Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Animal Science
On-line version ISSN 2221-4062
Print version ISSN 0375-1589
S. Afr. j. anim. sci. vol.52 n.6 Pretoria 2022
http://dx.doi.org/10.4314/sajas.v52i6.15
Seasonal effects of Rhus lancea and Celtis africana on intake, preference, and physiological responses in South African indigenous goats
F. PhiriI, #; A.T. KanengoniII; D. HattasIII; K.R. MbathaI
IDepartment of Agriculture and Animal Health, University of South Africa, Private Bag X6, Florida, 1709, South Africa
IIResearch and Veterinary Services Department, Johannesburg City Parks and Zoo, P.O. Box 2824, Johannesburg, 2000, South Africa
IIIDepartment of Biological Science, University of Cape Town, Private Bag X3, Rondebosch 7701, South Africa
ABSTRACT
This study investigated the seasonal effects of Rhus lancea and Celtis africana leaves on preference, intake, weight, and serum metabolites in South African indigenous, mature, male goats. Twelve mature, male goats weighing 34 ± 5.9 kg (mean ± SD) were randomly allocated to two groups of six and kept in metabolic crates for periods of 21 days in October 2015 and March, May, and August of 2016. A browser diet of R. lancea and C. africana and a control diet (lucerne and concentrates) were randomly allocated to each group. Measurements taken included nutritional composition of browse per season, and browse preference, intake, weight changes, and serum metabolites in the goats. The acid detergent fibre (2436%) and neutral detergent fibre (26.9-70.4 %) in R. lancea over the months were greater than in C. africana (50.3-53.2% and 49.4-55.4%, respectively). In the preference study, the goats preferred C. africana more in October (51.2 vs 48.8%), March (51.4 vs 48.6%), and May (54.3 vs 45.7%). Goats on the browser diet lost weight in March, May, and in August whereas those on the control diet gained weight. The serum urea concentration of goats consuming browser diets in May and August (1.8-3.3 mmol/l) was lower than the normal range, consistent with animals failing to derive their protein requirements from the diet. Goats prefer to browse C. africana more than R. lancea. The study also indicated the need for supplementation to meet maintenance requirements in animals fed R. lancea and C. africana.
Keywords: serum metabolites, condensed tannins, fibre, maintenance, small ruminants
Introduction
Rhus lancea and Celtis africana are indigenous, dicotyledonous, woody trees, whose leaves are regularly offered to captive-managed wild ruminant mixed feeders (Lombard, 2016; Mbatha & Bakare, 2018). This practice emanated from anecdotal reports of wild ruminant mixed feeders' readily consuming R. lancea and C. africana leaves in free-ranging environments. Both trees are popular ornamental shade and roadside trees in suburbs around South Africa and become available after pruning. The R. lancea is an evergreen woody perennial tree that flowers during the dry season (Gundidza et al., 2008). Werekeh (2012) reported that fresh R. lancea leaves harvested in Gauteng province of South Africa over wet and dry seasons contained 109.4-130.6 g crude protein (CP)/kg, 352.3-431.7 g neutral detergent fibre (NDF)/kg, 172.2-249.8 g acid detergent fibre (ADF)/kg, 80.5125.5 g acid detergent lignin (ADL)/kg, and 43.3-66.4 g tannins/kg.
The C. africana, in dry or very cold climates, is a seasonal tree that flowers during the wet season, with leaves turning yellow in the early dry season and defoliating in the dry season (Gogh & Anderson, 1988; Ts'ehlana, 2005; Al-Taweel et al., 2012). Under ideal warm and moist conditions, C. africana keeps its foliage all year round (Ts'ehlana, 2005). In regions with wet, mild winters, at the coast, and occasionally inland, C. africana trees commonly retain their old leaves, dropping them all at once when the new spring leaf flush appears. A literature search found no published nutrient values of C. africana from South Africa. Shenkute et al. (2012) however reported values of 151 g CP/kg, 304 g NDF/kg, 249 g ADF/kg, and 90.2 g ADL/kg in trees from Ethiopia. The reported CP content of the leaves from both trees renders them as potential protein resources for mixed feeders, especially during dry seasons when herbaceous pasture grasses and legumes become senescent (Theart, 2015).
Goats have been used as models for mixed feeders in investigating feed intake before (Mkhize, 2015; Moyo et al., 2017). This is because extensively-kept indigenous goats exhibit dynamic foraging behaviour and versatility similar to impala (Aepycerous melampus), springbok (Antidorcas marsupialis), and kudu (Tragelaphus strepsiceros) in forage selection; equipping them to counter any fluctuation in dietary resources (Sponheimer et al., 2003; Alexandre & Madonnet, 2005; Hooimeijer et al., 2005). There are also strong similarities in physiological and anatomical morphologies between goats and impala or springbok. Indigenous goats are also amenable to confinement where they still exhibit selective behaviour when offered cut and carry forages, choosing the best available (Werekeh, 2012).
The incorporation of browser forages in the rations of wild ruminant mixed-feeders in captivity faces several challenges (Aganga & Monyatsiwa, 1999). There is a concern related to the browse-associated secondary plant metabolites (SPMs), such as lignin, terpenes, flavonoids, oxalates, saponins, and condensed tannins (CTs) (Hagerman, 1995; Bele et al., 2010; Lamy et al., 2011; Mbatha & Bakare, 2018), which have been reported to cause biological tissue damage (Lamy et al., 2011; Gattiker et al., 2014). In addition, the provision of browser forage in the captive-management setting is erratic at best and usually sudden, increasing the risks associated with SPMs. The sudden exposure of these ruminant mixed-feeders to diets containing SPMs without time to adapt causes damage in the intestines, liver, and kidneys (Lamy et al., 2011). Rogosic et al. (2007) reported that microbes in the gut of ruminant mixed-feeders could adapt to tannins, enabling them to use tannin-rich plants more efficiently, provided they have had an appropriate adaptation period. Mbatha & Bakare (2018) posited that, under free-ranging conditions, the risk of mixed-feeders suffering SPM toxic effects is low, because they seldom consume large quantities of pods or leaves with SPMs. Bhat et al. (2013) also reported that free-ranging mixed-feeders feed on SPM-rich plants and later dilute the effects by feeding on plants with less SPMs, as a way of coping. The ruminant mixed-feeders can also detect and avoid intoxication from SPMs through post-ingestive feedback from nutrients and toxins (Burritt & Provenza, 1996). Not all these coping mechanisms may be available to mixed-feeders kept under captive management, and it is thus critical to evaluate their intake, preference, and physiological responses when given R. lancea and C. Africana leaves to improve their nutritional management.
While a few studies have evaluated the nutrient composition and preference intake of R. lancea in goats (Werekeh, 2012; Theart, 2015), no literature could be found on C. africana. Information from preference studies may assist nutritionists to identify and source new suitable browser forage for captive mixed-feeders. The objective of the current study was to simulate feed intake, browser forage preference, weight changes, and serum metabolites in captive-managed ruminant mixed-feeders using South African indigenous Nguni-type goats fed R. lancea and C. africana leaves over four seasons.
Materials and methods
The study was conducted at the Agricultural Research Council-Animal Production Institute (ARC-API) in Irene, ~22 km south of central Pretoria (25° 53' 59.6" S; 28° 12' 516.6" E), South Africa. The area has an average annual rainfall of 646 mm. The wet season extends from October to March, while the dry season is from April to September (Conradie, 2017). The study protocol was reviewed and approved by the Animal Ethics Committee of the ARC-API (Ref: APIEC 15/028) and the Animal Research Ethics committee of the University of South Africa (Ref: 2015/CAES/046).
The study involved feeding mature, South African, indigenous, Nguni-type, male goats for 21 days in the early wet, late wet, early dry, and late dry seasons. The goats belonged to the ARC-API breeding herd kept uniformly in semi-extensively in paddocks and fed commercial pellets and eragrostis hay throughout the year. Twenty-three different goats, aged between two and three years, with body weights of 34 ± 5.9 kg (mean ± SD) participated in the study across the four seasons. Ten goats could not continue from the early wet to the late wet seasons as they were receiving treatment for haemonchosis and were replaced; and subsequently one goat was replaced for the early dry and one for the late dry seasons as they was not healthy enough to continue in the study. Efforts were made to ensure that replacement goats were of similar weight and age. However, goats continuing in the study remained on the same diet throughout subsequent seasons. In-between the feeding trials the goats returned to the breeding herd where they were maintained under uniform husbandry conditions.
The seasonal periods were: 12th November to 1st December 2015 representing the early wet season (EWS); 15th February to 6th March 2016 for the late wet season (LWS); 16th May to 6th June 2016 for the early dry season (EDS); and 20th August to 16th September 2016 for the late dry season (LDS). This resulted in a 4 χ 2 (season χ diet) factorial experimental design. Nested within the design, each goat on the browser diet received R. lancea and C. africana in separate feeding troughs within the same metabolic crate to evaluate preference. In each season, 12 goats were randomly allocated to one of two dietary treatments (a browser and a control diet), ensuring that the treatment groups were uniform in terms of body weight. The goats stayed individually in metabolic crates (1.2 m long χ 0.74 m wide χ 0.92 m high) placed side by side, a metre apart, in an alternating pattern in a well-ventilated room. The goats adapted to the diets and the metabolic crates over a period of ten days before data collection. The goats received treatment against internal and external parasites (Ivermax® 1% injectable solution, Norbrook Laboratories Limited Newry, County Down, Northern Ireland) over the four seasons.
The R. lancea and C. africana leaves were harvested every second day from the ARC Vegetable and Ornamental Plant Institute (ARC-VOPI), which is located ~23.7 km north of central Pretoria (25° 56" S; 28° 35" E), to ensure that they were as fresh as possible. The browser forage was chopped into small pieces (approximately 2-5 mm in length) using a hammer mill crusher (Drotsky Hammer Mills and Feed Mixers (Pty) Ltd, 33 Barium St, Alrode, Alberton 1451, South Africa). A portion of the browser forage was fed to the goats immediately and the remainder was kept at room temperature and fed to them the next day. Goats on the browser diet were each offered 1 000 g (as is) of R. lancea and 1 000 g (as is) of C. africana daily, in separate, identical feeding troughs that were placed side by side. The browser forage was alternated daily between the troughs to minimise positional bias. The feeders were checked and adjusted twice each day to ensure constant access to fresh feed and to minimise any possible wastage. An extra 10% of feed was provided for the animals that finished the offered meal. Each goat from the control group was offered 1 600 g (as is) of baled lucerne hay and 400 g of commercial pellets (Epol Lamb and Ewe 13®) (Table 1). The control diet provided the nutrient requirements recommended by the NRC (2007), in relation to the weight of the goats and the physiological condition. Feed offered and refusals were weighed daily to determine intake. Preference for each browser forage (R. lancea or C. africana) was calculated by dividing the amount of the particular browser forage offered by the total browser forage consumed by each goat daily. Both groups had ad libitum access to fresh drinking water.
Body weight was recorded three times during data collection for all four seasons (on day 1 before the adaptation period, on day 11 after the adaptation period, and on Day 21 at the end of the data collection period) to reduce stress on the goats. Feed samples were randomly collected before feeding and dried at 60 °C in a drying oven until a constant weight was achieved and, thereafter, milled to pass through a 1-mm sieve (Wiley Mill, Standard Model 3, Arthur H. Thomas Co., Philadelphia, PA, USA) and then stored, pending analysis. A veterinarian collected approximately 5 ml of blood from each goat via the jugular vein at the end of each season. The blood was collected into plain tubes and centrifuged in a Biofuge Primo® centrifuge for 15 min, at 1 500 rpm, at room temperature (~23 °C). An aliquot of the serum was collected and stored at -20 °C for biochemical analysis following the protocol of Gwaze et al. (2010). There were technical problems with processing and storage of the EWS serum samples and they were discarded.
Feed samples were analysed for dry matter (DM), organic matter (OM), and crude protein (CP) in accordance with the standard methods of the Association of Official Analytical Chemists (AOAC) (2005). Dry samples were ground through a 1-mm screen (Wiley mill, Standard Model 3, Arthur H. Thomas Co., Philadelphia, PA, USA) for chemical analyses. Moisture content was determined gravimetrically by drying the samples in an oven at 100 °C to a constant weight. Ash content was assayed by incinerating the samples in a muffle furnace at 550 °C (Method No. 930.05) (AOAC, 2005). The OM was determined as the difference between ash and DM. Crude protein content (N χ 6.25) was determined in accordance with the Kjeldahl method (Method No. 978.04) (AOAC 2005).
The neutral detergent fibre (NDF) and acid detergent fibre (ADF) contents were determined following the procedures of Van Soest et al. (1991), using the ANKOM Fibre Analyser 4 (ANKOM, Macedon, NY, USA), with sodium sulphite as inclusive in the NDF solution. The NDF and ADF contents were corrected for residual ash content. Extractions were performed using filter bags, and the ANKOM200 Fibre Analyzer equipment. Acid detergent lignin was determined using the Daisy11 Incubator (ANKOM Technology Corp., Fairport, NY), and the 08/05 ANKOM protocol, including the final ash of entire bags. Condensed tannins in the browse were extracted with 70% acetone, at room temperature, and determined according to the method of Hagerman (1995). The extract was decanted into conical flasks and hand-shaken to mix thoroughly. An aliquot of the supernatant was added to 6 ml of butanol-HCl reagent in triplicate test tubes. They were transferred to a dry block heater (Grant Instruments, Cambridge, UK) that had been pre-heated to warm the samples and maintained at 100 °C for 1 h (Mupangwa et al., 2000). Afterwards, they were removed and allowed to cool at room temperature before decanting into vials. They were diluted with distilled water to form 2, 4, 6, 8, and 10 (v/v) concentrations and absorbance was read at 550 nm using a Cecil CE 2030 single beam spectrophotometer (Cecil Instruments, England) (Mupangwa et al., 2000). Total protein (TP), albumin (ALB), globulin (GLB), serum urea, alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma glutamyl transferase (GGT), cholesterol (CHOL), creatinine (CREAT), and serum glucose in the sera were determined by IDEXX Laboratories (Pty) Ltd, in Johannesburg, South Africa.
The seasonal effects on nutrient composition were analysed using the generalised linear models in SAS (2010). The relationships among the nutrients, browser forage, and seasons were then mapped using the biplot command in Stata (16.1, Stata Corp LLC, College Station, TX). Biplots consist of lines and dots. The lines are used to reflect the variables of the dataset (nutrients in this study), and the dots are used to show the observations (the browser forage per season). In a biplot, the length of the lines approximates the variance of the variables. The longer the line, the higher the variance. The angle between the lines approximates the correlation between the variables they represent. The closer the angle is to 90°or to 270°, the smaller the correlation. An angle of 0°or 180°reflects a correlation of 1 or -1, respectively. The distance between two points approximates the Euclidean distance between two observations in the multivariate space. Observations that are far away from each other have a high Euclidean distance and vice versa.
Preference, feed intake, weight change, and serum metabolites were analysed using the mixed-effects model command of Stata with restricted maximum likelihood (REML). The mixed-effects model (adapted for the different parameters) was:
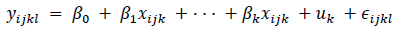
where yyki = parameter (preference, feed intake, weight change, and blood metabolites) for browse or treatment i, in season j, for goat k; ß0, ß1, . . ., ßk are fixed effects; uk are random effects; and uj- N(0, a2u) and eijkl- N(0, a2e). Predictions of preference and intake were made per day and plotted for each season using Stata (16.1, Stata Corp LLC, College Station, TX). Mixed-effects models accommodate the correlations among observations; are more flexible regarding ignorable, missing data and the correlation structure of the residuals; and can accommodate longitudinal data where measurements are taken in different schedules (such as the seasons in this study). Significance was set at P <0.05 and tendency was reported at 0.1 <P >0.05.
Results
The concentrations of CP, ADL, ADF, NDF, and OM in R. lancea and C. africana measured across the four seasons on a dry matter basis are presented in Table 2. There were browser forage χ season interactions in CP, ADF, and NDF concentrations (P <0.05). The CP concentration differed between the two browser forages in EWS and LDS, being higher in C. africana than R. lancea, but CP concentration was similar in LWS and EDS. The ADF and NDF concentrations in C. africana were fairly similar across the seasons (P >0.05). The ADF and NDF concentrations in R. lancea were highest in EWS and lowest in LDS. There was a season χ browser forage tendency (P = 0.061) for CT concentration. The CT concentrations in R. lancea in the dry season (EDS, LDS) were higher than in the wet season (EWS, LWS). The CT concentrations in R. lancea were higher than in C. africana across all the seasons (P <0.05).
A biplot displaying the browser forage per season and the distances between the observations indicated a clustering of C. africana across the seasons and a greater variation in R. lancea (Figure 1). In LDS, R. lancea appeared to be an outlier. The length of the lines showed that CT had the greatest variance among the variables. The angles between the variables showed that refusals were highly correlated with NDF, ADF and ADL and less so with CTs.
Daily and seasonal preferences of R. lancea and C. africana in goats are shown in Figure 2. In early wet season (EWS), the goats showed a preference (P <0.05) for R. lancea during the first three days and C. africana in the last four days. There were no distinct preferences (P >0.05) for either browser forage in the LWS and the EDS. In the LDS, the goats showed a preference (P <0.05) for R. lancea during the first week, but the preference for the two browser forages was similar for the last three days.
The average daily feed intake (as fed) of goats fed R. lancea and C. africana and the control diet in four different seasons are shown in Figure 3. The goats consumed more browser forage (P <0.05) than control feed in the EWS and more control feed than browser forage in the LDS. The goats consumed more (P <0.05) C. africana than R. lancea, especially in the EWS, LWS, and EDS. During the LDS, the goats consumed more R. lancea.
Table 3 shows the initial body weights, weight changes, average daily feed intake (ADFI, as fed) and final body weights (FBW) of goats over the four seasons. There were season χ diet interactions for ADFI, a seasonal effect on ADFI, a tendency (P = 0.06) for weight change, and a dietary effect on FBW, weight change, and ADFI. The goats on the control diet had higher FBW (P <0.05) than those on the browser diet across all seasons. Goats fed browser forage during the LWS, EDS, and LDS lost body weight. The ADFI in the goats consuming browser forage and the control diets were similar across all the seasons except for the goats on browser forage in LDS, which consumed less feed (P <0.05).
The effects of browser forage and the control diet on the concentration of blood metabolites in goats during three seasons are presented in Table 4. The metabolite concentrations in the goats in the study were compared with the reference ranges for goats as reported by Kaneko et al. (2008) and Radin et al. (2017). Some of the serum glucose, serum urea, albumin, ALP, GGT, ALB/GLOB ratio, and ALT values fell within the reference ranges for goats. The values for creatinine and cholesterol were all lower than the reference values and TP and globulin values were all higher than the reference values. There were season χ diet interactions for serum urea (P <0.05). Serum urea concentrations in goats consuming browser forage decreased in a linear trend from LWS to EDS and the lowest value was in LDS (P <0.05). Serum urea concentrations of goats on the control diet displayed a positive quadratic trend with the lowest values in EDS. There was a seasonal effect on serum glucose concentrations with goats in the LWS having lower concentrations than those in EDS and LDS (P <0.05). The LWS serum glucose concentration was also lower than the reference range (2.78-4.16 mmol/l) (Kaneko et al., 2018). The goats that consumed the control diet had higher serum glucose and urea concentrations (P <0.05) than the goats that consumed browser forage across the three seasons. Conversely, goats consuming the browser diet had higher creatinine concentrations (P <0.05) than those fed the control diet across the three seasons. The goats consuming browser forage had a tendency (P = 0.07) towards having a higher TP than those on the control diet. There were dietary effects on globulin and cholesterol, with goats consuming browser forage having higher globulin and cholesterol values (P <0.05) than those on the control diet. There were dietary effects on ALP and GGT concentration with goats on the control diet having higher values (P <0.05) than those on the browser diet.
Discussion
Celtis africana exhibited a smaller variation in ADF and NDF concentrations (50.3-53.2% and 49.455.4%, respectively) across the seasons than R. lancea (36% and 44%, respectively), suggesting that feeding captive mixed-feeders with C. africana may result in a more predictable supply of nutrients. The goats' consumption of more C. africana than R. lancea, especially in the EWS, LWS, and EDS, may reflect the narrow ranges of ADF and NDF concentrations in C. africana across the seasons. The goats' preference for C. africana over R. lancea in the EWS, LWS, and EDS may point to the potential role of C. africana in contributing to the nutrition of goats and mixed-feeders. Werekeh (2012) reported that goats had a high preference and intake of R. lancea in both wet and dry seasons, compared with other common browser species, including several Acacia spp. However, in this study, the goats showed a gradual increase in their preference for C. africana, which is indicative of an adaptive feeding behaviour, influenced by the fact that the CP values were higher and ADL and CT were lower in C. africana than in R. lancea.
The goats consumed more browser forage in the EWS and more of the control diet in the LDS, displaying seasonal intake patterns. This is consistent with the fact that, during the EWS, the browser forage is still succulent and nutritious whereas in the LDS, it is more mature and higher in fibre (Yayneshet et al., 2009). The R. lancea intake fluctuated somewhat, a further indication of the influence of higher ADL, ADF, NDF, and CT concentrations compared to those in C. africana. The consistently high intake of the browser forages in general across the seasons showed that palatability and the voluntary feed intake of R. lancea and C. africana were not issues with the goats in this study. This may be explained by the premise that goats utilise the proline-rich protein substances (PRPS) in saliva to buffer CT-induced bitterness (Bele et al., 2010), thus improving intake. The fact that the goats fed browser diets lost weight (LWS, EDS, and LDS) is consistent with the lower DM and CP intake compared to the control diet and implies that the browser forage consumed was not sufficient to meet the nutrient maintenance requirements of the goats (Aganga & Monyatsiwa, 1999). These results suggest a need to supplement animals on a R. lancea and C. africana diet with other dietary ingredients. This therefore necessitates an analysis of more parameters including energy and mineral contents of the browser leaves to determine how best to supplement them. That the weight loss was not greater may be attributed to the fact that goats have also been reported to be able to minimise maintenance energy requirements during periods of low energy intake (Silanikove, 2000).
The voluntary intake results also show that CTs do not necessarily adversely affect voluntary feed intake and palatability, but that other SPMs not determined in this study, could. This concurs with the findings of Turner et al. (2005), who reported that goats fed a Sericea lespedeza-based diet with high CTs, were still able to maintain a higher DM intake than the goats that were offered a lucerne (Medicago sativa)-based diet, with lower CT (42.1 vs. 38.7 g/kg BW, respectively). This is because tannins in forage plants can have positive or negative effects on intake, digestion in the rumen, and overall performance, depending on the type and concentration of compounds that are present in the diet (Turner et al., 2005). Min & Solaiman (2018) reported that optimum levels of CT concentrations in goat diets may be 4.5-10% CT (DM). The R. lancea CT concentration in this study of 4-9.6 % would therefore fall into this range and should not have detrimental effects on intake. Therefore, the results suggest that R. lancea can help bridge the seasonal nutrient gap for mixed-feeders when other dietary sources are scarce during the dry season (Owen-Smith, 1994).
The goats in the study were clinically healthy and interpretations of all results (including blood metabolites) assumed this. Gwaze et al. (2012) posited that some biochemical reference ranges are inappropriate for the local breeds and hence the interpretations in the current study were cognisant of this. Turner et al. (2005) stated that comparisons of blood parameters between individual goat breeds that are maintained on a wide variety of forages and supplements are tenuous. The findings of the current study show that serum glucose improved more by the season than by the diets they consumed. This is corroborated by Pambu-Gollah et al. (2000) and Gwaze et al. (2010; 2012), who reported that season improved the serum glucose of goats more than dietary sources did. The serum urea concentrations in the goats on the control diet were higher than the goat reference values during the LWS and LDS. A surplus of protein and non-protein nitrogen quantities in the diets combined with insufficient energy have been reported to increase urea concentrations in goats (Turner et al., 2005; Radović et al., 2011) and may explain this finding in this study. The goats on the browser diets exhibited serum urea concentrations that were lower than the reference values during the EDS and LDS. This is consistent with lower protein in the diets than is required by the goats, similar to what occurs when there is a combined deficit of energy and protein (Turner et al., 2005; Radin et al., 2017). Urea blood levels may indicate short-term metabolic changes and are normally interpreted together with serum TP and ALB levels (Zobel et al., 2015). Although all the goats exhibited hyperproteinaemia in the current study, this was characterised by hyperglobinaemia, indicating either stress, infection or inflammation (Sykes et al., 1980).
The CTs concentrations of R. lancea of 96.1 mg STE/g in the EDS and 90.3 mg STE/g during the LDS corresponded to the substantially higher globulin concentrations of 52 g/L and 50.5 g/L in the goats fed the browser diets compared to the control (globulin concentration of 47.0 g/L). These high globulin values in the current study, regardless of season or diet, warrant further investigation. All the goats had lower than normal creatinine concentrations: 70.0-71.8 μmol/L for the browser diets and 54.8-55.2 μmol/L in the control diets. Creatinine is a product of the metabolism of creatine in muscle. Urinary creatinine is an index of total muscle mass or the turnover of the protein pool in the body (Xue et al., 1988). The higher creatinine levels in the goats that consumed browser forage could be a consequence of the CTs in browse thwarting protein digestion and absorption from the small intestine and triggering muscle catabolism to meet the amino acid deficit required to support gluconeogenesis (Turner et al., 2005). This is reflected in the serum urea concentrations. In ruminants, some blood urea moves into the forestomach and is used by microbes as a non-protein source of nitrogen, decreasing the serum urea concentration. This gastrointestinal route of utilization accelerates during times of inadequate protein intake. The findings that some goats in the browser group had lower than normal levels of ALT, GGT, ALP, and CHOL could be indicative of a genotype trait rather than liver disease. Liver disease would have manifested as lower ALB levels and other clinical signs such as jaundice, none of which were observed in this study. The higher ALP activity corresponds with goats that gained weight, which concurs with the findings by Gwaze et al. (2012), who reported an association between high ALP and growth.
Conclusion
Goats' preference for C. africana over R. íancea means that C. africana should be included in the browser species used in feeds for goats and mixed-feeders. Although goats will readily consume C. africana and R. íancea without adverse effects on physiological parameters, the browser forage on its own is insufficient to meet their maintenance requirements. More research is required to investigate how much supplementation is required for R. íancea and C. africana to be used for mixed-feeders.
Acknowledgements
The researchers received financial assistance from the University of South Africa and the National Research Foundation (NRF ZA; Grant No: TTK1207183394).
Authors' contribution
FP designed the study and was involved in the data collection, chemical analysis, interpretation of results, and manuscript write-up. ATK advised on the design, interpreted the results, and edited the manuscript. DH assisted with the chemical analysis of samples and the editing of the manuscript. KRM participated in the design of the study, the interpretation of results, and the editing of the manuscript.
Conflict of interest
The authors declare that there was no conflict of interest in undertaking this study.
References
Aganga, A.A. & Monyatsiwa, C.B. 1999. Use of browse (Terminaíia serecia, Combretum apicuíatum or Eucíea schimperi) as a supplement for growing Tswana goats. Trop. Anim. Health Prod. 31, 295-305. [ Links ]
Alexandre, G. & Madonnet, N. 2005. Goat meat production in harsh environments. Small Rumin. Res. 60, 53-66. [ Links ]
Al-Taweel, A.M., Perveen, S., El-Shafae, A.M., Fawzy, G.A., Malik, A., Afza, N., Iqbal, L. & Latif, M. 2012. Bioactive phenolic amides from Ceítis africana. Mol. 17, 2675-2682. doi 10.3390/molecules17032675 [ Links ]
AOAC International. 2005. Official Method of Analytical Chemists International. 18th ed. Association of Official Analytical Chemistry. Arlington, Virginia, USA. [ Links ]
Bele, A.A., Jadhav, V.M. & Kadam, V.J. 2010. A review: Potential of tannins. Asian J. Plant Sci. 9, 209-214. [ Links ]
Bhat, T.K., Kannan, A., Singh, B. & Sharma, O.P. 2013.Value addition of feed and fodder by alleviating the antinutritional effects of tannins. Agric. Res. 2, 89-206. doi 10.1007/s40003-013-0066-6 [ Links ]
Burritt, E.A. & Provenza, F.D. 1996. Amount of experience and prior illness affect the acquisition and persistence of conditioned food aversions in lambs. Appl. Anim. Behav. Sci. 48, 73-80. doi.org/10.1016/0168-1591(95)01004-1 [ Links ]
Clauss, M. & Dierenfeld, E.S. 2008. The nutrition of browsers. In: Zoo and Wild Animal Medicine: Current Therapy, volume 6. Ed: Fowler, M.E., Miller, R.E. & Murray, E., University of Zurich. St. Louis, USA. pp 444-454. [ Links ]
Conradie, D.C. 2017. Bioclimatic techniques to quantify mitigation measures for climate change with specific reference to Pretoria. Smart Sustainable Cities & Transport Seminar, 12-14 July 2017, CSIR, Pretoria. [ Links ]
Gattiker, C., Espie, I., Kotze, A., Lane, E.P., Codron, D. & Clauss, M. 2014. Diet and diet-related disorders in captive ruminants at the National Zoological Gardens of South Africa. Zoo Biol. 9999, 1-7. doi 10.1002/zoo.21150 [ Links ]
Gogh, J. & Anderson, J. 1988. Trees and Shrubs of Witwatersrand, Magaliesburg and Pilanesburg. Struik Publishers. Cape Town. pp 51. [ Links ]
Gundidza, M., Gweru, N., Mmbengwa, V., Ramalivhana, N.J., Magwa, Z.A. & Samie, A. 2008. Phytoconstituents and biological activities of essential oil from Rhus lancea L. F. Afr. J. Biotech. 7, 2787-2789. doi10.5897/AJB08.136 [ Links ]
Gwaze, F.R., Chimonyo, M. & Dzama, K. 2010. Relationship between nutritionally-related blood metabolites and gastrointestinal parasites in Nguni goats of South Africa. Asian-Australas. J. Anim. Sci. 23, 1190-1197. doi 10.5713/aja.2010.90547 [ Links ]
Gwaze, F.R., Chimonyo, M. & Dzama, K. 2012. Effect of season and age on blood minerals, liver enzyme levels, and faecal egg counts in Nguni goats of South Africa. Czech J. Anim. Sci. 57, 443-453. doi 10.17221/6345-CJAS [ Links ]
Hagerman, A.E. 1995. Tannins Analysis: A Handbook. Department of Chemistry, Miami University, Oxford, Ohio, USA. [ Links ]
Hooimeijer, J.F., F.A. Jansen, W.F. de Boer, D. Wessels, C. van der Waal, C.B de Jong, N.D. Otto & L. Knoop. 2005. The diet of kudus in a mopane-dominated area, South Africa. Pretoria. Koedoe 48, 93-102. [ Links ]
Kaneko, J.J., Harvey, J.W. & Bruss, M.L. 2008. Clinical Biochemistry of Domestic Animals, 6th ed. Academic Press. London. [ Links ]
Lamy, E., Rawel, H., Schweigert, F.J., Capela e Silva, F.C., Ferreira, A., Costa, A.R., Antunes, C., Almeida, A.M., Coelho, A.V. & Baptista, E.S. 2011. The effect of tannins on Mediterranean ruminant ingestive behavior: The role of the oral cavity. Mol.16, 2766-2784. doi 10.3390/molecules16042766 [ Links ]
Lombard, L. 2016. Got prunings? Joburg Zoo Giraffes need your help. News24, 14 Jan, 2016. https://www.news24.com/news24/travel/got-prunings-joburg-zoo-giraffes-need-your-help-20160114 [ Links ]
Mbatha, K.R. & Bakare, A.G. 2018. Browse silage as potential feed for captive wild ungulates in southern Africa: A review. Anim. Nutri. 4, 1-10. doi.org/10.1016/j.aninu.2017.12.003 [ Links ]
Min, B.R. & Solaiman S. 2018. Comparative aspects of plant tannins on digestive physiology, nutrition, and microbial community changes in sheep and goats: A review. J. Anim. Physiol. Anim. Nutr. 102, 1181-1193 [ Links ]
Mkhize, N.R. 2015. Unlocking resources in savannas: How goats and other mixed feeders overcome the negative effects of tannins. PhD thesis, Wageningen University, Wageningen. ISBN: 978-94-6257-427-2 [ Links ]
Moyo, M., Gueguim Kana, E.B. & Nsahlai, I.V. 2017. Modelling of digesta passage rates in grazing and browsing domestic and wild ruminant herbivores. S Afri. J. Anim. Sci. 47, 362-377. doi.org/10.4314/sajas.v47i3.13 [ Links ]
Mupangwa, J.F., Acamovic, T., Topps, J.H., Ngongoni, N.T. & Hamudikuwanda, H. 2000. Content of soluble and bound condensed tannins of three tropical herbaceous forage legumes. Anim Feed Sci. Tech. 83, 139-144. doi.org/10.1016/S0377-8401(99)00117-0 [ Links ]
National Research Council. 2007. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids. Washington, DC: The National Academies Press. doi.org/10.17226/11654. [ Links ]
Owen-Smith, N. 1994. Foraging responses of kudu to seasonal changes in food resources: Elasticity in constraints. Ecol. 75, 1050-1062. [ Links ]
Pambu-Gollah, R., Cronjé, P.B. & Casey, N.H. 2000. An evaluation of the use of blood metabolite concentrations as indicators of nutritional status in free-ranging indigenous goats. S. Afri. J. Anim. Sci. 30,115-120. doi 10.4314/sajas.v30i2.3859 [ Links ]
Radin, L., ShekVugrovečki, A.S., PejakovićHlede, J.P., Vince, S., Ljubičić, I. & Šimpraga, M. 2017. Blood metabolites of extensively reared Croatian multi-coloured goats during early lactation and early gravidity. Vet. Arh. 87, 273-280. doi 10.24099/vet.archiv.151223 [ Links ]
Radović, B., Jotanović, S., Savić, Đ. & Nitovski, A . 2011. Blood biochemical parameters of Simmental cows in different phases of reproductive cycle. Vet. Glasnik. 65, 191-201. doi 10.2298/VETGL110491R [ Links ]
Rogosic, J., Estell, R.E., Ivankovic, S., Kezic, J. & Razov, J. 2007. Potential mechanisms to increase shrub intake and performance of small ruminants in Mediterranean shrubby ecosystems. Small Rumin. Res. 74,1-15. doi.org/10.1016/j.smallrumres.2007.07.006 [ Links ]
SAS. 2010. Statistical Analysis System Institute Inc. SASSTAT Programme, Cary, NC: SAS Institute Inc. [ Links ]
Silanikove, N. 2000. The physiological basis of adaptation in goats to harsh environments. Small Rumin. Res. 35, 181 -194. [ Links ]
Shenkute, B., Hassen, A., Assafa, T., Amen, N. & Ebro, A. 2012. Identification and nutritive value of potential fodder trees and shrubs in the mid Rift Valley of Ethiopia. J. Anim. Pla. Sci. 22, 1126-1132. [ Links ]
Solaiman, S. G. & Owens, F. N. 2010. Digestive physiology and nutrient metabolism. In: Solaiman, S.G. Goat Science and Production, Wiley-Blackwell, pp 157-178. [ Links ]
Sponheimer, M., Grant, C.C., de Ruiter, D.J., Lee-Thorp, J.A., Codron, D.M. & Codron, J. 2003. Diets of impala from Kruger National Park: Evidence from stable carbon isotopes. Pretoria. Koedoe. 46, 101-106. [ Links ]
StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC. [ Links ]
Sykes, A.R., Coop, R.L. & Robinson, M.G. 1980. Chronic subclinical ovine fascioliasis: Plasma glutamate dehydrogenase, gamma-glutamyl transpeptidase, and aspartate aminotransferase activities and their significance as diagnostic aids. Res. Vet. Sci. 28, 71-75. doi.org/10.1016/S0034-5288(18)32775-9 [ Links ]
Theart, J.J.F. 2015. Forage quality of some Kalahari browse species and its ability to reduce methane emission. MSc (Agric) thesis. University of Pretoria. South Africa. [ Links ]
Tsehlana M. T. 2005. Comparative evaluation of Celtis africana In Lesotho with that in Kwazulu-Natal, South Africa. Submitted in fulfilment of the academic requirements for the degree of Master of Science in the Forestry Programme School of Agricultural Science and Agribusiness, Faculty of Science and Agriculture. University of Kwazulu-Natal, South Africa. [ Links ]
Turner, K.E., Wildeus, S. & Collins, J.R. 2005. Intake, performance, and blood parameters in young goats offered high forage diets of lespedeza or alfalfa hay. Small Rumin. Res. 59, 15-23. doi.org/10.1016/j.smallrumres.2004.11.007 [ Links ]
Van Soest, P.J., Robertson, J.B. & Lewis, B. 1991.Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583-3597. [ Links ]
Werekeh, F.S. 2012. Diet selection and foraging efficiency of Nguni goats in the bushveld of Gauteng, South Africa. MSc (Agric) thesis. University of KwaZulu-Natal, Pietermaritzburg, South Africa. [ Links ]
Xue, G.P., Snoswell, A.M. & Fishlock, R.C. 1988. Quantitative study on creatine metabolism in sheep tissues. Biochem. Int. 16, 623-627. [ Links ]
Yayneshet, T., Eik, L.O. & Moe, S.R. 2009. Seasonal variation in the chemical composition and dry matter degradability of exclosure forages in the semi-arid region of northern Ethiopia. Anim. Feed. Sci. Tech. 148: 12-33. [ Links ]
Zobel, G., Weary, D.M., Leslie, K., Chapinal, N. & Von Keyserlingk, M.A.G. 2015. Technical note: Validation of data loggers for recording lying behaviour in dairy goats. J. Dairy Sci. 98, 1082. doi.org/ 10.3168/jds.2014-8635 [ Links ]
Submitted 16 July 2022
Accepted 8 September 2022
Published 6 March 2023
# Corresponding author: phirif@unisa.ac.za














