 2005. Establishment of a bovine leukemia virus-free dairy herd in Korea. J. Vet. Sci. 6 (3): 227-230. doi.org/10.4142/jvs.2005.6.3.227. [ Links ]
Villalobos-Cortes, A., Franco, S., Gonzalez, R. & Jaén, M. 2017. Nested polymerase chain reaction (nPCR)-based diagnosis of bovine leukemia virus in Panama. Afr. J. Biotechnol.16 (11): 528-535. doi. org/10.5897/AJB2016.15849. [ Links ]
Yang, Y., Fan, W., Mao, Y., Yang, Z., Lu, G., Zhang, R., Zhang, H., Szeto, C. & Wang, C. 2016. Bovine leukemia virus infection in cattle of China: Association with reduced milk production and increased somatic cell score. J. Dairy Sci. 99 (5): 3688-97. doi.org/10.3168/jds.2015-10580. [ Links ]
Submitted 20 June 2022
Accepted 16 December 2022
Published 6 April 2023
# Corresponding author: amar.nasir@uvas.edu.pk














