Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Animal Science
versión On-line ISSN 2221-4062
versión impresa ISSN 0375-1589
S. Afr. j. anim. sci. vol.52 no.6 Pretoria 2022
http://dx.doi.org/10.4314/sajas.v52i6.01
Influence of reduced dietary protein level on quality of pork carcasses in Windsnyer pigs
V. A. HlatiniI; C. N. NcobelaI; M. ChimonyoII, #
IAgricultural Research Council, Animal Production Institute (Nutrition Building), Private Bag X2, Irene 0062, South Africa
IIAnimal Science, Faculty of Science, Engineering and Agriculture, University of Venda, P/Bag X5050, Thohoyandou, Limpopo, 0950, South Africa
ABSTRACT
To promote the sustainable production of local pigs, their dietary protein requirements need to be determined. Meat production from these pigs when fed on appropriate diets, coupled with their adaptability to climatic extremes and disease and parasite challenges, could be of huge benefit to the pork industry. The objective of the study was to determine the carcass traits, primary pork cuts, and internal organ weights of pigs fed decreasing dietary protein levels. Thirty, slow-growing, Windsnyer male pigs were randomly allocated to six dietary treatments in a complete randomized design. There were five replications for each of the six treatments. Dietary crude protein levels in the six experimental diets were 193, 174, 154, 135, 116, and 97 g/kg, respectively. The diets were formulated to contain similar net energy levels of ~9.5 MJ/kg. Lysine, methionine, threonine, and tryptophan levels were the same for all diets. A two week adaptation was followed by an 8w feeding phase. At slaughter, pigs had an average weight of ~39.13±0.85 kg. Pigs were humanely slaughtered at the end of feeding period to determine carcass characteristics, primary pork cuts, and internal organ size. A negative linear relationship was observed between protein levels and cooler shrink. There was a positive linear relationship between protein level and dressing percentage, cooler shrink, and shoulder fat. There was a quadratic relationship between dietary protein level and shoulder fat, ham diameter, P2(3) backfat depth, and kidney weight. The thickness of dorsal fat at the last rib, the thickness of back fat, and the width of back fat at P2(2) increased linearly as protein level decreased. The reduction in dietary protein level had an influence on carcass traits, primal pork cuts, and internal organs in slow-growing Windsnyer pigs. A reduction in dietary protein level below 116 g/kg compromised ham diameter, P2(3) width of back fat thickness, shoulder fat, and kidney weight.
Keywords: back fat, dressing percentage, ham diameter, kidney weight, ideal protein level
Introduction
African countries have a large number of local pigs (Halimani et al., 2012). The Kolbroek and Windsnyer pigs are commonly found in South Africa, Swaziland, Lesotho, Malawi, and Zimbabwe. These slow-growing pigs face extinction, and there is a need to conserve them through sustainable use (Hlatini et al., 2020). Local pigs have high thermotolerance, low feed requirements, low labour requirement, and are adapted to endemic parasites. These attributes are beneficial to resource-limited farmers (Kanengoni et al., 2004; Halimani et al., 2012). Slow-growing indigenous pigs are rejected by mainstream economies, but they have the potential to alleviate poverty in rural communities and improve human dietary nutrition (Halimani et al., 2012). The human demand for a good source of protein and their responsiveness to the healthy meat consumption are other factors influencing the need to improve the production of local pigs. The intense demand for pork recently has put pressure to increase pork production in South Africa. The strong demand for pork implies a desperate need for protein-rich feedstuffs to meet a potential growth rate of pigs. However, conventional protein-rich feedstuffs are prohibitively expensive and not always available (Pham et al., 2010; Ncobela et al., 2018). This is due to climate change and competition with human and biofuels. The commercial pig industry in South Africa prioritises fast-growing breeds with high feed conversion rates such as the Large White. The pork industry only relies on fast-growing imported pigs (Wood et al., 2004). These breeds, however, have high feeding requirements and need high inclusion levels of specific nutrients, particularly protein (NRC, 2012). There is a need to reconsider harnessing local pigs such as Windsnyer pigs, which may require less protein needs than fast-growing pigs.
The Windsnyer is a slow-growing pig indigenous to southern Africa and is adapted to survive under low planes of nutrition; they have the ability to reproduce at low dietary protein levels. Indigenous pigs are avoided in pork production due to their low growth rate and high feed conversion ratio (Ncobela et al., 2018). As a result, South Africa opt to imports pork every year instead of paying attention on the commercial value of Windsnyer pigs. This occurrence could change by including traits from indigenous pigs through crossbreeding. Indigenous pigs have been used to produce pork for fresh consumption (Hoffman et al., 2005). Hence, improving the performance and pork quality of indigenous pigs to meet the market expectations is necessary. Lean meat is in high demand among consumers as an excellent addition to a healthy diet (Peres et al.,2014). The challenge in the pig industry is to improve the nutritional value, quality of pork, and meet consumer demand. To improve economic meat production in indigenous pigs, the correct supply of nutrients is critical, and should not exceed or be below the requirement.
Dietary protein is the most important and expensive nutrient in pig diets (Whittemore et al., 2001). The rate of protein and lipid deposition is largely determined by the ability of the pig to grow lean tissue and the protein to energy ratio of the diet (Ruusunen et al., 2007). In improved pig genotypes, reduced dietary protein levels enhance carcass traits, pork quality, and pork cuts (Alonso et al., 2010). Estimated protein levels for slow-growing pigs are valuable in ration formulation because maximizing the dietary content of crude protein is a viable strategy to increase profitability. It has been shown that dietary protein content can be reduced from 193 g/kg to 135 g/kg without affecting the nitrogen balance and growth potential of growing Windsnyer pigs (Hlatini et al 2020; 2021). Reports on the relationship between decreasing levels of protein and pork quality traits in slow-growing pigs are sparse. Zhang et al. (2008) reported that reduced protein levels were associated with more intramuscular fat and greater meat tenderness. Determining the relationship between dietary protein level and pork characteristics would help to identify the threshold value for protein. To determine the optimal protein level in pigs, there is need to examine the relationship between crude protein and carcass traits. Optimising protein levels for slow-growing pigs is a viable strategy for increasing profitability of pig enterprises. For the slow-growing Windsnyer pig, the protein requirements for growth have not been accurately estimated, as is the case for improved pig genotypes (NRC, 2012). In addition to Windsnyer growth performance (Hlatini et al., 2021) and nitrogen balance (Hlatini et al., 2020), carcass traits and meat quality needs to be understood because incorrect supply of some nutrients such as protein, could increase fat deposition (Monteiro et al., 2017; N0rgaard et al., 2014). Data on the effect of a decreasing protein level on carcass characteristics and primal pork cuts in the slow-growing Windsnyer pigs is not available. Such information can assist in reducing fat deposition while meeting protein requirements for Windsnyer pigs. The objectives of the current study were to determine carcass traits, primary pork cuts, and internal organ weight responses to decreasing dietary protein levels. It was hypothesized that decreasing the dietary protein level would not affect carcass traits, primary pork cuts, and internal organ weight in native Windsnyer pigs until a certain inclusion level of dietary protein where these variables would be negatively affected.
Materials and Methods
The study was conducted at the Agricultural Research Council (ARC), Animal Production Institute, Irene, South Africa. The institute lies at 25°34'0" S; 28°22'0" E and is ~1526 m above sea level. The average annual temperature during the period of the study was 20.7 °C. The care and use of experimental animals was according to the approved standards of the Animal Ethics Committee of the Agricultural Research Council-Animal Production Institution (Reference number APIEC 17/12)
The experimental diets contained 193, 174, 154, 135, 116, and 97 g/kg crude protein. The six diets were formulated to contain similar net energy values of ~9.5 MJ/kg. All diets were supplemented with synthetic lysine, methionine, threonine, and tryptophan levels to match the content of the control diets. Diets were allocated randomly to pens and each pig was treated as an experimental unit in a complete, randomized design. There were five pigs per treatment. Pigs were fed experimental diets in mash form for 8 w during the rainy season.
The control diet containing the NRC (2012) recommended CP (193 g/kg) for growing pigs was formulated. Low protein diets were formulated by reducing the CP content through partial substitution of soybean meal by yellow maize and wheat bran. The experimental diets were formulated to meet and exceed NRC requirements for all other nutrients for growing pigs (NRC, 2012). Diets for the current study, were formulated using the software program Bestmix® (Adifo, Belgium). The major ingredients used in the diets included yellow maize, soybean meal, wheat bran, sunflower oil, limestone, monocalcium phosphate, salt, lysine HCL, methionine, threonine, tryptophan, and a mineral-vitamin premix (Table 1). Diet samples were taken from all the feeders per dietary treatment three days after the beginning and three days before the end of the experiment and kept in a cool dry place until they were homogenised, subsampled, and prepared for chemical composition analysis. The composition and chemical analyses of the different diets are given in Table 1.
The dry matter, ash, crude protein, and ether extract were determined using AOAC (1990) Official Methods 945.15, 942.05, 979.09, and 920.39, respectively. Nitrogen content was determined using the Dumas Combustion method in a Leco Truspec Nitrogen Analyser (St Joseph MI, USA). Crude protein was then calculated as N χ 6.25. Neutral detergent fibre (NDF) and acid detergent fibre (ADF) were analysed using ANKOM Fibre Analyser (Ankom Macedon, NY, USA), according to Van Soest (1973) and Van Soest et al. (1991). Samples for mineral analyses were ashed at 450 °C overnight (Abdou et al., 2011). The bulk density was determined according to the method described by Kyriazakis and Emmans (1995).
Thirty, clinically healthy, slow-growing Windsnyer pigs, with an initial body weight of 23 ± 0.622 kg were randomly allocated into individual pens measuring 1.5 χ 1 m2. The animals were ear tagged and housed in a room with artificial heating, lighting, and proper ventilation systems. The house temperature and humidity were maintained at 21.9 ± 2.24 oC and 45.2 ± 6.85%, respectively. A 12 h dark-12 h artificial light cycle was applied. Each pen was provided with water through a low-pressure nipple drinker and feed was supplied in a self-feeder trough. Feed and water were supplied ad libitum. The pigs received no antibiotics or growth promoters.
At the end of the experiment, Windsnyer pigs were transported to the processing plant, located 1.5 km from the pig trial facility. Each pig was weighed to determine slaughter weight. Windsnyer pigs were weighed 8 h before transportation after being feed fasted for 8 h, but with ab libitum access to clean water. The final live body weight at slaughter was 39.13 ± 0.85 kg. Pigs were gently driven from the pens to slaughter. At the entrance, pigs were electrically stunned with electrodes at the base of each ear and exsanguinated using a thoracic puncture. Afterwards Windsnyer pigs were bled and processed following the standard procedures of the slaughterhouse. After dehairing and evisceration, warm carcass weight was measured after dressing using an overhead scale. The dressing percentage was calculated by taking the warm carcass weight as a percentage of slaughter weight. The carcasses were then driven to a chilling room set at 2-4 °C for 24 h, after which cold carcass weights were measured. Cooler shrink, which is the amount of water lost from a carcass in the first 24-48 h after harvest (Schweihofer, 2011), was calculated using the following formula:
Cooler shrink (%) = (1 - (cold carcass weight / warm carcass weight) x100).
The pH of the homogenate of 3.0 g muscle was measured in 27 ml of deionized water, using a pH meter (MP230, Mettler Toledo, Switzerland).
After the heads and tails of each carcass were removed, the kidneys, kidney fat, glands, and remaining parts of the diaphragm were removed, as well as flare fat, kidneys, kidney fat, and remaining diaphragm. Using a carcass splitting bandsaw, carcasses were then divided along the median plan from the remaining sacral vertebra to the first cervical vertebra. A measuring tape was used to measure the length of the carcass from the first rib to the pubic bone in the median plane. Back fat measurements were taken at the first rib (dorsal fat thickness at first rib [DFT1]), last rib (dorsal fat thickness at last rib [DFT2]), and last lumbar vertebra (dorsal fat thickness at last lumbar vertebra [DFT3]) off the median plane cut surface. For all other carcass measurements, the left side was used. There was a cut between the 10th and 11th rib followed by a cut through the spinal column. Over the eye muscle, 60 mm from the carcass midline, Vernier callipers were used to measure the P2, P2(1) length, P2(1) width, P2(2) width and P2(3) width fat on each carcass. The cut interface yielded three measurements of the eye muscle length (EML) and three measures of eye muscle width (EMW). The following formula from Bruwer (1992) was used to compute the lean meat percentage:
Lean % = 72.511 - 0.418V + 0.0547S,
where V is the fat thickness (mm), and S is the muscle depth (mm).
The eye muscle area (EMA) was calculated using the equation of Zhang et al. (2007):
EMA (mm2)= EML χ EMW χ 0.7,
where EML is measured in millimetres and EMW is the average of the three width measurements (mm) of the eye muscle.
A sample joint (measuring 2.5 cm thick and 16 cm long) along the surface of the rear of the eye muscle was cut off and weighed from the same cut where P2 values were taken. This sample joint was placed in a netlon bag and inserted into a small plastic bag, which was then secured in such a way that the sample joint did not come into contact with the bottom of the bag or air. They were then stored in a refrigerator between 0-5 °C for 24 h, after which the mass of the water lost was calculated from the weight of the water in the bag and was used to calculate drip loss. Following that, the primary cuts (shoulder, ham, and rib) were made. The shoulder was removed by cutting between the third and fourth ribs caudally and the junction of the caudal edge of the second rib with the sternum cranially, and the front trotter was removed by cutting through metacarpal region (at the joint of the carpal bones and the radius and ulna) and weighed to determined shoulder weight (SW). The rib was sliced along a parallel line 16 cm from the spinal cord midline ventrally, between the fourth and twelfth thoracic vertebrae dorsally. The rib weight (RW) was determined by weighing this.
The hind leg was removed between the second and third sacral vertebrae perpendicular to the stretched leg and at the hock joint distally and weighed to get the hindquarter weight (HQW). It was also measured to get the hindquarter length (HQL) from the ischiopubic symphysis to the hock joint and the hindquarter circumference (HQC), in maximum amplitude near the base of the tail. The RW, SW, and HQW were then each presented as a proportion of CCW to give RW proportion (RWP), SW proportion (SWP), and HQW proportion (HQWP), respectively. The selected primal pork cuts are important commercial pork cuts in South Africa. The weight of the heart, lungs, kidneys, liver, spleen, small intestine, large intestine, and stomach was recorded. The weights of the internal organs were expressed relative to the weight of each pig at slaughter.
A PROC GLM (SAS, 2008) procedure was used to examine the response of carcass traits, pork cuts, and internal organs to reduced dietary protein level. A polynomial regression (PROC REG) procedure (SAS, 2008) was used to determine the relationships between protein levels in the diet and carcass traits, pork cuts, and internal organs. Least square means were compared using the probability difference (PDIFF) option in the LS Means statement of SAS (2008). The model that was used was:
Y = Bo + B1A + B2A2+ E,
where Y = is the response variable (pork quality parameters), Bo= is the intercept, B1A = is the linear regression component, B2A2 = is the quadratic regression component, and E = is the error.
A piecewise regression (broken stick) analysis using the PROC NLIN procedure in SAS was used to determine the optimal inclusion levels of dietary protein on carcass traits and pork cuts. A level of P <0.05 was set at the criterion for statistical significance. The piecewise regression model used was:
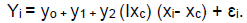
Using parameters (yo, y1, y2) and the xc, the two segmented simple regression functions were:
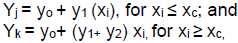
where Yi is the response variable when crude protein inclusion is inhibiting carcass traits and pork cuts; Yj is the response variable before crude protein in the diet inhibits carcass traits and pork cuts; Yk is the response variable when crude protein in the diet exceeds the optimal level;
Yo = yo- y2 xc; when xi = 0; yo is the intercept or minimum Yi when xc < 0; y1 is the rate of change of Yi when xi < xc; y2 is the rate of increase in Yi when xi > xc; xi is the level of crude protein in the diet; xc is the optimal level below which carcass traits and pork cuts are compromised by the decrease in the crude protein content in the diet; and Ixc is a dummy variable with a value = 0 when xi < xc and 1 when xi > xc.
Results
Table 2 shows the relationship between dietary protein level and pork traits. The relationship between protein content and warm carcass weight and cold carcass weight was not significant. A negative linear relationship was observed between protein content and cooler shrink in pigs (P <0.01). Cooler shrink decreased with dietary protein content (P <0.01). Protein content and dressing percentage in growing pigs were linearly related (P <0.05). As the protein content in the diet decreased, dressing percentage increased (P <0.05). A quadratic relationship between dietary protein content and shoulder fat was observed (P <0.05). As the protein content dropped, shoulder fat increased at a decreasing rate (P <0.05). There was no relationship between protein content and carcass length in pigs (P >0.05). Reduced protein had no significant influence on drip loss, lean percentage, eye muscle area, and ultimate pH.
The relationship between dietary protein content and primary pork cuts in Windsnyer pigs is shown in Table 3. There was a positive quadratic relationship between protein content and ham diameter (P <0.05). The ham diameter decreased at an increasing rate as the dietary protein content decreased (P <0.05). The reduction in dietary protein content had no effect on ham length, ham circumference, hind quarter mass, shoulder mass, and rib mass (P >0.05). There was a linear relationship between protein content and dorsal fat thickness at the last rib (P2) in pigs (P <0.05). As the dietary protein content decreased, dorsal fat thickness at the last rib (P2) declined. A negative linear relationship between protein content and P2 back fat thickness was observed (P <0.01). P2 back fat thickness decreased with protein content (P <0.01). A decreasing linear relationship was observed between protein content and P2(2) back fat depth width in pigs (P <0.05). The relationship between protein content and dorsal fat thickness at rib (f1), and dorsal fat thickness at the last lumbar vertebra (fat) was not significant in pigs (P >0.05). Dietary protein content did not influence P2(1) back fat depth length, P2(1) back fat depth width, and P2(2) back fat depth width (P >0.05). There was a quadratic relationship between dietary protein content and P2(3) back fat width (P <0.05). As the protein content in the pig diet decreased, P2(3) back fat depth width decreased at an increasing rate (P <0.05). Reducing the protein content in the diets had no influence on shoulder weight proportions, rib weight proportions, and hindquarter weight proportions (P >0.05).
Table 4 shows the relationship between dietary protein content and internal organ weight in slow-growing Windsnyer pigs. There was a quadratic relationship between protein content and kidney weight (P <0.05). As the protein content in the pig diet decreased, the kidney weight increased at a decreasing rate. Protein content was not related to heart weight, lung weight, spleen, liver weight, small intestine, large intestine, and stomach weight in pigs (P >0.05).
Table 5 shows the optimal levels below which the shoulder fat, ham diameter, and P2(3) width (back fat depth), together with kidney weight of growing Windsnyer pigs was compromised by low dietary protein content. The optimum protein content for shoulder fat of Windsnyer pigs was estimated to be 136.8 g/kg and the plateau was estimated at 24.7 mm (P >0.05). For ham diameter and P2(3) backfat depth width, the threshold levels of protein were estimated at 111.3 and 115.3 g/kg, respectively. Their plateau was estimated at 44.6 cm and 24.8 mm (P <0.05), respectively. The optimum dietary protein content for kidney weight was estimated at 114.7 g/kg and the plateau was at 0.19 kg in Windsnyer pigs (P <0.05).
Discussion
Decreasing the dietary crude protein content led to an increase in the dressing percentage of slow-growing Windsnyer pigs. The observed linear relationship between protein content and dressing percentage may suggest well-muscled meat type breeds, because the heavier the pig, the better the dressing percentage. Castell et al. (1994) reported that increasing the protein content in the diet led to a decline in the dressing percentage. Reducing lysine and nutrient content for pigs at ~60 kg body weight causes a decrease in dressing percentage (Zhang et al., 2008). Such findings suggest that low protein diets have adequate nutrient density. The linear relationship between protein content and cooler shrink suggests that at high protein contents, there is a larger water loss from the carcass than at a lower dietary protein content. The decrease in dietary protein content with cooler shrink could be associated with the increase in the full subcutaneous fat cover. Carcass with excess fat lose less water at chilling than trimmer carcasses (Apple et al., 2017).
The carcass length depends more upon genetic selection than dietary specifications. Hence, it was expected that carcass length would not be affected by the dietary protein content. The carcass lengths are associated with genes because slow-growing Windsnyer pigs have a small body frame. Kanengoni et al. (2004) reported that the primary pork cuts are affected by the carcass length. The quadratic relationship between protein content and shoulder fat is difficult to explain. The decrease in protein content up to 136.8 g/kg caused changes in shoulder fat, after that, a further improvement in shoulder fat was not observed. Slow-growing Windsnyer pigs fed a fibre-rich diet responded linearly (Ncobela et al., 2018). Reducing dietary protein caused changes resulted in a linear response on the right full side; the observed relationship can be explained by the dressing percentage and cooler shrink outcomes.
A quadratic relationship with protein content caused changes; ham diameter was attributed to shoulder yields. At 111.3 g/kg of protein, ham diameter reached a plateau of 44.6 cm, i.e., for further decreases in the protein content, the ham diameter stayed the same. Our results suggest that a higher protein content in the diet results in carcass leanness and more intramuscular fat. Ruusunen et al. (2007) reported that high dietary protein contents lead to higher carcass lean meat and lower fat content than low protein diets. The protein level supplied to pigs can largely influence muscle growth. The quadratic influence of protein content in the exotic pigs on the total weight of the ham has been reported (Young et al., 1967). An increase in total ham as the dietary protein caused changes decreased has also been reported in exotic pigs (Apple et al., 2017). This indicates that the ham diameter can be influenced by several factors including dietary composition, slaughter weight, and breed type.
The linear increase in dorsal fat thickness at the last rib (f2) as the protein content decreased suggests that the rate of fat deposition is inversely proportional to dietary protein content. A lower protein content in the diet increases fat deposition because it provides more net energy due to a reduction in amino acid deamination, urea excretion, and a lower heat production (Noblet et al., 2001). Reducing the protein content in the diet leads to an increase in intramuscular fat content (Teye et al., 2006). Zhang et al. (2008) reported that dietary nutrient levels have an influence on the last rib back fat depth. The available information suggests that the regulation of fat deposition is depot-dependent and is regulated by protein inclusion.
The decreasing linear relationship of P2 back fat and P2(2) width of backfat depth against protein could be due to the reduced dietary protein content. Needham and Hoffman (2015) reported the effect of dietary protein content on back fat thickness. The back fat thickness tends to decrease as dietary protein content is lowered (Hong et al., 2016). The quadratic relationship between dietary protein content and P 2(3) width of backfat depth is not clear. At 115.3 g/kg dietary crude protein, the P2(3) width of backfat depth reach a plateau of 24.8 mm, i.e., a further decrease in protein led to no improvement. Back fat has been reported to be influenced by several factors such as dietary specifications, genotype, sex, and body weight (Needham and Hoffman, 2015; Hong et al., 2016; Apple et al., 2017). The response in back fat in slow-growing Windsnyer pigs may be related to the genotype and dietary composition.
The quadratic relationship between dietary protein content and kidney weight is poorly understood. Organ weights are usually affected by the protein content, so the present results indicate that that the response in kidney weight to dietary protein is not uniform. A decrease in protein level up to 114.7 g/kg had an influence on kidney weight with a plateau at 190 g; beyond that limit, no positive change in kidney weight was observed. Dietary protein content and live body weight have been reported to contribute to the organ weights in slaughter pigs (Ruusunen et al., 2007). The type of diet fed to pigs affects the genetic potential to grow, as well as overall pork quality. It is a common practice in nutritional experiments to use the weights of internal organs as indicators of the influence of diet in animals (Ahamefule et al., 2006). The production level of slow-growing Windsnyer pigs is influenced by the diets that are insufficient or excessive. Our study is the first to report on the effects of a reduced dietary protein content (designed to achieve optimal dietary protein content) on carcass traits, primary pork cuts, and internal organ weights of Windsnyer pigs. There were very few quadratic results in the carcass parameters measured. Due to this, it is difficult to place a strong recommendation on optimum protein levels for Windsnyer pigs without compromising their carcass yield. There are very few studies that focus on the Windsnyer pig carcass and pork quality because of the need to sacrifice numerous pigs for research, despite the fact that their number is declining. The sample size of the study was further limited due to the scarcity of purebred Windsnyer pigs.
Conclusions
The reduction in dietary protein level resulted in linear relationships with cooler shrink, dressing percentage, shoulder fat, dorsal fat thickness at last rib (f2), P2 back fat thickness, and P2(2) width of backfat depth. Decreasing dietary protein content led to a quadratic relationship with few parameters such as shoulder fat, P2(3) width of backfat depth, ham diameter, and kidney weight. Some responses in carcass traits, primal pork cuts, and internal organs are assumed to be related to genetics of the Windsnyer pigs rather than to diet. To optimise carcass performance of the slow-growing Windsnyer pigs, the protein content in the diet should not be reduced below 116 g/kg. The obtained protein content value is needed for use in formulating swine rations for indigenous Windsnyer breed. Knowledge of the protein requirement for indigenous pigs obtained from the present study would enable feed manufacturers and producers to avoid protein deficits and strategically offer supplementary sources of protein. However, further studies should be done to validate the present findings.
Acknowledgements
The authors thank the UKZN competitive grant (P530) and National Research Foundation for funding this research (grant number; 102702).
Authors' Contributions
VAH designed the study, collected, and analysed the data, drafted, and revised the manuscript. NCN collected the data and revised the manuscript. MC was responsible for supervising and editing the written manuscript.
Conflict of Interest Declaration
In this study, the authors declare that they have no conflict of interest.
References
Abdou, N., Nsahlai, I.V. & Chimonyo, M., 2011. Effect of groundnut haulms supplementation on millet stover intake, digestibility, and growth performance. Anim. Feed Sci. Technol. 169: 176-184. [ Links ]
Ahamefule, F.O., Eduok, G.O., Usman, A., Amaefule, K.U., Obua, B.E. & Oguike, S.A., 2006. Blood biochemistry and haematology of weaner rabbits fed sundried, ensiled, and fermented cassava peel-based diets. Pak J Nutr. 5: 248- 253. [ Links ]
Alonso, V.,Campo, M.M., Provincial, L., Roncales, P., & Beltran, J.A., 2010. Effect of protein level in commercial diets on pork meat quality. Meat Sci. 85: 7-14. [ Links ]
AOAC. 1990. Official Methods of Analysis. Association of Official Analysis Chemists (15th ed.), DC, U.S.A. [ Links ]
Apple, J. K. Maxwell, C. V.Bass, B. E. Yancey, J. W. S. Payne, R. L. & Thomson, J., 2017. Effects of reducing dietary crude protein levels and replacement with crystalline amino acids on growth performance, carcass composition, and fresh pork quality of finishing pigs fed ractopamine hydrochloride. J. Anim. Sci. 95: 4971-4985. [ Links ]
Castell A. G., Cliplef R. L., Poste-Flynn L. M., & Butler G. 1994. Performance, carcass and pork characteristics of castrates and gilts self-fed diets differing in protein content and lysine:energy ratio. Can J Anim Sci. 74: 519-528. [ Links ]
Halimani, T. E., Muchadeyi, F. C., Chimonyo, M., & Dzama, K., 2012. Opportunities for conservation and utilisation of local pig breeds in low-input production systems in Zimbabwe and South Africa. Trop. Anim. Health Prod, 45: 81-90. [ Links ]
Hlatini, V.A., Ncobela, C.N. & Chimonyo, M., 2020. Nitrogen balance response to varying levels of dietary protein in slow-growing Windsnyer pigs. S. Afr. J. Anim. 50, 643-653. [ Links ]
Hoffman, L.C., Styger, W.F., Brand, T.S. & Muller, M., 2005. The growth, carcass yield, physical and chemical characteristic of two South African indigenous pig breeds. S. Afr. J. Anim. 6, 25. [ Links ]
Hong, J.S., Lee, G.I., Jin, X.H. & Kim Y.Y.,2016 Effect of dietary energy levels and phase feeding by protein levels on growth performance, blood profiles and carcass characteristics in growing-finishing pigs. J. Anim. Sci. Technol. 58:37. DOI 10.1186/s40781-016-0119-z. [ Links ]
Kanengoni, A. T., Dzama, K., Chimonyo, M., Kusina, J., & Maswaure, S. M., 2004. Growth performance and carcass traits of Large White, Mukota and Large White X Mukota F 1 crosses given graded levels of maize cob meal. Anim Sci. 78: 61-66. [ Links ]
Kanengoni, A.T., Chimonyo, M., Erlwanger, K.H., Ndimba, B.K. & Dzama, K., 2014. Growth performance, blood metabolic responses, and carcass characteristics of grower and finisher South African Windsnyer-type indigenous and Large White* Landrace crossbred pigs fed diets containing ensiled corn cobs. J Anim Sci. 92: 5739-5748. [ Links ]
Monteiro, A.N.T.R., Bertol, M.T.,de Oliveira, P.A.V., Dourmad, J.Y., Coldebella, A. & Kessler, A.M., 2017. The impact of feeding growing-finishing pigs with reduced dietary protein levels on performance, carcass traits, meat quality and environmental impact. Livest Sci. 198: 162-169. [ Links ]
Ncobela, C. N., Kanengoni, A. T., & Chimonyo, M., 2018. Response in nutritionally related blood metabolites, carcass traits and primal pork cuts of slow growing Windsnyer pigs fed on varying levels of potato hash silage. S. Afr. J. Anim. 48:770-776. [ Links ]
Needham & L. C. Hoffman., 2015. Carcass traits and cutting yields of entire and immunocastration pigs fed increasing protein levels with and without ractopamine hydrochloride supplementation1-3T. J Anim Sci. 93: 4545-4556. [ Links ]
Noblet, J., Le Bellego, L., van Milgen, J., & Dubois, S., 2001. Effects of reduced dietary protein level and fat addition on heat production and nitrogen and energy balance in growing pigs. Anim Res. 50: 227-238. [ Links ]
N0rgaard, J.V., Hansen, M.J., Soumeh, E.A., Adamsen, A.P.S. & Poulsen, H.D., 2014. Effect of protein level on performance, nitrogen utilisation and carcass composition in finisher pigs. Acta Agriculturae Scandinavica, Section A-Animal Science, 64: 123-129. [ Links ]
NRC, 2012. Nutrient Requirements of Swine. 12th revised edition. National Academy of Science. Washington, DC. USA. [ Links ]
Peres, L.M., Bridi, A.M., da Silva, C.A., Andreo, N., Barata, C.C.P., & Dário, J.G.N., 2014. Effect of supplementing finishing pigs with different sources of chromium on performance and meat quality, Revista Brasileira de Zootecnia-Brazilian J. Anim. Sci. 43, 369-375. [ Links ]
Pham, K. T., Hoang, N. D., Duc, N. L., Hendricks, W. H., van de Peet-Schwering, C. M. C. & Verstegen, M. W. A., 2010. Effects of genotype and dietary protein level on the growth performance and carcass characteristics of fattening pigs in Central Vietnam. Asian-australas. J. Anim. Sci. 23:1034-1042. [ Links ]
Ruusunen M., Partanen K., Posó R., & Puolanne E., 2007. The effect of dietary protein supply on carcass composition, size of organs, muscle properties and meat quality of pigs. Livest Sci. 107: 170-181. doi:10.1016/j.livsci.2006.09.021 [ Links ]
SAS (2008) SAS User's Guide: Statistics, Version 9.3. SAS Institute, Cary, NC, USA. [ Links ]
Schweihofer, J.P., 2011.Carcass dressing percentage and cooler shrink vary among species and type of animals. Michigan State University. [ Links ]
Teye G. A., Sheard P. R., Whittington F. M., Nute G. R., Stewart A., & Wood J. D., 2006. Influence of dietary oils and protein level on pork quality. 1. Effects on muscle fatty acid composition, carcass, meat and eating quality. Meat Sci. 73:157-165. doi: 10.1016/j.meatsci.2005.11.010 [ Links ]
Van Soest P.J., Robertson J.B., & Lewis B.A., 1991. Methods for dietary fibre, neutral detergent fibre and non-starch polysaccharides in relation to animal nutrition. J Dairy Sci. 3583-3597. [ Links ]
Van Soest. P. J., 1973. Collaborative study of acid-detergent fiber and lignin. J. Assoc. Official Analy. Chem. 56: 781-784. [ Links ]
Whittemore, C.T., Green, D. M. & Knap, P. W., 2001. Technical review of the energy and protein requirement of growing pigs: food intake. Anim Sci. 73: 3-17. [ Links ]
Whittemore, E.C., Emmans, G.C. & Kyriazakis, I., 2003. The relationship between live weight and the intake of bulky foods in pigs. Anim Sci. 76, 89-10. [ Links ]
Wood, J.D., Nute, G.R., Richardson, R.I., 2004. Effects of breed, diet and muscle on fat deposition and eating quality in pigs. Meat Sci. 67:651-667. [ Links ]
Young, L. G., Asrrror, G. C., Fons, f. R. P. eNu, R. H., 1967. Relationship of dietary protein levels to performance and carcass merit of market swine. Can J Anim Sci. 48: 71-82 [ Links ]
Zhang, H.J., Xiong, Z.Y.,Zuo,G.M.,Lei,G.M.,Jiang, E.F.,Li, R.,Zheng, L.J.,Li, Q. and Xu, D.,2007. Quantitative trait loci for carcass traits on pig chromosomes 4,6,7,8 and 13, J Appl Gene. 48:363-369. [ Links ]
Zhang, J., Yin J., Zhou, X., Li, F., Ni, J. and Dong, B., 2008. Effects of lower dietary lysine and energy content on carcass. Asian-australas. J. Anim. Sci. 21: 1785-1793. [ Links ]
Submitted 14 February 2022
Accepted 30 June 2022
Published 30 January 2023
# Corresponding author: Michael.chimonyo@univen.ac.za














