Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Animal Science
versão On-line ISSN 2221-4062
versão impressa ISSN 0375-1589
S. Afr. j. anim. sci. vol.52 no.5 Pretoria 2022
http://dx.doi.org/10.4314/sajas.v52i5.15
A field study of Bacillus licheniformis-fermented products on growth performance and faecal microbiota of weaning piglets
K.H. Lin; Y.H. Yu
Department of Biotechnology and Animal Science, National Ilan University, Yilan, Taiwan
ABSTRACT
This study investigated the effects of Bacillus licheniformis-fermented products (BLFPs) on the growth performance, faecal microbiota, and antibiotic resistance gene (ARG) expression in weaning piglets on a commercial farm. Ninety-six weaning piglets were randomly assigned to four treatments as follows: basal diet as control (C), basal diet plus 30 mg/kg of antibiotics (bacitracin methylene disalicylate) (A), basal diet plus 1 g/kg of BLFPs (F), and basal diet plus 15 mg/kg of antibiotics and 0.5 g/kg of BLFPs (AF), with six replicate pens per treatment and four pigs per pen. Results showed that, similar to antibiotics, replacing all or half the antibiotics with BLFPs improved the feed conversion ratio of weaning piglets from 15-28 d. Microbiota analysis showed that microbial community composition in the faeces showed a clear separation between groups. Replacing all the antibiotics with BLFPs increased the abundance of the genus, Streptococcus, in the faeces compared with the other groups. Half replacement of antibiotics with BLFPs increased the chloramphenicol resistance gene levels in the faeces compared with the C group, whereas full replacement of antibiotics with BLFPs reduced the streptomycin resistance gene levels compared with the C group. A trend of decreased levels of formic acid and acetic acid was observed in the group treated with BLFPs in combination with antibiotics compared with the C group. In conclusion, the field study demonstrates that replacing all or half the antibiotics with BLFPs can improve feed conversion ratio, modulate faecal microbiota, and alter ARG expression in weaning piglets.
Keywords: feed additive, probiotic, pig, 16S rRNA gene sequencing, antibiotic resistance gene
Introduction
Weaning has been considered as a critical period that affects piglet health and growth. Post-weaning diarrhoea caused by pathogens and environmental stress has an important economic impact on pig production worldwide (Rhouma et al., 2017). Gut microbiota dysbiosis is highly associated with a high incidence of diarrhoea in weaning piglets, resulting in poor growth rate (Dou et al., 2017). Antibiotic growth promoters have been widely used for the prevention of post-weaning diarrhoea and improving the growth of weaning piglets (Rhouma et al., 2017). Multidrug-resistant bacteria and antibiotic residues in animal products are becoming a major threat to human health since antibiotic growth promoters are overused in animal production. Therefore, the use of antibiotics as growth promoters in animal feeds has been prohibited in many countries (Maron et al., 2013). It is important to find potential alternatives to antibiotics to improve gut health and the growth of weaning piglets.
The establishment of a beneficial gut microbiota early in the life of piglets can improve health and growth (Gresse et al., 2017). Probiotics have been extensively used as a strategy for antibiotic alternatives in pig production and many studies suggest that probiotics exert beneficial effects on piglets through the modulation of gut microbiota (Guevarra et al., 2019). It has been demonstrated that probiotic supplementation protects weaned pigs against pathogenic bacteria and improves performance similar to antibiotics (Kritas & Morrison, 2005). Bacillus licheniformis is a spore-forming probiotic that promotes nutrient utilization and inhibits pathogen growth through the production of the digestive enzymes and antimicrobial peptides (Rozs et al., 2001 ; Thaniyavarn et al., 2003; Horng et al., 2019). Supplementation with B. licheniformis reduces diarrhoea incidence in weaning piglets (Zong et al., 2019). Recent studies demonstrate that, similar to antibiotic growth promoters, B. licheniformis-fermented products (BLFPs) can alleviate diarrhoea incidence and improve the growth performance of weaning piglets (Hung et al., 2019; Lin & Yu, 2020). BLFP supplementation in the diet of sows also improves the piglet body weight at weaning (Yu et al., 2020). In addition, microbial communities are different in terms of the caecal digesta or faeces between the weaning piglets treated with BLFPs and antibiotics (Hung et al., 2019; Lin & Yu, 2020).
It has been reported that the antibacterial activity with the combined use of antibiotics and probiotics is higher than when using the antibiotic or probiotic alone (Soleymanzadeh Moghadam et al., 2018). A 50% replacement of antibiotics with probiotics improves digestive enzyme activity, antioxidant activity, and growth performance in weaning piglets (Wang et al., 2017; Hu et al., 2018). Our previous study demonstrated that a 50% replacement of antibiotics with BLFPs was able to decrease the incidence of diarrhoea and modulate caecal microbiota composition in weaning piglets (Lin & Yu, 2020). To the best of our knowledge, little is known about the efficacy of parallel and combined supplementation with BLFPs and antibiotics in the diet of weaning piglets on growth performance and gut microbiota in the commercial farm. Furthermore, the effect of BLFPs on the abundance of antibiotic resistance gene (ARG) in the faeces of weaning piglets still remains to be evaluated. For practical application, these results can provide valuable information about the effect of BLFPs on weaning piglets for development as an alternative to antibiotics.
Therefore, the current study aimed at evaluating and comparing the effects of BLFPs and antibiotics, as well as their combination on growth performance, faecal microbiota, and ARG expression on a commercial pig farm.
Materials and Methods
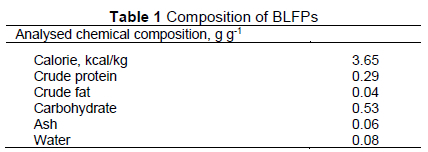
Protocols for BLFP preparation in this study were performed as described in a previous study (Lin et al., 2019). Antibiotics (bacitracin methylene disalicylate) were purchased from Nice Garden Industrial (Taipei, Taiwan). The concentration of B. licheniformis spores in BLFPs was 5 χ 1011 CFU/g. The detailed composition of BLFPs is listed in Table 1. All experiments were performed in accordance with an animal protocol approved by the Institutional Animal Care and Use Committee of National Ilan University (IACUC, protocol number 109-4). The study was carried out from April to July 2020 on a commercial farrow-to-finish pig farm (Miaoli, Taiwan) with a breeding stock of 100 sows. The pig farm had its own feed mill. A total of 96, twenty-eight-day-old weaning pigs [Duroc χ (Yorkshire χ Landrace)] with an average body weight (BW) of 9.0 ±0.03 kg were used in a 42-day trial. All pigs were randomly allotted to four experimental diets based on initial BW and sex (six replicate pens per treatment; two gilts, and two barrows/pen). Dietary treatments included: a basal diet as control (C), basal diet + 30 mg/kg of antibiotics (bacitracin methylene disalicylate) (A), basal diet + 1.0 g/kg of BLFPs (F), and basal diet + 15 mg/kg of antibiotics + 0.5 g/kg of BLFPs (af). Diets (Table 2) were formulated to meet or exceed the nutrient requirements recommended by National Research Council (2012). In F and AF groups, the soybean meal in the basal diet was replaced with BLFPs equally. All the pigs were housed in an environmentally-controlled room with a slatted metal floor (2.5 m χ 4.0 m). Each pen was equipped with a one-sided self-feeder and a nipple waterer to allow the pig ad libitum access to feed and water throughout the experimental period. The experimental period was 42 days. Room temperature during week 1 was maintained at 30 °C and was then gradually decreased and kept at 24°C until the end of the experiment. The photoperiod was controlled to provide 10 h of light and 14 h of dark in the shed throughout the experiment. The morbidity and mortality of piglets were monitored daily. The feed offered and refused was weighed daily to calculate the average daily feed intake (ADFI). The piglets were weighed weekly to calculate average BW and average daily weight gain (ADG). The feed conversion ratio (FCR) was calculated every week.
At the end of experiment (day 42), faeces from two piglets (male and female) per replicate were freshly collected and then pooled. Three replicates (n = 3) were used for faecal microbiota analysis. Faecal DNA was purified using the QIAamp DNA Stool Mini Kit (QIAGEN, Germany) and quantified on the Qubit 2.0 Fluorometer (Thermo Scientific, Waltham, MA, USA).
The V3 and V4 hypervariable region of the 16S rRNA gene was amplified using a 341F-805R primer. PCR products were purified using the QIAquick Gel Extraction kit (QIAGEN, Germantown, MD, USA). The library was constructed using TruSeq Nano DNA Library Prep kits (Illumina, San Diego, CA, USA). The constructed library was sequenced using the paired-end method on Illumina MiSeq platform (San Diego, CA, USA) after being subjected to DNA quantification and library testing. The low-quality part of the reads was cut, and in each sample, probes were split from the high-quality reads. The clean reads of all samples were clustered and classified into the same operational taxonomic unit (OTU) with an identity of 97% similarity using the UCHIME software (version 4.2) and Mothur software (version 1.39.5). The obtained sequences were aligned to the Genomes Online Database (gold.jgi.doe.gov) to determine the phylogeny of the OTUs. Taxonomic assignments, alpha diversity, and principal component analysis (PCA) were calculated by Quantitative Insights Into Microbial Ecology 2 (QIIME2, version 1.9.1). Unweighted UniFrac metric and weighted principal coordinate analysis (PCoA) were performed using QIIME 2 software. A Venn diagram (version 1.6.17) was constructed to show the number of common and unique OTUs among groups. The R package, corrplot (version 0.84), was used for visualizing correlation matrices.
At the end of experiment (day 42), faeces from two piglets (male and female) per replicate were freshly collected and then pooled. Three replicates (n = 3) were used for ARG analysis. Faecal DNA was purified using the QIAamp DNA Stool Mini Kit (QIAGEN) and quantified on the Qubit 2.0 Fluorometer (Thermo Scientific). Quantitative polymerase chain reaction (qPCR) was performed using a MiniOpticon Real-Time PCR detection system (Bio-Rad, Hercules, CA) and KAPA SYBR FAST qPCR Kit (Kapa Biosystems, Boston, MA). Primer pairs specific for each ARG were as follows: sull (sulphonamide-resistant dihydropteroate synthase) forward: 5'-GGA TCA GAC GTC GTG GAT GT-3', and reverse: 5'-GTC TAA GAG CGG CGC AAT AC-3'; cat (chloramphenicol acetyltransferase) forward: 5'-TCC ATG AGC AAA CTG AAA CG-3', and reverse: 5'-GGG AAA TAG GCC AGG TTT TC-3'; aadA (aminoglycoside adenylyltransferase) forward: 5'-CAG CCC GTC TTA CTT GAA GC-3', and reverse: 5'-GAT CTC GCC TTT CAC AAA GC-3'; strA (streptomycin phosphotransferase) forward: 5'-CCA GTT CTC TTC GGC GTT AG-3', and reverse: 5'-ACT CTT CAA TGC ACG GGT CT-3'; tetA (tetracycline resistance determinant, class A) forward: 5'-CGA TCT TCC AAG CGT TTG TT-3', and reverse: 5'-CCA GAA GAA CGA AGC CAG TC-3'; tetB (tetracycline resistance determinant, class B) forward: 5'-TAC AGG GAT TAT TGG TGA GC-3', and reverse: 5'-ACA TGA AGG TCA TCG ATA GC-3'; tetG (tetracycline resistance determinant, class G) forward: 5'-GTG TTC CCG ATT CTG TTG CT-3', and reverse: 5'-GAT TGG TGA GGC TCG TTA GC-3'. Amplification of the 16S rRNA gene (forward: 5'-GTG STG CAY GGY TGT CGT CA-3', and reverse: 5'-ACG TCR TCC MCA CCT TCC TC -3') was used as a reference to determine the total amount of DNA in each sample. After quantitative PCR, the relative expression of ARG in the total bacterial population was finally calculated using the formula 2-ΔΔα.
At the end of experiment (day 42), faeces from two piglets (male and female) per replicate were freshly collected and then pooled. Three replicates (n = 3) were used for short-chain fatty acid analysis. Short-chain fatty acids from faeces were extracted and analysed using gas chromatography-mass spectrometry (Bruker GC-MS System, Burker Corp., Billerica, MA, USA), as described previously (Cheng et al., 2021).
Replicates were used as the experimental unit. The differences among the dietary treatment groups were analysed using one-way ANOVA followed by Tukey's honestly significant difference test using SAS (version 9.4, 2012; SAS Institute, Cary, NC, USA). A P-value between 0.05 and 0.1 was considered a trend, and a P-value of less than 0.05 was statistically significant. The PCoA and PCA were performed using UniFrac distances coupled with standard multivariate statistics.
Results
The piglets were healthy during the experimental period. No significant difference was found in BW, ADG, and ADFI between groups, except for FCR (Table 3). Antibiotic supplementation improved the FCR in weaning piglets at 15-28 days compared with the C group (P = 0.011) (Table 3). Similar to antibiotics, the FCR was improved at 15-28 days in the F and AF groups compared with the C group (P = 0.011) (Table 3).
After stringent quality trimming of raw data, the averages of high-quality reads from the faeces of C, A, F, or AF were 26951, 22360, 22133, and 22044, respectively (Table 4). Replacing all antibiotics with BLFP decreased OTUs in the faeces of weaning piglets compared with the C group (P = 0.022) (Table 4). No significant difference was observed in the bacterial species richness (Chao1 and Fisher alpha) and species evenness (Shannon and Enspie) between groups (Table 4).
The Venn diagram showed an overlap (199 OTUs, core) that was shared by four of the plotted groups (Figure 1). In total, 70, 19, 8, and 49 unique OTUs were found in the four aforementioned groups, respectively; 11 OTUs were found in both the C and A groups; 3 OTUs were found in both the C and F groups. By contrast, 134 OTUs were found in both the C and AF groups.
Unweighted PCoA of qualitative traits indicated that the microbiota of faecal samples was not well-separated among the groups (Figure 2A). In contrast, weighted PCoA of quantitative traits and PCA revealed significant discrimination among the groups (Figure 2B and 2C).
The result of bacterial taxonomy in the faeces of weaning piglets is shown in Table 5. No significant differences were observed in the abundance at the phylum level between groups. At the class level, the abundance of the class, Bacilli, was increased in the F group compared with the other groups (P = 0.007). The abundance of class, Methanobacteria, was higher in the AF group compared with the other groups (P = 0.002). At the order level, the abundance of the order, Lactobacillales, was higher in the F group compared with the other groups (P = 0.007). The abundance of the order, Methanobacillales, was increased in the AF group compared with the other groups (P = 0.002). Antibiotic supplementation increased the abundance of order, Spirochaetales, compared with the other groups (P <0.001). The abundance of order, Spirochaetales, was reduced in the F group compared with the AF group (P <0.001). At the family level, the abundance of the family, Streptococcaceae, was increased in the F group compared with the other groups (P <0.001). The abundance of the family, Ruminococcaceae, was lower in the F group compared with the A group (P = 0.05). Replacing all antibiotics or half the antibiotics with BLFPs decreased the abundance of the family, Lactobacillaceae, compared with the C and A groups (P = 0.002). The abundance of the family, Muribaculaceae, was increased in A and AF groups compared with the C and F groups (P = 009). Replacing all antibiotics with BLFPs increased the abundance of the family, Methanobacteriaceae, compared with the other groups (P = 002). The abundance of the family, Christensenellaceae, was decreased in the F group compared with other groups (P = 007). At the genus level, the abundance of the genus, Streptococcus, in the F group was higher compared with the other groups (P <0.001). Replacing all antibiotics or half the antibiotics with BLFPs decreased the abundance of the genus, Lactobacillus, compared with the C and A groups (P = 0.002). Antibiotic supplementation increased the abundance of the genera, Lachnospiraceae_unclassified and Treponema 2, compared with the other groups (P = 0.004 and P <0.001). The abundance of the genus, Bíautia, in the AF group was lower compared with the other groups (P = 0.046). Replacing all antibiotics with BLFPs decreased the abundance of the genus, Christenseneííaceae R-7 group, compared with the other groups (P = 0.007). The abundance of the genus, Treponema 2, in the F group was lower compared with the A and AF groups (P <0.001). Replacing all antibiotics with BLFPs increased the abundance of the genus, Methanosphaera, compared with the other groups (P = 0.001).
The results of the heat map show that similar bacterial community clusters, such as genera Ruminococcaceae UCG-008, Ruminococcaceae NK4A214 group, Christensenellaceae R-7 group, and Clostridium sensu stricto 6, were observed between the C, A, and AF groups (Figure 3). Some bacterial community clusters were specifically decreased in the AF group, such as the genera, [Eubacterium] hallii group, Blautia, Faecalibacterium, and Subdoligranulum (Figure 3). Some bacterial community clusters were specifically increased in the A group, such as genera, Turicibacter, Alloprevotella, and Lachnospiraceae_unclassified (Figure 3). Replacing all antibiotics with BLFPs resulted in unique bacterial community clusters compared with other groups, such as the genera, Streptococcus, Dialister, and Oribacterium (Figure 3).
No significant difference in the sulphonamide resistance gene (sul1) and aminoglycoside resistance gene (aadA) in the faeces was found among the groups (Figure 4). The chloramphenicol resistance gene (cat) levels were increased in the AF group compared with the C group (P <0.05) (Figure 4). Replacing all antibiotics with BLFPs decreased the levels of the streptomycin resistance gene (strA) in the faeces compared with the C group (P <0.05) (Figure 4). Two tetracycline resistance gene (tetA and tetB) levels in the faeces were not altered among the groups (Figure 4). The level of the tetG gene in the faeces of the A group was increased compared with the C group (P <0.05) (Figure 4). The result of short-chain fatty acid levels in the faeces of weaning piglets is shown in Table 6. Relative to the control group, a trend of the decreased levels of formic acid and acetic acid was observed ( P = 0.084 and P = 0.067) in the group treated with BLFPs in combination with antibiotics.
Discussion
The application of probiotics or probiotic-derived functional metabolites for growth promotion has been investigated in weaning piglets (Hu et al., 2014; Cheng et al., 2019; Hung et al., 2019; Lin & Yu, 2020). A field study has reported that supplementation of Bacillus species-based probiotics (B. íicheniformis and B. subtiíis) in the diet of piglets can improve ADG and FCR at the weaning stage (Alexopoulos et al., 2004). Multi-strains of Baciííus species-based probiotics (B. íichenformis, B. subtiíis, and B. coaguíans) also ameliorate the ADG and feed efficiency of growing-finishing pigs (Balasubramanian et al., 2016). The combined use of B. íichenformis and zinc oxide in the diet of weaning piglets improves the ADG and FCR (Zong et al., 2019). Our previous study demonstrated that the replacement of half the antibiotics with BLFPs increased the ADG of weaning piglets (Lin & Yu, 2020). In this field study, similar to the effects of antibiotics, we further demonstrated that full replacement of antibiotics or half replacement of antibiotics with BLFPs improved the FCR of weaning piglets. Our previous studies have demonstrated that the antimicrobial peptides isolated from BLFPs exhibit antibacterial activity against pathogens, such as Cíostridium perfringens and Brachyspira hyodysenteriae (Lin et al., 2019; Horng et al., 2019). Therefore, we speculate that BLFP-derived antimicrobial peptides may exert the antimicrobial effect of antibiotics in the gut of weaning piglets, thereby improving the growth of piglets. It has been reported that B. íicheniformis spores are able to germinate in the gastrointestinal tract of pigs (Leser et al., 2008). Germination and outgrowth of B. íicheniformis spores in the gut can reduce the pathogens by competitive exclusion (Xu et al., 2018). Thus, the B. íicheniformis spores of fermented products may also eliminate the gut pathogens of weaning piglets by competitive exclusion in the present study. In addition, B. íicheniformis can increase nutrient utilization by the production of digestive enzymes (Rozs et al., 2001). Taken together, these findings demonstrate that the dietary supplementation of BLFPs has beneficial effects on FCR in piglets.
The mechanism of BLFPs on the improvement of FCR in weaning piglets may be different and complicated compared to antibiotic supplementation.
The environmental and nutritional changes present huge challenges for the early life of pigs. The establishment of beneficial gut microbiota can ensure the health and growth of weaning pigs (Gresse et al., 2017). However, the weaning pigs are susceptible to pathogen infection since gut microbial composition is still developing in the weaning stage (Guevarra et al., 2019). Probiotics have been shown to have a broad range of beneficial effects in weaning pigs through the modulation of gut microbiota, thereby improving health and growth (Kritas & Morrison, 2005; Gresse et al., 2017; Guevarra et al., 2019). Our previous studies have demonstrated that gut microbiota imbalance is observed in broilers and weaning piglets in response to antibiotic prophylaxis (Hung et al., 2019; Chen & Yu, 2020; Lin & Yu, 2020). BLFP supplementation in the diet of weaning piglets can re-shape gut microbial composition and diversity and this microbiota modulation has a positive effect on health and growth (Hung et al., 2019; Lin & Yu, 2020). In this field study, BLFPs could modify the gut microbiota based on the PCA and weighted PCoA results, which is in agreement with previous studies (Hung et al., 2019; Lin & Yu, 2020). Interestingly, a differential bacterial community structure is also observed in the group treated with antibiotics and the group treated with BLFPs in combination with antibiotics in the present study, which is also in agreement with the previous study (Lin & Yu, 2020). The results imply that BLFP supplementation can still regulate the microbiota in the gut even though the antibiotics are simultaneously supplied. Further, simultaneous supplementation of BLFPs and antibiotics can ameliorate the FCR of weaning piglets in the present study. These findings demonstrate that BLFPs, alone or in combination with antibiotics, show a positive effect on the gut microbiota of weaning piglets.
The use of antibiotics as growth promoters in animal feeds has been banned in many countries, but livestock are more susceptible to pathogen infection, resulting in a negative impact on production. Developing a strategy to gradually replace antibiotics may be an effective and acceptable approach to maintain animal health and production. A previous study has reported that the combined use of B. íichenformis and antibiotics in the diet of weaning piglets does not promote growth performance (Collinder et al., 2003). BW and gut microbiota of weaning piglets were not improved by Baciííus species-based probiotics in combination with antibiotic supplementation (Poulsen et al., 2018). In contrast, a full replacement or half replacement of antibiotics with B. amyíoíiquefaciens ameliorated growth performance, digestive enzyme activity, and antioxidant capacity compared with the antibiotic-alone group (Wang et al., 2017; Hu et al., 2018). Our previous study demonstrated that half replacement of antibiotics with BLFPs can decrease the incidence of diarrhoea, regulate caecal microbiota composition, and improve ADG in weaning piglets (Lin & Yu, 2020). In this field study, we further confirmed that half or total replacement of antibiotics with BLFPs ameliorate the FCR of weaning piglets. Moreover, similar to the previous study (Lin & Yu, 2020), a clear separation of faecal bacterial communities between the groups treated with antibiotics alone, BLFPs alone, or both were also observed in this field study. These results imply that antibiotics alone, a full replacement or half replacement of antibiotics with BLFPs still have a different impact on the gut microbiota of weaning piglets although there is no significant difference in FCR among the groups (A, F, and AF). However, how these treatments differentially modulate microbiota in the gut and whether the microbiota regulated by these treatments have a direct impact on the health and growth of weaning piglets remains to be investigated.
Lactobaciííus species have been considered as the major bacterial population found in the porcine gastrointestinal tract (Guevarra et al., 2019). Feeding of Lactobaciííus species to weaning piglets resulted in an increased growth performance due to better FCR and improved gut health (Dowarah et al., 2016). It has been reported that the number of Lactobaciííus species increased in the faeces of weaning piglets with an increasing dose of B. subtiíis (Hu et al., 2014). However, low-doses of a B. íicheniformis and B. subtiíis mixture can increase the abundance of the genus, Lactobaciííus, in the faeces of weaning piglets challenged with Escherichia coli, whereas high-doses of a B. íicheniformis and B. subtiíis mixture reduce the abundance of the genus, Lactobaciííus (Zhang et al., 2017). In this field study, replacing all antibiotics or half the antibiotics with BLFPs decreased the abundance of the genus, Lactobaciííus, in the faeces of weaning piglets. This finding is in agreement with the results of Hung et al. (2019), who observed that high-doses of BLFPs could reduce the abundance of Lactobaciííus species in the faeces of weaning piglets. A previous study demonstrated that Lactobaciííus species supplementation can promote the growth and utilization rate of the feed in weaning piglets (Guevarra et al., 2019). However, our previous results (Hung et al., 2019; Lin & Yu, 2020) and present field study demonstrate that the abundance of the genus, Lactobaciííus, in the faeces is not strongly correlated with growth performance (BW, ADG, ADFI, and FCR). These findings may indicate that the effect of Lactobaciííus species in the gut on the health and growth of weaning piglets remains to be confirmed. Furthermore, whether antagonistic interactions between B. íicheniformis and Lactobaciííus species occur in modifying the porcine gut microbiome also needs to be investigated. It has been reported that the genus, Streptococcus, is enriched in the faeces of more feed-efficient pigs (Yang et al., 2017). Our previous study demonstrated that replacing all antibiotics with BLFPs increased the abundance of the genus, Streptococcus, in the cecal digesta of weaning piglets (Lin & Yu, 2020). The average abundance of the genus, Streptococcus, is negatively correlated with BW and the ADG (Lin & Yu, 2020). Similar to the previous study (Lin & Yu, 2020), we also confirmed that replacing all the antibiotics with BLFPs increased the abundance of the genus, Streptococcus, in the faeces and the abundance of the genus, Streptococcus, is negatively correlated with growth performance (BW, ADG, ADFI, and FCR). Furthermore, the combined use of B. lichenformis and antibiotics in the diet of weaning piglets can normalize the abundance of the genus, Streptococcus, in the faeces of weaning piglets. However, the effect of the genus, Streptococcus, in the gut on the health and growth of weaning piglets in response to BLFP supplementation remains to be elucidated in the future.
In the past, bacitracin was used widely as a growth promoter in pig feed and to control the spread of necrotic enteritis. It has been reported that bacitracin treatment can promote the resistance and virulence of Streptococcus species (Ma et al., 2019). Bacitracin-fed broilers have higher levels of bacitracin resistance genes and of vancomycin-resistant Enterococcaceae (Gupta et al., 2021). Antibiotic-resistant bacteria will remain in the gut of healthy pigs even when antibiotics are not used (Joyce et al., 2019). Probiotics have been considered as alternatives to antibiotics to prevent antibiotic resistance. In this study, the tetG (tetracycline resistance determinant, class G) gene expression was increased in the faeces of weaning piglets in response to bacitracin treatment, whereas full replacement or half replacement of antibiotics with BLFPs partially reduced the tetG gene expression. Interestingly, a full replacement of antibiotics with BLFPs decreased the strA (streptomycin phosphotransferase) gene expression in the faeces of weaning piglets. Taken together, BLFP supplementation is able to reduce ARG expression in the faeces of weaning piglets. A longitudinal study using metagenome sequencing on the effects of BLFPs on the dynamics of ARG in the faeces of weaning piglets is needed in the future.
Conclusion
Replacing all or half the antibiotics with BLFPs has beneficial effects on the FCR of weaning piglets from days 15 to 28. BLFPs and antibiotics differentially regulate the gut microbiota and ARG expression of weaning piglets. Therefore, based on our field study, BLFPs may be used as a natural alternative to antibiotics in weaning piglets.
Acknowledgements
This work was supported by the Council of Agriculture (109AS-12.1.2-ST-a4) in Taiwan.
Authors' contributions
KHL and YHY collected the data, conducted the statistical analyses, collaborated in interpreting the results, wrote the initial draft of this manuscript, and finalized the manuscript. YHY developed the original hypothesis and designed the experiments. The authors have read and approved the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
References
Alexopoulos, C., Georgoulakis, I.E., Tzivara, A., Kyriakis, C.S., Govaris, A. & Kyriakis, S.C., 2004. Field evaluation of the effect of a probiotic-containing Bacillus licheniformis and Bacillus subtilis spores on the health status, performance, and carcass quality of grower and finisher pigs. J. Vet. Med. A. Physiol. Pathol. Clin. Med. 51, 306312. DOI: 10.1111/j.1439-0442.2004.00637.x [ Links ]
Balasubramanian, B., Li, T. & Kim, I.H., 2016. Effects of supplementing growing-finishing pig diets with Bacillus spp. probiotic on growth performance and meat-carcass grade quality traits. R. Bras. Zootec. 45, 93-100. DOI: 10.1590/S1806-92902016000300002 [ Links ]
Chen, Y.C. & Yu, Y.H., 2020. Bacillus licheniformis-fermented products improve growth performance and the faecal microbiota community in broilers. Poult. Sci. 99, 1432-1443. DOI: 10.1016/j.psj.2019.10.061 [ Links ]
Cheng, Y.H., Su, L.W., Horng, Y.B. & Yu, Y.H., 2019. Effects of soybean meal fermented by Lactobacillus species and Clostridium butyricum on growth performance, diarrhoea incidence and faecal bacteria in weaning piglets. Ann. Anim. Sci. 19, 1051-1062. DOI: 10.2478/aoas-2019-0042 [ Links ]
Cheng, Y.H., Horng, Y.B., Chen, W.J., Hua, K.F., Dybus, A. & Yu, Y.H., 2021. Effect of fermented products produced by Bacillus licheniformis on the growth performance and cecal microbial community of broilers under coccidial challenge. Animals 11, 1245. DOI: 10.3390/ani11051245. [ Links ]
Collinder, E., Cardona, M.E., Berge, G.N., Norin, E., Stern, S. & Midtvedt, T., 2003. Influence of zinc bacitracin and Bacillus licheniformis on microbial intestinal functions in weaned piglets. Vet. Res. Commun. 27, 513-26. DOI: 10.1023/a:1026043623194 [ Links ]
Dowarah. R., Verma, A.K. & Agarwal, N., 2016. The use of Lactobacillus as an alternative to antibiotic growth promoters in pigs: A review. Anim. Nutr. 3, 1-6. DOI: 10.1016/j.aninu.2016.11.002 [ Links ]
Dou, S., Gadonna-Widehem, P., Rome, V., Hamoudi, D., Rhazi, L., Lakhal, L., Larcher, T., Bahi-Jaber, N., Pinon- Quintana, A., Guyonvarch, A., Huërou-Luron, I.L. & Abdennebi-Najar, L., 2017. Characterisation of early-life faecal microbiota in susceptible and healthy pigs to post-weaning diarrhoea. PLoS One. 12, e0169851. DOI:10.1371/journal.pone.0169851 [ Links ]
Gresse, R., Chaucheyras-Durand, F., Fleury, M.A., Van de Wiele, T., Forano, E. & Blanquet-Diot, S., 2017. Gut microbiota dysbiosis in postweaning piglets: Understanding the keys to health. Trends Microbiol. 25, 851-873. DOI: 10.1016/j.tim.2017.05.004 [ Links ]
Guevarra, R.B., Lee, J.H., Lee, S.H., Seok, M.J., Kim, D.W., Kang, B.N., Johnson, T.J., Isaacson, R.E. & Kim, H.B., 2019. Piglet gut microbial shifts early in life: Causes and effects. J. Anim. Sci. Biotechnol. 10, 1. DOI: 10.1186/s40104-018-0308-3 [ Links ]
Gupta, C.L., Blum, S.E., Kattusamy, K., Daniel, T., Druyan, S., Shapira, R., Krifucks, O., Zhu, Y.G., Zhou, X.Y., Su, J.Q. & Cytryn, E., 2021. Longitudinal study on the effects of growth-promoting and therapeutic antibiotics on the dynamics of chicken cloacal and litter microbiomes and resistomes. Microbiome. 9, 178. DOI: 10.1186/s40168-021-01136-4 [ Links ]
Horng, Y. B., Yu, Y.H., Dybus, A., Hsiao, F.S. & Cheng, Y.H., 2019. Antibacterial activity of Bacillus species-derived surfactin on Brachyspira hyodysenteriae and Clostridium perfringens. AMB Express 9, 188. DOI: 10.1186/s13568-019-0914-2 [ Links ]
Hu, S., Cao, X., Wu, Y., Mei, X., Xu, H., Wang, Y., Zhang, X., Gong, L. & Li, W., 2018. Effects of probiotic Bacillus as an alternative of antibiotics on digestive enzymes activity and intestinal integrity of piglets. Front. Microbiol. 9, 2427. DOI: 10.3389/fmicb.2018.02427 [ Links ]
Hu, Y., Dun, Y., Li, S., Zhao, S., Peng, N. & Liang, Y., 2014. Effects of Bacillus subtilis KN-42 on growth performance, diarrhoea and faecal bacterial flora of weaned piglets. Asian-Australas. J. Anim. Sci. 27, 1131-1140. DOI: 10.5713/ajas.2013.13737 [ Links ]
Hung, D.Y., Cheng, Y.H., Chen, W.J., Hua, K.F., Pietruszka, A., Dybus, A., Lin, C.S. & Yu, Y.H., 2019. Bacillus licheniformis-fermented products reduce diarrhoea incidence and alter the faecal microbiota community in weaning piglets. Animals 9, 1145. DOI: 10.3390/ani9121145 [ Links ]
Joyce, A., McCarthy, C.G.P., Murphy, S. & Walsh, F., 2019. Antibiotic resistomes of healthy pig faecal metagenomes. Microb. Genom. 5, e000272. DOI: 10.1099/mgen.0.000272 [ Links ]
Kritas, S.K. & Morrison, R.B., 2005. Evaluation of probiotics as a substitute for antibiotics in a large pig nursery. Vet. Rec. 156, 447. DOI: 10.1136/vr. 156.14.447 [ Links ]
Leser, T.D., Knarreborg, A. & Worm, J., 2008. Germination and outgrowth of Bacillus subtilis and Bacillus licheniformis spores in the gastrointestinal tract of pigs. J. Appl. Microbiol. 104, 1025-1033. DOI: 10.1111/j.1365-2672.2007.03633.x [ Links ]
Lin, K.H. & Yu, Y.H., 2020. Evaluation of Bacillus licheniformis-fermented feed additive as an antibiotic substitute: Effect on the growth performance, diarrhoea incidence, and cecal microbiota in weaning piglets. Animals 10,1649. DOI: 10.3390/ani10091649 [ Links ]
Lin, E.R., Cheng, Y.H., Hsiao, F.S.H., Proskura, W.S., Dybus, A. & Yu, Y.H., 2019. Optimization of solid-state fermentation conditions of Bacillus licheniformis and its effects on Clostridium perfringens-induced necrotic enteritis in broilers. R. Bras. Zootec. 48, e20170298. DOI: 10.1590/rbz4820170298 [ Links ]
Ma, J., Liu, J., Zhang, Y., Wang, D., Liu, R., Liu, G., Yao, H. & Pan, Z., 2019. Bacitracin resistance and enhanced virulence of Streptococcus suis via a novel efflux pump. BMC Vet. Res. 15, 377. DOI: 10.1186/s12917-0192115-2 [ Links ]
Maron, D.F., Smith, T.J. & Nachman, K.E, 2013. Restrictions on antimicrobial use in food animal production: An international regulatory and economic survey. Global Health 9, 48. DOI: 10.1186/1744-8603-9-48 [ Links ]
Poulsen, A.R., Jonge, N., Nielsen, J.L., H0jberg, O., Lauridsen, C., Cutting, S.M. & Canibe, N., 2018. Impact of Bacillus spp. spores and gentamicin on the gastrointestinal microbiota of suckling and newly weaned piglets. PLoS One 13, e0207382. DOI: 10.1371/journal.pone.0207382 [ Links ]
Rhouma, M., Fairbrother, J.M., Beaudry, F. & Letellier, A., 2017. Post weaning diarrhoea in pigs: Risk factors and non-colistin-based control strategies. Acta Vet. Scand. 59, 31. DOI: 10.1186/s13028-017-0299-7 [ Links ]
Rozs, M., Manczinger, L., Vagvölgyi, C. & Kevei, F., 2001. Secretion of a trypsin-like thiol protease by a new keratinolytic strain of Bacillus licheniformis. FEMS Microbiol. Lett. 205, 221-224. DOI: 10.1111/j.1574-6968.2001.tb10951.x [ Links ]
Soleymanzadeh Moghadam, S., Khodaii, Z., Fathi Zadeh, S., Ghooshchian, M., Fagheei Aghmiyuni, Z. & Mousavi Shabestari., 2018. Synergistic or antagonistic effects of probiotics and antibiotics- alone or in combination- on antimicrobial-resistant Pseudomonas aeruginosa isolated from burn wounds. Arch. Clin. Infect. Dis. 13, e63121. DOI: 10.5812/archcid.63121 [ Links ]
Thaniyavarn, J., Roongsawang, N., Kameyama, T., Haruki, M., Imanaka, T., Morikawa, M. & Kanaya, S., 2003. Production and characterization of biosurfactants from Bacillus licheniformis F2.2. Biosci. Biotechnol. Biochem. 67, 1239-1244. DOI: 10.1271/bbb.67.1239 [ Links ]
Wang, Y., Wu, Y., Wang, B., Cao, X., Fu, A., Li, Y. & Li, W., 2017. Effects of probiotic Bacillus as a substitute for antibiotics on antioxidant capacity and intestinal autophagy of piglets. AMB Express 7, 52. DOI: 10.1186/s13568- 017-0353-x [ Links ]
Xu, S., Lin, Y., Zeng, D., Zhou, M., Zeng, Y., Wang, H., Zhou, Y., Zhu, H., Pan, K., Jing, B. & Ni, X., 2018. Bacillus licheniformis normalize the ileum microbiota of chickens infected with necrotic enteritis. Sci. Rep. 8, 1744. DOI: 10.1038/s41598-018-20059-z [ Links ]
Yang, H., Huang, X., Fang, S., He, M., Zhao, Y., Wu, Z., Yang, M., Zhang, Z., Chen, C. & Huang, L., 2017. Unravelling the faecal microbiota and metagenomic functional capacity associated with feed efficiency in pigs. Front. Microbiol. 8, 1555. DOI: 10.3389/fmicb.2017.01555 [ Links ]
Yu, Y.H., Hsu, T.Y., Chen, W.J., Horng, Y.B. & Cheng, Y.H., 2020. Effect of Bacillus licheniformis-fermented products and postpartum dysgalactia syndrome on litter performance traits, milk composition, and faecal microbiota in sows. Animals 10, 2044. DOI: 10.3390/ani10112044 [ Links ]
Zhang, W., Zhu, Y.H., Zhou, D., Wu, Q., Song, D., Dicksved, J. & Wang, J.F., 2017. Oral administration of a select mixture of Bacillus probiotics affects the gut microbiota and goblet cell function following Escherichia coli challenge in newly weaned pigs of genotype MUC4 that are supposed to be enterotoxigenic E. coli F4ab/ac receptor negative. Appl. Environ. Microbiol. 83, e02747-16. DOI: 10.1128/AEM.02747-16 [ Links ]
Zong, X., Wang, T.H., Lu, Z.Q., Song, D.G., Zhao, J. & Wang, Y.Z., 2019. Effects of Clostridium butyricum or in combination with Bacillus licheniformis on the growth performance, blood indexes, and intestinal barrier function of weanling piglets. Livest. Sci. 220, 137-142. DOI: 10.1016/j.livsci.2018.12.024 [ Links ]
Submitted 25 February 2022
Accepted 22 August 2022
Published 28 January 2023
# Corresponding author: yuyh@niu.edu.tw














