Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Animal Science
On-line version ISSN 2221-4062
Print version ISSN 0375-1589
S. Afr. j. anim. sci. vol.52 n.6 Pretoria 2022
http://dx.doi.org/10.4314/sajas.v52i6.05
Effect of extrusion with molasses on the rumen undegradable protein fraction of canola oilcake meal and sweet lupins
T.S. BrandI, II, #; L. JordaanII; O.DreyerII
IDirectorate: Animal Sciences, Department of Agriculture, Western Cape Government, Private Bag X1, Elsenburg, 7607, South Africa
IIDepartment of Animal Sciences, University of Stellenbosch, Private Bag X1, Matieland, 7602, South Africa
ABSTRACT
The effect of the extrusion of canola oilcake meal (COM) and crushed sweet lupins (CSL) with molasses on the dry matter (DM) and crude protein (CP) degradability was determined in situ. Locally sourced (SOILL Moorreesburg, South Africa) COM and CSL, with the addition of 6% molasses, were extruded at a maximum temperature of 116 °C. A total of six Dohne Merino wethers (± 80 kg), fitted with rumen cannula, were used in this trial. Samples in polyester bags (5 g) were incubated in the rumen of the sheep at intervals of 0, 2, 4, 8, 16, 24, and 48 h. The DM and CP disappearances from the rumen were determined and degradability parameters were estimated. Extrusion increased the potentially de-gradable CP fraction by 43.5%. At each outflow rate, the average CP effective degradability of COM (68.2%) was lower than that of CSL (78.0%). Extrusion substantially lowered the CP effective degradability for both protein sources at every outflow rate tested. The biggest effect was seen at 0.08/h, where effective degradation was lowered by 25.6%. Extrusion with molasses was found to modify ruminal degradation parameters of both canola oilcake meal and crushed sweet lupins, while also decreasing the effective rumen degradation, especially at faster outflow rates. Thereby, the rumen undegradable protein fraction was increased by 85.4%. This study shows that COM and CSL extruded with 6% molasses can substantially increase RUP.
Keywords: bypass protein, crude protein degradability, digestion, in sacco, in situ, protein source
Introduction
Improving the productivity of livestock and meeting future demands for animal products and food security can be achieved if high producing animals are fed according to their specific nutrient requirements (González et al., 2018). The increased demand for animal products leads to the strategic use of nutrition for enhancing production (Martin & Kadokwa, 2006). This increased demand for animal and plant protein products may lead to the availability of protein meals becoming limited or cost-prohibitive. In addition, geopolitical crises and weakening economies in particular regions may also limit or prevent trade. This could be especially detrimental in parts of the world where soybeans (the most popular plant protein sources in animal feeds) do not grow or where soybeans and soybean meals have to be imported. Therefore, it is important to have local feed ingredient alternatives and the processing capacity to produce feed for livestock (Albin, 2015).
Canola oilcake meal (COM) is a by-product of oil production from canola seeds and is commonly incorporated in ruminant rations as a protein supplement due to its favourable protein profile (Newkirk et al., 2003; Santos, 2011). The protein content of canola meal differs depending on variety, growth conditions, and oil extraction method but its crude protein (CP) content is widely reported to be 36-40% (Paula et al., 2018). Solvent and expeller oil-extracted canola meal produced in South Africa contain 31.6% and 42.8% CP, respectively, on a dry matter (DM) basis (Brand et al., 2001). Sweet lupins are also widely used as a source of protein and energy in livestock feeds. Sweet lupins are largely free of anti-nutritional factors and have low risk of causing acidosis due to low starch levels and highly fermentable carbohydrates (Dixon & Hosking, 1992). The relatively high CP content of lupins (34% of DM (Brand et al., 2004) makes it a valuable resource for ruminant nutrition as they are also cost competitive. Sweet lupins are nitrogen fixating and can be used in crop rotation systems with small cereals to reduce the need for inorganic nitrogen fertilizers (Abraham et al., 2019).
Both COM and crushed sweet lupins (CSL) can be used to partially replace soybean meal in ruminant diets, but inclusion is restricted due to high rumen degradable protein (RDP) content of these ingredients. The RDP fraction of COM and CSL is ~77 and 81%, respectively. These extremely high RDP values limit the use of COM and CSL as a substitute for soybean meal (NRC, 2001). Rumen degradable proteins are soluble proteins that are degraded extensively in the rumen by microbes. This results in large scale ammonia production in ruminants. Ammonia is either directly absorbed via the rumen wall, passed out the rumen via the fluid digesta phase, or is converted to microbial crude protein (Kempton & Leng, 1971). Only a small portion of urea is recycled in the rumen to contribute nitrogen that will be used to produce microbial protein. In order to increase the efficiency of protein utilisation from the highly degradable protein sources, these proteins need to be protected from excessive ruminal degradation, allowing the protein to bypass the rumen (rumen undegradable protein, RUP). The RUP then gets digested to amino acids, which will be absorbed from the small intestine of the ruminant and will be directly available for production (Walli, 2005; Makkar & Beever, 2013).
Highly degradable protein sources such as COM and lupins could possibly be extruded before inclusion in ruminant diets to decrease the rumen degradability thereof and increase the RUP fraction. Extrusion is a heat treatment with added pressure and moisture that leads to the Maillard reaction and partial protein denaturing, which reduces the rumen degradability of protein sources without impairing protein digestibility in the small intestine, thus increasing the supply of RUP and intact amino acids to the small intestine. Extrusion processing conditions regarding the temperature application can alter the quality of protein. Chang & Perry (2011) suggested that the optimum heat application is dependent mainly on the moisture content and carbohydrate composition, due to extrusion resulting in the gelati-nization of starches. Inclusion of heat-treated canola for dairy cows improves milk production due to higher RUP (Jones et al., 2001; Wright et al., 2005). Therefore, improving efficiency of feed conversion to meat or milk can have a substantial impact on the profitability of a ruminant enterprise (Makkar & Beever, 2013).
Kumar et al., (2015) stated that protein degradation was decreased through the Maillard reaction, which occurs between sugar aldehyde groups and free amino acids, and adversely increases the amount of protein escaping protein degradation. The treatment of soybean meal and (COM) with xylose was successful in decreasing the RDP fraction thereof (Harstad & Prestl0kken, 2000; Tuncer & Sacakli, 2003).
Paula et al. (2017) added 2-3% molasses to canola meal before extrusion to increase the browning reaction. The aim of this study was to determine the effect of extrusion with 6% molasses on the in situ DM and CP degradabilities of locally produced COM and CSL with the objective of decreasing rumen protein degradation.
Materials and Methods
Ethical clearance for this research was granted by the Animal Care and Use Research Ethics Committee of the University of Stellenbosch (Ethical clearance number #0378 and #0379). Six Dohne Merino wethers (± 80 kg), fitted with rumen cannula, were housed in individual pens (1 m χ 2 m) at the Welgevallen Experimental Farm of the University of Stellenbosch during the trial. The animals had ad íibitum access to clean drinking water and were supplied a basal diet of wheat straw and lucerne hay (50:50) ad íibitum during the experimental period. The feed was replenished twice daily (every morning and evening) as necessary. The daily intake was estimated as 3% of the body weight. The wethers were already adapted to the feed before the in situ trial started.
The experimental feedstuffs (COM and CSL) were obtained from SOILL, Moorreesburg (Western Cape, South Africa) and then, to execute the extrusion experiments, 6% molasses (Kalori 3000, Yara Animal Nutrition) was added. Half of each batch was kept and the other half was extruded with temperatures reaching a maximum of 116 °C (at 13% moisture) with a Millbank extruder that is based on the Insta Pro 2000RC model (maximum capacity of 1 ton per hour, motor at 55 kW, and feeder motors at 1.5 kW). The following four feeds were tested in this trial: raw (unprocessed) COM (RCOM) and CSL (RCSL) and extruded COM (ECOM) and CLS (ECSL). These four feeds were used for further analysis after being milled through a 2-mm size screen using a Wiley mill (Thomas Scientific, Swedesboro, NJ, USA).
The DM and CP degradabilities of RCOM, ECOM, RCSL, and ECSL were determined using the in situ technique described by 0rskov & McDonald (1979). The feed samples were dried in a force draught oven for a minimum of 48 h at 60 °C, after which 5-g samples were weighed on a digital scale and inserted into each of a series of previously dried, weighed, and marked polyester bags (12 cm χ 9 cm) with mean pore size of 15 μm The bags were tied off by nylon strings of different lengths to prevent the strings from knotting in the rumen, as well as to ensure easy retrieval of the bags. Bags were incubated in the rumen at different time intervals, being 2, 4, 8, 16, 24, and 48 h. An incubation series started when the first bag was inserted into the rumen cannula at 14h00. The incubation was ended when all of the bags were removed at the same time after 48 h. After bag removal, the bags were submerged in ice water to rapidly stop further degradation. The bags were washed under running tap water until water squeezed from it was clear. The 0-hour bag was prepared in the same way and was washed under the tap like the rest, without being placed in the rumen. All bags were dried after incubation in a force draught oven for a minimum of 48 h at 60 °C.
The treatments were randomly assigned to wethers during each period. This procedure was repeated in six periods as a completely randomised design, giving a total of six observations for each variable studied.
After drying the bags for 48 h at 60 °C, the nylon strings were removed and the dried bags were weighed to determine the DM residue. The nitrogen content (%) of the residue was determined using the Dumas combustion method (Method 990.03; AOAC, 2002) using a LECO TruMac N Nitrogen De-terminator, version 1.3X (LECO Corporation, Michigan, USA). The CP content of the dry matter was determined by multiplying the percentage nitrogen by a factor of 6.25.
The chemical properties of the studied feedstuffs before rumen incubation were determined with the official methods as described by the Association of Official Analytical Chemists (AOAC, 2002) for DM (method 934.01), ash (method 942.05), CP (method 990.03), and crude fat (method 2003.06). Their NDF and ADF contents were determined according to Van Soest et al. (1991). Calcium and phosphorus contents were determined using method 6.1.1 of the Agri Laboratory Association of Southern Africa guidelines (ALASA, 1998).
Rumen DM and CP disappearances were expressed as percentages of the amount remaining after rumen incubation. The percentage material degraded was fitted to the following one-compartment model, as proposed by 0rskov & McDonald (1979), using the non-linear regression procedure of SAS 9.4 software (SAS Institute Inc., Cary, NC, USA, 2014) to determine DM and CP degradability parameters:
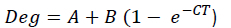
Where Deg = actual degradation at time, T (%)
A = rapidly soluble fraction, intercept of degradation curve at T = 0 (%)
B = the fraction that will degrade over time, potential degradable fraction, or asymptote (%)
e = the base of natural logarithms
C = the rate constant for degradation of the B fraction (%/h)
Ruminal retention time affects the extent of degradation and therefore a fractional outflow rate of undegraded protein from the rumen (k) was taken into account when the percentage effective degradation (Degeff) was calculated as:
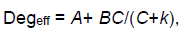
at chosen values of 0.02 (low intake level), 0.04, 0.05 (medium intake level), 0.06, 0.08/h (high intake level).
Non-linear parameters A, B, and C, as well as the Degeff values, were submitted to a two-way analysis of variance (ANOVA) based on a 2x2 factorial design using SAS 9.4 software (SAS Institute Inc., Cary, NC, USA, 2014). Significance was declared at P <0.05 and tendencies at P <0.10 using Bonferroni tests.
Results
The chemical composition of the feedstuffs used in this study is presented in Table 1. Extrusion did not seem to have a noticeable effect on the chemical composition of canola oilcake meal, with CP content of 33.6% for COM and 34.3% for ECOM. The CP content of ECOM was 29.6% while that of the RCOM was 26.6%. The NDF content of extruded crushed sweet lupins seemed to be different to unprocessed samples (18.9% and 22.14%, respectively).
The in situ DM degradability parameters showed an interaction between the main effects of protein source and processing for the rapidly soluble fraction, but not for the potential degradable fraction and the rate of degradation of B. Thus, the results are presented in Table 2 as main effects and with an interaction table. Canola oilcake meal (RCOM) had the highest rapidly soluble fraction (42.5%) which differed substantially from ECOM (34.5%), RCSL (34.7%), and ECSL (32.7%), which in turn did not differ from each other. Between protein sources, COM had a lower potential degradable fraction than CSL (51.2% and 67.8%, respectively). Extrusion increased the potential degradable fraction for DM from 53.6% to 65.4%. No differences were observed between protein sources for the rate of degradation of the degradable fraction, while extrusion did lower the rate of degradation from 0.071 to 0.046%/h.
The DM effective degradability showed interactions at each of the outflow rates, except for 0.08/h and for that reason it is presented in Table 3 as a main effect of protein source and processing, as well as an interaction table. At an outflow rate of 0.08/h, canola oilcake meal was substantially lower (58.8%) than crushed lupins (61.4%). At outflow rates of 0.02, 0.04, 0.05, and 0.06/h, there were no significant differences between RCOM and ECOM but there were significant differences between RCSL and ECSL where extrusion lowered the DM effective degradability, on average, from 76.4% to 55.2%.
The in situ crude protein degradability parameters are presented in Table 4 as main effects and an interaction table, as an interaction was observed for the rapidly soluble fraction as well as the rate of degradation for the potential degradable fraction but not for the potential degradable fraction. The rapidly soluble values of unprocessed COM (49.2%) and CSL (46.0%) did not differ from each other (P >0.05). Extrusion was found to markedly lower the soluble fraction from 49.2% for RCOM to 18.6% for ECOM. On the other hand, the effect of extrusion had no significant impact on the soluble fraction of RCSL. No difference was found for the potential degradable fraction between protein sources, whereas extrusion increased (P <0.001) the potential degradability from 51.3% to 73.6%. The highest rate of degradation of the potential degradable fraction was found for RCSL (0.130%/h), which differed substantially from ECSL (0.044%/h), RCOM (0.063%/h), and ECOM (0.053%/h). The expected CP disappearance from the rumen over time, derived from the in situ CP degradability parameter estimates, is presented graphically in Figure 1.
No interactions were observed between the main effects for crude protein effective degradability and thus the main effects are presented in Table 5. At each outflow rate, the effective degradability was lower (P <0.001) for canola oilcake meal than for crushed sweet lupins. The average effective crude protein degradability for canola meal was 68.2% and 78.0% for crushed lupins. Extrusion substantially lowered the CP effective degradability at every tested outflow rate. The biggest effect was seen at an outflow rate of 0.08/h, where effective degradation was lowered (P <0.001) by 25.6%, from 74.5% to 55.4%. This is expected as 0.08/h is the outflow rate representing high producing animals at a faster outflow rate of digesta from the rumen.
Discussion
ECL showed a slightly lower NDF content than that of the unprocessed crushed lupins. This could be because of partial depolymerization of polysaccharides of the cell wall, which makes it more soluble in the acid and alkali solutions used during measurements (Solanas et al., 2005) during the extrusion process. This has been observed by other authors using extruded lupins (Cros et al., 1992; Barchiesi-Ferrari & Anrique, 2011). The CP content of ECSL seemed markedly higher than unprocessed lupins (26.6% and 29.5%), which may be due to the release of peptides from cell walls during the extrusion process. The CP value of sweet lupins in this study was lower than the value of 35.5% reported by Brand et al. (1992). One possible reason could be the addition of molasses in this study, which would also explain the lower DM of lupins in this study as molasses (Kalori 3000, Yara Animal Nutrition) has a high hygroscopicity.
The reason for the observed interaction in the DM soluble fraction could be due to extrusion lowering the soluble fraction for COM, whereas this was not observed for CSL. Dry matter effective degradability showed interaction at every outflow rate except 0.08%/h. This could be due to no difference being observed between RCOM and ECOM, whereas decreases were observed for ECSL compared to RCLS at outflow rates of 0.02, 0.04, 0.05, and 0.06%/h. The DM effective degradation at 0.08%/h for COM was lower than CSL, i.e., 58.8% and 61.4% respectively. Extrusion decreased the DM effective degradation at 0.08%/h by 10.7%.
Therefore, by increasing the RUP fraction, protein utilization and subsequent animal performance is improved. Extrusion at 116 °C in the current study markedly lowered the CP rapidly soluble fraction of COM by 62%. Similar results for canola oilcake meal heated at 125 °C were found by Mustafa et al. (1997) and Griffiths (2004), who extruded canola meal at 120 °C. Similar results were also observed with extrusion at higher temperatures. Michalak & Potkanski (2005) showed that extrusion at 140 °C of rapeseed oilcake meal lowered the soluble fraction by 13%. Brand & Jordaan (2020) reported a 48.4% decrease in the rapidly soluble fractions of sweet lupins extruded at 116 °C. Kibelolaud et al. (1993) extruded white L. albus seeds and reported a 4% increase in the soluble fraction when extruded at 110 °C, but a 19.3% decrease at 130 °C. Griffiths (2004) extruded lupins at 120 °C and found a 6% increase in the rapidly soluble fraction. Barchiesi-Ferrari & Anrique (2011) extruded dehulled L. albus at 130 °C (20% moisture), which decreased the soluble fraction by 12.4% (from 42.7 to 37.4%). Barchiesi et al. (2018) extruded dehulled L. albus at 140 °C (20% moisture), which led to a decrease of 29% in the soluble fraction. Thus Brand & Jordaan (2020) justified that a decrease in the rapidly soluble fractions of sweet lupins could be reached at relatively lower extrusion temperatures, in contrast to the previous research mentioned.
In the current study, extrusion increased the CP potential degradable fraction by 30.3% while no effect was seen between protein sources. Kibelolaud et al. (1993) observed an 18.6% decrease in the potential degradable fraction for lupins extruded at 110 °C. However, a 43.1% increase was seen for extrusion at 130 °C, which indicates that a higher temperature produced better results for extrusion in that particular study. Solanas et al. (2008) extruded L. albus at 140 °C, which resulted in no difference in the potential degradable fraction (62.1% and 64.2%), while Barchiesi et al. (2018) found a 4% increase at the same temperature. Barchiesi-Ferrari & Anrique (2011) extruded dehulled L. albus at 130 °C, which led to a 4.7% increase in the potential degradable fraction. Mustafa et al. (1997) heated canola meal at 125 °C and found an 18.9% increase in the potential degradable fraction. Michalak & Potkanski (2005) showed that extrusion at 140 °C of rapeseed oilcake meal increased the potential degradable fraction by only 4.9%. Barchiesi-Ferrari & Anrique (2011) extruded rapeseed meal at 120 °C (20% moisture) and found no difference in the potential degradable fraction (45.1% and 45.7%).
Extrusion did not affect the rate of degradation of the potential degradable fraction for canola oilcake meal in this study. Barchiesi-Ferrari & Anrique (2011) also observed no difference when extruding rapeseed meal at 120 °C. However, Mustafa et al. (1997) extruded canola meal at 125 °C for 20 min and found a decrease in the rate of degradation from 6%/h to 1.4%/h. The rate of degradation lowered substantially to 66.2% for crushed sweet lupins in this study. The same trend was seen in lupins by other authors (Kibelolaud et al., 1993; Griffiths, 2004; Solanas et al., 2008; Brand & Jordaan, 2020). Barchiesi et al. (2018) extruded dehulled L. albus at 140 °C (20% moisture), which led to no difference in the rate of degradation (0.17% and 0.15%).
A lower CP effective degradability of COM at every outflow rate tested compared to CSL (respective averages, 68.1% and 78%) indicated or suggested that extrusion markedly lowered the CP effective degradability at every outflow rate tested, 0.02, 0.04, 0.05, 0.06 and 0.08%/h, by 10.6%, 18%, 20.3%, 22.6%, and 25.6%, respectively. The CP effective degradation of ECSL in this study is lower than the observed degradabilities of lupins by Brand & Jordaan (2020), which might be due to the addition of molasses, which promotes the Maillard reaction, lowering rumen degradability. The effect could also be seen in lower CP rapidly soluble fractions and the rate of degradation, as well as higher potential de-gradable fraction values, in this study. Previous studies found that heating rapeseed at high temperatures (150 °C and 200 °C; Lindberg, 1982) and lupins (195 °C; Benchaar & Moncoulon, 1993) decreased effective degradability while no effect was found at 100 °C by Lindberg (1982). Kibelolaud et al. (1993) extruded white L. albus seeds at 110 °C and 130 °C, which decreased the CP effective degradation at the 0.06%/h outflow rate by 3.9% and 14.5%, respectively. In contrast, McKinnon et al. (1995) found that heating canola meal to 145 °C for 30 min decreased intestinal and total tract digestibility. However, heating at 125 °C lowered ruminal degradability without negative effects on intestinal digestibility. Similarly, Michalak & Potkanski (2005) showed that extrusion at 140 °C of rapeseed oilcake meal reduced effective degradability by 30% and shifted protein digestion from the rumen to the small intestine, producing similar total tract protein digestibilities. This shows that higher temperatures are not necessarily needed to achieve the benefits of extrusion and heat treatment. High temperatures could possibly lead to heat damage and increased production costs.
Paula et al. (2017) extruded canola meal with 2-3% added molasses and fed it to lactating dairy cows with no effect on milk yield and milk components. It did, however, decrease urinary nitrogen percentage, faecal nitrogen, and milk urea concentration, and thus may reduce environmental impact. An increase in nitrogen utilisation was also seen by Huhtanen et al. (2011) and Broderick et al. (2015).
Differences between studies could be due to intrinsic differences in the feeds or extrinsic differences such as smaller particle size and different fistulated animals (steers vs sheep), basal diets, in situ techniques, and are mostly due to processing conditions (Habib et al., 2013). Van Soest (1987) suggested that the non-protein nitrogen and rapidly degradable true protein fractions denature at lower temperatures and become intermediately or slowly degradable, while the slowly degradable protein fraction responds at higher temperatures and usually becomes unavailable due to heat damage from the Maillard reaction. Optimum temperature and conditions vary from one dietary protein to another.
Extrusion with 6% molasses at 116 °C more than doubled the RUP fraction (calculated from the CP content on a dry matter basis and the effective degradability at 0.08%/h outflow rate) of the protein sources tested, which resulted in a more favourable ratio of RDP:RUP for inclusion in ruminant diets. The RDP fraction was decreased in favour of increasing RUP fraction of canola oilcake meal by 70.1% and crushed sweet lupins by 107.8% at an outflow rate of 0.08%/h. The intestinal degradability and the effect on bypass amino acid composition of these protein sources was not covered in this study.
Conclusion
Extrusion with 6% molasses modified ruminal degradation parameters of canola oilcake meal and crushed sweet lupins, while also decreasing the effective rumen degradation, especially at faster outflow rates. Thereby, the RUP fraction of canola oilcake meal and crushed sweet lupins was increased by extrusion with molasses compared to no extrusion (from 3.3% to 6.5%).
Regionally grown and properly processed feed materials, such as those produced from canola and lupins, play important roles in supporting livestock production, which is a major and reliable source of animal protein in the human diet. Thus, locally produced canola oilcakes and lupins could be extruded, increasing the quantity of local produced feeds being used and alternatively supporting the production of these commodities, ultimately decreasing the South African imports of canola oilcake and lupin.
Further research is required to examine the effect of extrusion with molasses of canola oilcake meal and crushed sweet lupins on the intestinal digestibility, particularly its effect on the availability of essential amino acids. This could be done through bioassays to generate true ileal digestibility values of crude protein to be used in feeds.
Acknowledgements
Acknowledgements are hereby made to the Western Cape Department of Agriculture for providing facilities to do the study as well as joint funding of the study. In addition, the Western Cape Agricultural Research Trust is thanked for the joint funding of the study.
Authors' contributions
Concept and design: TSB; Source of funding: TSB; data collection and analysis: TSB & LJ; drafting of initial paper: LJ; critical revision and final approval of version to be published: TSB. This statement is to certify that all the authors of this paper made substantial contributions to conception and design, acquisition of data, and analysis and interpretation of data. All the authors have seen and approved the manuscript being submitted.
Conflict of interest declaration
The authors certify that they have no affiliations with or involvement in any organization or entity with financial or non-financial interests in the subject matter and materials discussed in this manuscript.
References
Abraham, E.M., Ganopoulos, I., Madesis, P., Mavromatis, A., Mylona, P., Nianiou-Obeidat, I., Parissi, Z., Polidoros, A., Tani, E. & Vlachostergios, D., 2019. The use of lupin as a source of protein in animal feeding: Genomic tools and breeding approaches. Int. J. Mol. Sci. 20, 851. DOI 10.3390%2Fijms20040851 [ Links ]
ALASA, 1998. Method 6.1.1 - Dry ashing. In: Handbook of Feeds and Plant Analysis. Ed: Palic, D., Hatfield, Pretoria, South Africa [ Links ]
Albin, D., 2015. Further canola processing makes better meal for livestock feed. Feed Strategy. (Accessed 11 February 2020) https://www.feedstrategy.com/anima;l-feed-manufacturing/further-canola-processing-makes-better-meal-for-livestock-feed/ [ Links ]
AOAC, 2002. Official Methods of Analysis (17th Ed.). Association of Official Analytical Chemists, Inc., Arlington, Virginia, USA [ Links ]
Barchiesi-Ferrari, C. & Anrique, R., 2011. Ruminal degradability of dry matter and crude protein from moist dehulled lupin and extruded rapeseed meal. Chilean J. Agr. Res. 71, 430-436. DOI 10.4067/S0718-58392011000300014 [ Links ]
Barchiesi, C., Williams, P. & Velàsquez, A., 2018. Lupin and pea extrusion decreases the ruminal degradability and improves the true ileal digestibility of crude protein. Cien. Inv. Agr. 45, 231-239. DOI 10.7764/rcia.v45i3.1762 [ Links ]
Benchaar, C. & Moncoulon, R., 1993. Effect of extrusion at 195 °C on the in situ disappearance of lupin amino acids in the rumen and in the intestine in the dairy cow. Ann. Zootech. 42, 128-129. DOI 10.1051/animres:19930211 [ Links ]
Brand, T.S., Franck, F., Brand, A.A., Durand, A. & Coetzee, J., 1992. An evaluation of faba bean (Vicia faba) and lupin (Lupinus albus) stubble and seed for sheep. S. Afr. J. Anim. Sci. 22, 170-173. http://www.sasas.co.za/journals/an-evaluation-of-fababean-vicia-faba-andlupin-lupinus-albus-stubble-and-seed-for-sheep-short-communication/ [ Links ]
Brand, T.S., Brandt, D.A. & Cruywagen, C.W., 2001. Utilisation of growing-finishing pig diets containing high levels of solvent or expeller oil extracted canola meal. New Zeal. J. Agr. Res. 44, 31-35. DOI 10.1080/00288233.2001.9513459 [ Links ]
Brand, T.S., Brandt, D.A. & Cruywagen, C.W., 2004. Chemical composition, true metabolisable energy content and amino acid availability of grain legumes for poultry. S. Afr. J. Anim. Sci. 34, 116-122. DOI 10.4314/sajas.v34i2.3815 [ Links ]
Brand, T.S. & Jordaan, L., 2020. Effect of extrusion on the rumen undegradable protein fraction of lupins. S. Afr. J. Anim. Sci. 50, 779-785 DOI 10.4314/sajas.v50i6.2 [ Links ]
Broderick, G.A., Faciola, A.P. & Armentano, L.E., 2015. Replacing dietary soybean meal with canola meal improves production and efficiency of lactating dairy cows. J. Dairy Sci. 98, 5672-5687. DOI 10.3168/jds.2015-9563 [ Links ]
Chalupa, W., 1975. Rumen bypass and protection of proteins and amino acids. J. Dairy Sci. 58, 1198-1218. DOI 10.3168/jds.s0022-0302 (75)84697-2 [ Links ]
Chang, Y. H., & Perry, K. W. 2011. Effects of Extrusion process variables on quality properties of wheat-ginseng extrudates. Int. J. Food Prop. 14, 914-925 https://doi.org/10.1080/10942910903491173. [ Links ]
Cleale, R.M., Britton, R.A., Klopfenstein, T.J., Bauer, M.L., Harmon, D.L. & Satterlee, L.D., 1987. Induced non-enzymatic browning of soybean meal. II. Ruminal escape and net portal absorption of soybean protein treated with xylose. J. Anim. Sci. 65, 1319-1326. DOI 10.2527/jas1987.6551319x [ Links ]
Cros, P., Moncoulon, R., Bayourthe, C. & Vernay, M., 1992. Effect of extrusion on ruminal and intestinal disappearance of amino acids in white lupin seeds. Can. J. Anim. Sci., 72, 89-96. DOI 10.4141/cjas92-010 [ Links ]
Dixon, R.M. & Hosking, B.J., 1992. Nutritional value of grain legumes for ruminants. Nutr. Res. Rev. 5, 19-43. DOI 10.1079/NRR19920005 [ Links ]
González, L.A., Kyriazakis, I. & Tedeschi, L.O., 2018. Review: Precision nutrition of ruminants: Approaches, challenges, and potential gains. Animal. 12, s246-s261. DOI 10.1017/S1751731118002288 [ Links ]
Griffiths, J., 2004. The effect of extrusion on the degradability parameters of various vegetable protein sources. MSc (Agric) thesis, Department Animal Science, Stellenbosch University, South Africa. [ Links ]
Habib, G., Khan, N.A., Ali, M. & Bezabih, M., 2013. In situ ruminal crude protein degradability of by-products from cereals, oilseeds and animal origin. Livest. Sci. 153, 81-87. DOI 10.1016/j.livsci.2013.01.017 [ Links ]
Harstad, O.M. & Prestl0kken, E., 2000. Effective rumen degradability and intestinal indigestibility of individual amino acids in solvent-extracted soybean meal (SBM) and xylose-treated SBM (SoyPass®) determined in situ. Anim. Feed Sci. Tech. 83, 31-47. DOI 10.1016/S0377-8401(99)00114-5 [ Links ]
Huhtanen, P., Hetta, M. & Swensson, C., 2011. Evaluation of canola meal as a protein supplement for dairy cows: A review and a meta-analysis. Can. J. Anim. Sci. 91, 529-543. DOI 10.4141/cjas2011-029 [ Links ]
INRA-CIRAD-AFZ Feed Tables 2020. Feed tables of composition and nutritional values of feed materials. (Accessed 03 March 2020) https://www.feedtables.com/ [ Links ]
Jones, R.A., Mustafa, A.F., Christensen, D.A. & McKinnon, J.J., 2001. Effects of untreated and heat-treated canola presscake on milk yield and composition of dairy cows. Anim. Feed Sci. Technol. 89, 97-111. DOI 10.1016/S0377-8401(00)00219-4 [ Links ]
Kempton, T. J., & Leng, R. A. 1971. Principles for the use of non-protein-nitrogen and bypass proteins in diets of ruminants. Dep. Biochem. Nutr. Fac. Rural Sci. Univeristy New Engl., 160-181. [ Links ]
Kibelolaud, A.R., Vernay, M., Bayourthe, C. & Moncoulon, R., 1993. Effect of extruding on ruminal disappearance and lower gastrointestinal tract digestion of white lupin seeds. Can. J. Anim. Sci. 73, 571-579. [ Links ]
Kumar, S., Kumari, R., Kumar, K., & Walli, T. K. 2015. Roasting and formaldehyde method to make bypass protein for ruminants and its importance: A review. Indian J. Anim. Sci. 85, 223-230. [ Links ]
Lindberg, J.E., 1982. Ruminal flow rate of soya-bean meal, rapeseed meal and cottonseed meal in cows fed at maintenance and at three times maintenance. J. Agr. Sci. 98, 689-691. DOI 10.1017/S0021859600054502 [ Links ]
Makkar, H.P.S. & Beever, D., 2013. Optimization of feed use efficiency in ruminant production systems. Proceedings of the FAO Symposium, 27 November 2012, Bangkok, Thailand. FAO Animal Production and Health Proceedings, No. 16. Rome, FAO and Asian-Australasian Association of Animal Production Societies. pp. 33-44. http://www.fao.org/3/i3331e/i3331e.pdf [ Links ]
Martin, G.B. & Kadokawa, H., 2006. Clean, green and ethical animal production. Case study: reproductive efficiency in small ruminants. J. Reprod. Dev. 52, 145-152. DOI 10.1262/j rd.17086-2 [ Links ]
McDonald, I. W., 1948. The absorption of ammonia from the rumen of sheep. Biochem. J. 42, 584-587. DOI 10.1042/bj0420584 [ Links ]
McKinnon, J.J., Olubobokun, J.A., Mustafa, A., Cohen, R.D.H. & Christensen, D.A., 1995. Influence of dry heat treatment of canola meal on site and extent of nutrient disappearance in ruminants. Anim. Feed Sci. Tech. 56, 243-252. DOI 10.1016/0377-8401(95)00828-4 [ Links ]
Michalak, S. & Potkanski, A., 2005. Effect of extrusion of rapeseed oilmeal on rumen degradability and intestinal digestibility. J. Anim. Feed Sci. 14, 283-286. DOI 10.22358/jafs/70539/2005 [ Links ]
Mustafa, A.F., McKinnon, J.J., Thacker, P.A. & Christensen, D.A., 1997. Effect of borage meal on nutrient digestibility and performance of ruminants and pigs. Anim. Feed. Sci. Technol. 64, 273-285. DOI 10.1016/S0377-8401(96)01040-1 [ Links ]
Newkirk, R.W., Classen, H.L., Scott, T.A. & Edney, M.J., 2003. The digestibility and content of amino acids in toasted and non-toasted canola meals. Can. J. Anim. Sci. 83, 131-139. DOI 10.4141/A02-028 [ Links ]
National Research Council, 2001. Nutrient requirements of dairy cattle. 7th rev. ed. Natl. Acad. Press, Washington, D.C. [ Links ]
Ørskov, E.R. & McDonald, I., 1979. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agr. Sci. 92, 499-503. DOI 10.1017/S0021859600063048 [ Links ]
Paula, E.M., Monteiro, H.F., Silva, L.G., Benedeti, P.D.B., Daniel, J.L.P., Shenkoru, T., Broderick, G.A. & Faciola, A.P., 2017. Effects of replacing soybean meal with canola meal differing in rumen-undegradable protein content on ruminal fermentation and gas production kinetics using two in vitro systems. J. Dairy Sci. 100, 5281-5292. DOI 10.3168/jds.2016-12301 [ Links ]
Paula, E.M., Broderick, G.A., Danes, M.A.C., Lobos, N.E., Zanton, G.I. & Faciola, A.P., 2018. Effects of replacing soybean meal with canola meal or treated canola meal on ruminal digestion, omasal nutrient flow, and performance in lactating dairy cows. J. Dairy Sci. 101, 328-339. DOI 10.3168/jds.2017-13392 [ Links ]
Santos, J.E.P., 2011. Chapter 5: Nutritional management of lactating dairy cows. Dairy production medicine 1st ed., pp. 33-72. John Wiley & sons, Inc. DOI 10.1002/9780470960554.ch5 [ Links ]
Solanas, E., Castrillo, C., Balcells, J. & Guada, J.A., 2005. In situ ruminal degradability and intestinal digestion of raw and extruded legume seeds and soya bean meal protein. J. Anim. Physiol. Anim. Nutr. 89, 166-171. DOI 0.1111/j.1439-0396.2005.00555.x [ Links ]
Solanas, E.M., Castrillo, C., Jover, M. & de Vega, A., 2008. Effect of extrusion on in situ ruminal protein degrada-bility and in vitro digestibility of undegraded protein from different feedstuffs. J. Sci. Food Agric. 88, 25892597. DOI 10.1002/jsfa.3345 [ Links ]
Tuncer, S.D. & Sacakli, P., 2003. Rumen degradability characteristics of xylose treated canola and soybean meals. Anim. Feed Sci. Technol. 107, 211-218. DOI 10.1016/S0377-8401(03)00117-2 [ Links ]
Van Soest, P.J., 1987 Nutritional ecology of the ruminant. 2nd ed. Cornell University press. Ithaca, NY. DOI 10.7591/9781501732355 [ Links ]
Van Soest, P.J., Robertson, J.B. & Lewis, B.A., 1991. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583-3597. DOI 10.3168/jds.s0022-0302 (91)78551-2 [ Links ]
Walli, T.K., 2005. Bypass protein technology and the impact of feeding bypass protein to dairy animals in tropics: A review: Indian J. Anim. Sci., 75, 135-142. [ Links ]
Wright, C.F., Keyserlingk, M.A.G., Swift, M.L., Fisher, L.J., Shelford, J.A. & Dinn, N.E., 2005. Heat- and lignosul- fonate-treated canola meal as a source of ruminal undegradable protein for lactating dairy cows. J. Dairy Sci. 88, 238-243. DOI 10.3168/jds.S0022-0302(05)72681-3 [ Links ]
Submitted 3 June 2022
Accepted 14 September 2022
Published 6 February 2023
# Corresponding author: Tertius.Brand@westerncape.gov.za














