Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Animal Science
versão On-line ISSN 2221-4062
versão impressa ISSN 0375-1589
S. Afr. j. anim. sci. vol.52 no.5 Pretoria 2022
http://dx.doi.org/10.4314/sajas.v52i5.13
Effect of various pH levels on the sperm kinematic parameters of boars
Cindy U. RivasI, II; Maria E. AyalaIII; Andrés AragónI, #
ILaboratory of Gamete and Technology Development, Faculty of Superior Studies Iztacala, National Autonomous University of Mexico. Paseo de los Barrios Número 1, Los Reyes Iztacala, Tlalnepantla, México State, México, C.P. 54090
IIPosgraduate Studies in Biological Sciences, Postgraduate Unit, Building A, Postgraduate Circuit, 1st floor, Universitary City, Coyoacan, Mexico City, Mexico, C.P. 04510
IIIBiology of Reproduction Unit, Laboratory of Puberty, Faculty of Superior Studies Zaragoza, National Autonomous University of Mexico, Mexico City, México, AP 9-020, C.P. 15000
ABSTRACT
The objective of this work was to identify the effects of pH on the structure of kinematic sperm subpopulations using commercial doses of boar semen diluted in Androstar Plus extender. We adjusted the pH value of the diluted semen samples to 7.4, 7.6, 7.8, and 8.0, and evaluated motility parameters using an open-source computer-assisted sperm analysis system. We evaluated the dataset of the kinematic parameters of the individual sperm for the effects of pH on the average values overall and on the subpopulations of sperm. To identify these subpopulations, we reduced the dimensionality of the dataset through principal component analysis followed by hierarchical clustering. We then compared the results for the kinematic parameters across the dataset and in the subpopulations with the average of the corresponding control (i.e., the basal pH value). We found that increasing the pH did not affect the proportion of motile sperm but did affect the manner in which they moved, rendering their movement more linear. The progressive motility increased relative to the control at all pH values above 7.1, while the proportion of sperm with low motility increased at pH 7.8. We distinguished three sperm subpopulations. The proportion of sperm in Subpopulation 1 increased at pH values of 7.8 and 8.0, with the sperm being characterized by low velocity and high values for linearity. The sperm in Subpopulation 3, by contrast, were relatively few but showed relatively high curvilinear velocity. In this study, we demonstrated the presence of subpopulations of boar sperm that responded differentially to changes in pH.
Keywords: CASA system, kinematic sperm subpopulations, multivariate statistics, boar, sperm motility
Introduction
The use of computer-assisted sperm analysis (CASA) systems has confirmed statistically the existence of heterogeneity in the motility of sperm (Ramón & Martínez-Pastor, 2018), that is, of sperm subpopulations distinguished by their kinematic patterns. Heterogeneity is, essentially, a statistical property of cellular populations (Altschuler & Wu, 2010). The statistical strategies for the identification of sperm motility subpopulations described previously include distinct clustering with or without dimensionality reduction (Martínez-Pastor et al., 2011; Becerril et al., 2013; Ramón & Martínez-Pastor, 2018). An understanding of sperm subpopulations has clear implications for animal production (Ramón & Martínez-Pastor, 2018).
Sperm are excitable cells. Thus, their flagellar beating changes in response to the environment, altering their swimming patterns. Their motility integrates the biochemical events occurring within the cells in numerous ways (Martínez-Pastor et al., 2011). Nevertheless, the characterization of sperm features was long limited to recording a few average parameters per sample, such as motility and morphometry, with the result that valuable information about the natural variability of samples was not collected (Ramón & Martínez-Pastor, 2018). Currently, CASA systems produce a wealth of data that are frequently ignored, including motility parameters for individual sperm (Ramón & Martínez-Pastor, 2018), with studies using CASA systems tending to report only the average values for the motility parameters. When the structure of the subpopulations has been considered, however, strong associations with fertility have been observed (Ramón & Martínez-Pastor, 2018). Thus, a first step in assessing the possible biological significance of heterogeneity in a characteristic such as fertility is to pinpoint precisely and describe statistically the kinematic sperm subpopulations. In the case of boar sperm motility, three or four subpopulations have been identified with respect to treatments with bicarbonate (Henning et al., 2014) and caffeine (Abaigar et al., 1999) and physical factors such as freezing (de Mercado et al., 2020).
The pH within the female reproductive tract varies across regions (Mishra et al., 2018). Previous research has found that sperm motility is affected by the pH of the surrounding milieu, both in vivo and in vitro (Acott & Carr, 1984; Goltz et al., 1988; Lishko & Kirichok, 2010; Contri et al., 2013; Zhou et al., 2015; Mishra et al., 2018). However, the responsiveness of the various subpopulations within a sperm sample has not been reported. As noted, some in vitro studies have utilized CASA systems but reported only the average values for the motility parameters (Contri et al., 2013; Zhou et al., 2015). The extant studies of kinematic subpopulations of boar sperm have reported responsiveness to various other factors but not pH (Henning et al., 2014; Abaigar et al., 1999, Henning et al., 2014). A recent study did assess the effects of extenders and freezing on subpopulations within boar sperm, but the focus was on the influence of the alkalinity of the thawing extender after thawing on motility (de Mercado et al., 2020).
Sperm is, of course, the only human cell that performs its function outside the body that produces it (Zhou et al., 2015). In the inconsistent chemical milieu that sperm traverse, the pH is a critical factor in determining their quality. Thus, functions such as motility, viability, and capacitation are pH-dependent (Zhou et al., 2015). During the journey through both the male and female genital tracts, sperm cells show precise regulation of the proton gradient and, thereby, regulation of intracellular pH (Mishra et al., 2018). Studies have shown deviations in or disruption of sperm functions at high and low pH that indicate the existence of a dynamic pH regulatory system in the sperm (Zhou et al., 2015). However, no data are available for kinematic sperm subpopulations that allow for an assessment of how changes in motility relate to such cellular events as capacitation or fertilization. The purpose of the present study was to determine the kinematic properties of boar sperm subpopulations in response to pH.
Materials and Methods
The semen doses used in this study were initially produced for artificial insemination and were donated to us for the experiments described here. The Committee of the Facultad de Estudios Superiores Zaragoza, UNAM, approved the experimental protocols (Letter 04/22/2021).
The experimental unit in these experiments was a semen dose. We drew aliquots from each semen sample (N = 10) for adjustment to the specific pH values. We diluted the doses in Androstar Plus extender (Minitube, Tiefenbach, Germany).
The semen was collected at the Centro de Educación, Investigación y Extensión en Producción Porcina de la Facultad de Medicina Veterinaria y Zootecnia (UNAM) using the gloved-hand technique. The genotypes of the boars were Pietrain (n = 3), Yorkshire x Pietrain (n = 1), and Yorkshire χ Landrace (n = 1). The boars ranged in age from 1.8 to 2.3 years.
The analysis of the semen doses took place on the same day that they were collected. The samples were transported to the laboratory at 17 °C in keeping with the protocol used in commercial distribution. Once the samples arrived at the laboratory, we maintained aliquots of 1.5 ml at 38 °C in a dry bath for 25 min before use. We measured the sperm concentration with a Neubauer chamber (PROPER Lumicyte 1/100 mm depth) and the pH with an Oakton PC 700 pH meter (Eutech Instruments, Singapore).
The average pH of the ten, untouched semen doses used in this study was 7.13 ± 0.16. We used this basal pH as the control value (in the Results section below, we rounded the basal pH to 7.1). We adjusted the pH of aliquots of each semen dose using sodium bicarbonate (Sigma, St. Louis, USA) according to (Contri et al., 2013). We drew four aliquots of 500 μl from each semen sample and then adjusted the pH of the aliquots to 7.4, 7.6, 7.8, or 8.0, taking into account the basal pH.
We evaluated the motility of the sperm in the aliquots 5 min after adjusting the pH. Briefly, we placed 15 μl of the sperm suspension on a slide, covered the suspension with a clean coverslip (22 χ 22 mm), and conducted observations using a B3 Clinilab phase-contrast microscope (Motic, British Columbia, Canada) equipped with a customized stage prewarmed to 38 °C. All of the glass and plastic materials were prewarmed to 38 °C. For each sperm sample, we took three or four image sequences of separate fields at 100* magnification (our evaluations included at least 200 sperm in each sample). We captured the image sequences with a Stingray F-033B camera (Allied Vision Technologies Inc., Exton, PA, USA) and stored them digitally. We captured each image sequence with μManager version 1.4 software (Edelstein et al., 2014) at 60 frames per second (60 Hz) for two seconds. We looked at three to four fields per sample and analysed the image sequences using ImageJ software version 1.50d (Rasband, 1997) and the plugin developed by Wilson-Leedy and Ingermann (2007) and modified by Giaretta et al. (2017). We modified the settings of the plugin (CASA_bgm) for use with boar sperm. Thus, in brief, we adjusted the particle area to a range of 7-80 μηι and considered sperm motile for which the velocity average path (VAP) exceeded 15 μm/s. When determining the progressive motility, the cut-offs were 15 for the VAP and 30 for STR. The maximum VAP values for the categorization of the speed of the sperm as slow or medium were 50 μm and 100 μπι, respectively. As stated in other reports, before analysing the track sequence, we determined the trajectory of each sperm, assessing each field visually to eliminate possible debris and reduce the likelihood of including unclear tracks in the analysis (Cremades et al., 2005).
We evaluated the following parameters for each sperm: VAP (μm/sec), curvilinear velocity (VCL, μm/sec), straight-line velocity (VSL, μm/sec), beat cross frequency (BCF, Hz), linearity (LIN, VSL/VCL), straightness coefficient (STR, VSL/VAP), amplitude of the lateral head displacement (ALH, μm), and wobble (WOB, VAP/VCL). We also recorded such parameters as total motility (%), progressive motility (%), and the numbers of slow, medium, or rapid sperm. The kinematic data for individual sperm served to construct the dataset.
To investigate the effects of pH on the average of each kinematic parameter, we conducted multivariate analyses of variance (MANOVAs) of the kinematic parameters grouped as follows: 1) VCL, VAP, and VSL; 2) LIN and STR; and 3) ALH, BCF, and WOB. When the MANOVA yielded significant results, we conducted one-way ANOVAs for the unbalanced models (since the number of sperm evaluated in each treatment varied) and applied linear (orthogonal) contrasts.
We used a two-step process to identify the sperm subpopulations. First, we reduced the dimensionality of the data using principal component analysis (PCA), determining the number of principal components (PCs) by following the Kaiser criterion (i.e., retaining only PCs with eigenvalues greater than 1). Next, we performed hierarchical clustering and partitioning of the PCs using Ward's criterion (Husson et al., 2010). Ward's criterion is based on the Huygens theorem, which facilitates decomposition of the total inertia (total variance) into between and within-group variance by aggregating two clusters so as to minimize the growth of within-inertia at each step of the algorithm (Husson et al., 2010). We determined the number of clusters (subpopulations) based on inertial gain (total variance gain). We used the chi-square test to compare the proportions of sperm in each subpopulation with the pH values and compared the kinematic parameters in each subpopulation with the basal pH.
The statistical model that we used was:
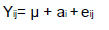
where Yy is the response variable (kinematic parameters of sperm in all dataset or in sperm subpopulations); μ is the overall mean; ai is the fixed effect of the various pH levels (i = 7.1, 7.4, 7.6, 7.8, 8.0); and eij is the random error effect.
We used R software version 3.4.3 (R Core Team, 2017) running on a MacBook Pro with Mac OS X version 10.11.6 to analyse all of the data. For all of the tests, we considered P-values less than 0.05 to be significant. We performed the MANOVAs with the R base and the ANOVAs with the car package version 2.1-0 (Fox & Weisberg, 2011). We performed the matrices of the Chi square with the vcd package version 1.3-2 (Meyer et al., 2014), and the PCA and hierarchical clustering with the plugin FactoMineR version 1.16 (Husson et al., 2011).
Results
The dataset on which the findings of this study was based is available at Harvard Dataverse (https://doi.org/10.7910/DVN/CRVVSI). It includes the kinematic data for 11,620 sperm. Each of the MANOVAs for the CASA-derived grouped kinematic parameters was significant (P <0.001), for which reason we performed the one-way ANOVAs. As the pH increased, the average values of the kinematic parameters changed. In general, the kinematic parameters of velocity diminished at high pH values (Table 1). However, the parameters describing "linearity" and the width of the sperm head's trajectory increased at high pH values (Table 1).
The pH did not induce changes in the proportions of motile sperm, but the proportions of progressive sperm were greater at the higher pH values (the P-values were 0.007, 0.023, 0.004, and < 0.001 for pH 7.4, 7.6, 7.8, and 8.0, respectively) (Figure 1A and B). For the control (pH 7.1), the percentages of sperm categorized as rapid were greater than those categorized as medium or slow; the proportions of sperm categorized as slow were greater at pH 7.8, but sperm categorized as rapid were less at this pH (the P-values appear on the right in Figure 1C). We observed no changes in the proportions of the distinct category of velocity for the pH values of 7.4, 7.6, and 8.0. The proportions of sperm in the distinct velocity category can be represented as medium > rapid > slow. The bivariate representation of data showed that some sperm linearized their paths at the higher pH values (Figure 2).
Having analysed the effects of pH on the average values of the kinematic parameters, we identified the kinematic subpopulations in the dataset. The two principal PCA components (PC1 and PC2) that we obtained explained 79.90% of the total variance of the dataset (Table 2). The other components, PC3 to PC8, explained 11.79%, 5.79%, 1.46%, 0.56%, 0.34%, and 0.14%, respectively, of the variance. The individual kinematic parameters were associated with particular PCs (Table 2).
Although the sperm were obtained from three distinct strains of boars, we observed no difference among the boars in the distribution of the PC data (Figure 3). The motility parameters VAP, ALH, and VCL were relatively high and correlated positively with PC1, whereas the parameters STR, LIN, and WOB correlated positively with PC2 (Figure 4A). The data for the distinct pH values did not form clear groupings (Figure 4B). The hierarchical clustering method identified three clusters corresponding to distinct subpopulations (Figure 4C), as depicted on the map of principal components (Figure 4D). We calculated and mapped the proportions of individuals belonging to each pH treatment in each cluster (Figure 4E). Notably, the proportion of sperm in Cluster 1 was substantially greater at pH values of 7.8 and 8.0 (Figure 4F).
Subpopulations 1, 2, and 3 contained kinematic data from 5,063, 5,830, and 727 sperm, respectively. We observed the greatest velocities in Subpopulation 3 and high linearity in Subpopulations 1 and 2 (Table 3). The VCL in all subpopulations diminished as the pH increased. The kinematic parameters (other than the VCL) in Subpopulation 3 were insensitive to the changes in pH value (Table 3). The increase in pH levels induced low values for VCL, VAP, and ALH in Subpopulations 1 and 2; and LIN, STR, and BCF increased in the same subpopulations. Linearity and velocities were greater in Subpopulation 2 than in Subpopulation 1 (Table 3).
Discussion
It is widely recognized that the pH of the surrounding medium affects the motility of sperm (Contri et al., 2013). However, the population of sperm in an ejaculate is heterogeneous, and not all sperm respond in the same way to changes in the surrounding medium (Ramón & Martínez-Pastor, 2018). Changes in average sperm motility and kinematic parameters induced by pH have been evaluated objectively using CASA systems (Contri et al., 2013; Zhou et al., 2015), but such research has not described the kinematic subpopulations within sperm samples. In this study, we helped to fill this gap in the literature by evaluating the effect of distinct pH values on the parameters obtained from CASA of boar sperm. We first determined the kinematic values at the population level and then identified kinematic subpopulations to assess the changes in the proportions of these subpopulations at each pH value.
The sperm in an ejaculate respond to changing pH in their environment by modifying their motility and swimming patterns. Researchers became aware of the kinematic effects of pH well before the availability of detailed CASA-derived descriptions. For instance, in bulls, the percentage of progressive sperm and their swimming speed increased at high levels of K+ and pH (Babcock et al., 1983) whereas, for ram and boars, the percentage of motile sperm and the flagellar beat frequency increased as pH increased from 7.0 to 8.0 (Gatti et al., 1993). For species such as sheep, boars, cattle, and humans, sperm motility decreases as the pH decreases (Babcock et al., 1983; Gatti et al., 1993; Rizvi et al., 2009; Contri et al., 2013; Zhou et al., 2015).
In studies using CASA systems, the mean of the kinematic sperm parameters is generally high within a defined range of pH values. In one study, for example, bull sperm showed high mean values of VCL, VSL, VAP, and the derived parameter, LIN, within a pH range of 7.0-7.5 (Contri et al., 2013), whereas the same kinematic parameters diminished below pH 7.2 in samples of human sperm (Zhou et al., 2015). After sperm were exposed to motility activators, such as bicarbonate, the tendencies of the derived kinematic parameters were unambiguous when subjected to bivariate analysis (for instance LIN versus VAP) (Satake et al., 2006). In the current study, we observed no changes in the percentages of total motile sperm as the pH increased, but we did observe changes in the manner in which the sperm moved. In particular, we detected a sperm subpopulation with relatively high LIN values but no change in VAP values at pH 7.6-8.0, a result consistent with reports on the treatment of boar sperm with bicarbonate (Satake et al., 2006; Henning et al., 2014) and maintaining it in liquid storage (Henning et al., 2014), almost the same result as that for LIN. The significance of these sperm subpopulations with high LIN values is unknown, though their presence could indicate that they are from high-quality samples in terms of linearity of displacement and maintaining motility, as previously reported (Satake et al., 2006; Mondéjar et al., 2012; Henning et al., 2014). Clearly, sperm with curved paths have no chance to reach the ovum; conversely, for example, bull sperm showing high values for velocity and linearity achieved relatively high rates of fertilization in vitro (Ferraz et al., 2014).
The genotype of sperm may play a role in their kinematic response to chemical factors (Ramón & Martínez-Pastor, 2018). Thus, variability in the genotype of the boars from which semen samples are obtained could introduce bias into the results of research into the effects of pH. For this study, we obtained the semen samples from boars of three distinct genotypes. Though the effect of genotype on sperm motility was beyond the scope of the present study, we note that we did not observe any effect of the boars on the distribution of data following the PCA. We observed an increase in the derived parameters, such as LIN, STR, WOB, and BCF, at the higher pH values (7.6-8.0). An increase in the BCF was previously reported for bovine sperm, and, though that research did not use a CASA approach (Babcock et al., 1983), our results are consistent with these earlier findings. The pH values in the reproductive tract of the sow become progressively more basic, increasing from ~7.0 in the vagina to ~8.0 near the ovaries (Nichol et al., 1997). Our results show the biological significance of the changes in pH with respect to kinematic sperm parameters.
To reach the ovum, the sperm must overcome adverse physical conditions within the female reproductive tract, including the obstacles posed by the micro-anatomy (e.g., mucosal folds) and fluids in counter-flow (Suarez, 2016). How the linear swimming pattern in the sperm subpopulation responsive to high pH, observed in this study, could help those sperm to overcome these obstacles is unknown. The kinematic evidence with the best fit among a number of sperm subpopulations, indicate 1) the few sperm found in the oviduct of swine (again, an alkaline environment) showed more linear swimming trajectories than in semen (Suarez et al., 1992); and 2) the fitness of spermatozoa swimming across a vertical plane has been observed at pH values of 7.6-7.8 (Rivas Arzaluz et al., 2021).
The data obtained from the CASA are characterized by a large number of kinematic variables (from 8 to more than 20) and by redundancy among the variables (Martínez-Pastor et al., 2011). Therefore, it was desirable to reduce the number of variables before feeding the data to the clustering algorithm so as to reduce both dimensionality and redundancy (Martínez-Pastor et al., 2011), and we did reduce the dimensionality of the data by PCA before the clustering process was employed. Various studies have observed either three or four clusters for boar sperm depending on the statistical procedure followed (Abaigar et al., 1999; Quintero-Moreno et al., 2004; Flores et al., 2009; Henning et al., 2014). Our finding of three clusters is thus in line with these previous reports.
In studies involving multiple subpopulations, heterogeneity (i.e., the proportions of the subpopulations within an overall population) may serve as an informative property regarding the physiology of the population and the prediction of responses to perturbations in the environment (Altschuler & Wu, 2010). The proportion of sperm in Cluster 1 at all pH values assayed was greater than at pH 7.0, ranging from 17% at pH 7.4 to 23% at pH 7.8. Whether these sperm subpopulations have some biological significance in the fertilization process is unknown. However, the changes in the kinematic pattern of a sperm subpopulation induced by the biochemical environment are indicative of changes in the functioning of intrinsic factors, such as proteins, in those sperm. A cellular mechanism involving ion channels regulating flagellar movement has been described for boar sperm (Vicente-Carrillo et al., 2017; Yeste et al., 2020), but ours is the first study to describe the kinematic effects of pH on boar sperm. It remains to be determined whether the ion channels are responsible for the changes that we observed in the motility and swimming patterns of various sperm subpopulations.
In order to have the opportunity to fertilize the ovum, mammalian sperm must, of course, reside in the female reproductive tract for some period of time. The physiological changes that render the sperm able to fertilize an ovum, known as capacitation, are associated with increases in intracellular pH, calcium, and the phosphorylation of various proteins (Escoffier et al., 2015). However, only a fraction of sperm undergoes capacitation (Escoffier et al., 2012). It is possible that the kinematic subpopulation that we identified as especially motile and linear in swimming patterns in this study is related to the subpopulation that undergoes capacitation.
Conclusions
In this work, we documented changes in the kinematic sperm parameters in the subpopulations of boar sperm exposed to various pH levels. At all of the pH values that we assayed, the VCL decreased in all of the subpopulations, while the BCF increased in Subpopulations 1 and 2. In Subpopulations 1 and 2, the increases in pH induced large increases in the linearity of the swimming paths. The potential roles of the kinematic subpopulations responsive to pH during fertilization remain to be determined.
Acknowledgments
We are grateful to Dr. Oscar Gutiérrez from CEIEEP, UNAM, for supplying us the boar semen doses. Cindy Rivas produced this work as a pre-requisite for doctoral graduation (Posgrado en Ciencias Biológicas, UNAM; "Investigación realizada gracias al programa UNAM-PAPIIT [Grant number IN221018]."
Authors' contributions
Conceptualization: AA, CR; methodology: AA, CR, MEA; formal analysis: AAA, CR, MEA; investigation: AAA, CR; original draft preparation: AA, CR, MEA; review & editing: AAA, MEA; supervision: AAA
Conflict of interest declaration
Authors declare no conflict of interest.
References
Abaigar, T., Holt, W. V., Harrison, R. A., & del Barrio, G. 1999. Sperm subpopulations in boar (Sus scrofa) and gazelle (Gazeíía dama mhorr) semen as revealed by pattern analysis of computer-assisted motility assessments. Biol. Reprod. 60, 32-41 https://doi.org/10.1095/biolreprod60.132. [ Links ]
Acott, T. S., & Carr, D. W. 1984. Inhibition of bovine spermatozoa by caudal epididymal fluid: II. Interaction of pH and a quiescence factor. Biol. Reprod. 30, 926-935 https://doi.org/10.1095/biolreprod30A926. [ Links ]
Altschuler, S. J., & Wu, L. F. 2010. Cellular heterogeneity: Do differences make a difference? Cell 141, 559-563 https://doi.org/10.1016/j.cell.2010.04.033. [ Links ]
Babcock, D. F., Rufo, G. A., & Lardy, H. A. 1983. Potassium-dependent increases in cytosolic pH stimulate metabolism and motility of mammalian sperm. Proc. Natl. Acad. Sci. U.S.A. 80, 1327-1331 https://doi.org/10.1073/pnas.80.5.1327. [ Links ]
Becerril, M., Huerta, C., Méndez, M., Hernandez, G., & Aragón, M. 2013. Use of multivariate statistics to identify unreliable data obtained using CASA. Syst. Biol. Reprod. Med. 59, 164-171 https://doi.org/10.3109/19396368.2013.766281. [ Links ]
Contri, A., Gloria, A., Robbe, D., Valorz, C., Wegher, L., & Carluccio, A. 2013. Kinematic study on the effect of pH on bull sperm function. Anim. Reprod. Sci. 136, 252-259 https://doi.org/10.1016/j.anireprosci.2012.11.008. [ Links ]
Cremades, T., Roca, J., Rodriguez-Martinez, H., Abaigar, T., Vazquez, J. M., & Martinez, E. A. 2005. Kinematic changes during the cryopreservation of boar spermatozoa. J. Androl. 26, 610-618 https://doi.org/10.2164/jandrol.05028. [ Links ]
Edelstein, A. D., Tsuchida, M. A., Amodaj, N., Pinkard, H., Vale, R. D., & Stuurman, N. 2014. Advanced methods of microscope control using μManager software. J. Biol. Methods 1, e10 https://doi.org/10.14440/jbm.2014.36. [ Links ]
Escoffier, J., Krapf, D., Navarrete, F., Darszon, A., & Visconti, P. E. 2012. Flow cytometry analysis reveals a decrease in intracellular sodium during sperm capacitation. J. Cell Sci. 125, 473-485 https://doi.org/10.1242/jcs.093344. [ Links ]
Escoffier, J., Navarrete, F., Haddad, D., Santi, C. M., Darszon, A., & Visconti, P. E. 2015. Flow cytometry analysis reveals that only a subpopulation of mouse sperm undergoes hyperpolarization during capacitation. Biol. Reprod. 92, 121 https://doi.org/10.1095/biolreprod.114.127266. [ Links ]
Ferraz, M. A. M. M., Morató, R., Yeste, M., Arcarons, N., Pena, A. I., Tamargo, C., Hidalgo, C. O., Muino, R., & Mogas, T. 2014. Evaluation of sperm subpopulation structure in relation to in vitro sperm-oocyte interaction of frozen-thawed semen from Holstein bulls. Theriogenology 81, 1067-1072 https://doi.org/10.1016/j.theriogenology.2014.01.033. [ Links ]
Flores, E., Fernández-Novell, J. M., Pena, A., & Rodríguez-Gil, J. E. 2009. The degree of resistance to freezing-thawing is related to specific changes in the structures of motile sperm subpopulations and mitochondrial activity in boar spermatozoa. Theriogenology 72, 784-797 https://doi.org/10.1016/j.theriogenology.2009.05.013. [ Links ]
Fox, J., & Weisberg, S. 2011. An R companion to applied regression. Second edition. Sage, Thousand Oaks, CA, USA. [ Links ]
Friendly, M. 1994. Mosaic displays for multi-way contingency tables. J. Am. Stat. Assoc. 89, 190-200 https://doi.org/10.2307/2291215. [ Links ]
Gatti, J. L., Chevrier, C., Paquignon, M., & Dacheux, J. L. 1993. External ionic conditions, internal pH and motility of ram and boar spermatozoa. J. Reprod. Fertil. 98, 439-449 https://doi.org/10.1530/jrf.0.0980439. [ Links ]
Giaretta, E., Munerato, M., Yeste, M., Galeati, G., Spinaci, M., Tamanini, C., Mari, G., & Bucci, D. 2017. Implementing an open-access CASA software for the assessment of stallion sperm motility: Relationship with other sperm quality parameters. Anim. Reprod. Sci. 176, 11-19 https://doi.org/10.1016/j.anireprosci.2016.11.003. [ Links ]
Goltz, J. S., Gardner, T. K., Kanous, K. S., & Lindemann, C. B. 1988. The interaction of pH and cyclic adenosine 3',5'-monophosphate on activation of motility in Triton X-100 extracted bull sperm. Biol. Reprod. 39, 1129-1136 https://doi.org/10.1095/biolreprod39.5.1129. [ Links ]
Henning, H., Petrunkina, A. M., Harrison, R. A. P., & Waberski, D. 2014. Cluster analysis reveals a binary effect of storage on boar sperm motility function. Reprod. Fertil. Dev. 26, 623-632 https://doi.org/10.1071/RD13113. [ Links ]
Husson, F., Josse, J., Le, S., & Mazet, J. 2011. FactoMineR: Multivariate exploratory data analysis and data mining with R. R package version 1.16. [ Links ]
Husson, F., Josse, J., & Pages, J. 2010. Principal components methods-hierarchical clustering-partitional clustering: Why would we need to choose for visualizing data. Technical Report-Agrocampus, 1-17. [ Links ]
Lishko, P. V., & Kirichok, Y. 2010. The role of Hv1 and CatSper channels in sperm activation. J. Physiol. 588, 4667-4672 https://doi.org/10.1113/jphysiol.2010.194142. [ Links ]
Martinez-Pastor, F., Tizado, E. J., Garde, J. J., Anel, L., & de Paz, P. 2011. Statistical Series: Opportunities and challenges of sperm motility subpopulation analysis. Theriogenology 75, 783-795 https://doi.org/10.1016/j.theriogenology.2010.11.034. [ Links ]
de Mercado, E., Tomás-Almenar, C., & Gómez-Izquierdo, E. 2020. Improvement of the motility of boar sperm after cryopreservation. Anim. Reprod. Sci. 222, 106610 https://doi.org/10.1016/j.anireprosci.2020.106610. [ Links ]
Meyer, D., Zeileis, A., & Hornik, K. 2014. vcd: Visualizing Categorical Data. Sage, Thousand Oak, CA, USA. [ Links ]
Mishra, A. K., Kumar, A., Swain, D. K., Yadav, S., & Nigam, R. 2018. Insights into pH regulatory mechanisms in mediating spermatozoa functions. Vet. World 11, 852-858 https://doi.org/10.14202/vetworld.2018.852-858. [ Links ]
Mondéjar, I., Acuña, O. S., Izquierdo-Rico, M. J., Coy, P., & Avilés, M. 2012. The oviduct: Functional genomic and proteomic approach. Reprod. Domest. Anim. 47 Suppl 3, 22-29 https://doi.org/10.1111/j.1439-0531.2012.02027.x. [ Links ]
Nichol, R., Hunter, R. H., & Cooke, G. M. 1997. Oviduct fluid pH in intact and unilaterally ovariectomized pigs. Can. J. Physiol. Pharmacol. 75, 1069-1074. [ Links ]
Quintero-Moreno, A., Rigau, T., & Rodriguez-Gil, J. E. 2004. Regression analyses and motile sperm subpopulation structure study as improving tools in boar semen quality analysis. Theriogenology 61, 673-690 https://doi.org/10.1016/s0093-691x(03)00248-6. [ Links ]
R Core Team. 2017. R: A Language and Environment for Statistical Computing. Vienna, Austria. [ Links ]
Ramón, M., & Martinez-Pastor, F. 2018. Implementation of novel statistical procedures and other advanced approaches to improve analysis of CASA data. Reprod. Fertil. Dev. 30, 860-866 https://doi.org/10.1071/RD17479. [ Links ]
Rasband, W. S. 1997. ImageJ, US National Institutes of Health. Bethesda, Maryland, USA. [ Links ]
Rivas Arzaluz, C., Ayala, M. E., & Aragón Martinez, A. 2021. A new open-source hardware device to measure vertical sperm motility and concentration. Cytometry A http://dx.doi.org/10.1002/cyto.a.24343. [ Links ]
Rizvi, A. A., Quraishi, M. I., Sarkar, V., DuBois, C., Biro, S., & Mulhall, J. 2009. The effect of pH and viscosity on bovine spermatozoa motility under controlled conditions. Int. Urol. Nephrol. 41, 523-530 https://doi.org/10.1007/s11255-008-9493-x. [ Links ]
Satake, N., Elliott, R. M. A., Watson, P. F., & Holt, W. V. 2006. Sperm selection and competition in pigs may be mediated by the differential motility activation and suppression of sperm subpopulations within the oviduct. J. Exp. Biol. 209, 1560-1572 https://doi.org/10.1242/jeb.02136. [ Links ]
Suarez, S. S. 2016. Mammalian sperm interactions with the female reproductive tract. Cell Tissue Res. 363, 185-194 https://doi.org/10.1007/s00441-015-2244-2. [ Links ]
Suarez, S. S., Dai, X. B., DeMott, R. P., Redfern, K., & Mirando, M. A. 1992. Movement characteristics of boar sperm obtained from the oviduct or hyperactivated in vitro. J Androl 13, 75-80. [ Links ]
Vicente-Carrillo, A., Álvarez-Rodríguez, M., & Rodríguez-Martínez, H. 2017. The CatSper channel modulates boar sperm motility during capacitation. Reprod Biol 17, 69-78 https://doi.org/10.1016/j.repbio.2017.01.001. [ Links ]
Wilson-Leedy, J. G., & Ingermann, R. 2007. Development of a novel CASA system based on open-source software for characterization of zebrafish sperm motility parameters. Theriogenology 67, 661-672 https://doi.org/10.1016/j.theriogenology.2006.10.003. [ Links ]
Yeste, M., Llavanera, M., Mateo-Otero, Y., Catalan, J., Bonet, S., & Pinart, E. 2020. HVCN1 Channels are relevant for the maintenance of sperm motility during in vitro capacitation of pig spermatozoa. Int J Mol Sci 21,E3255 https://doi.org/10.3390/ijms21093255. [ Links ]
Zhou, J., Chen, L., Li, J., Li, H., Hong, Z., Xie, M., Chen, S., & Yao, B. 2015. The semen pH affects sperm motility and capacitation. PLoS ONE 10, e0132974 https://doi.org/10.1371/journal.pone.0132974. [ Links ]
Submitted 6 January 2022
Accepted 30 July 2022
Published 28 January 2023
# Corresponding author: Dr. Andrés Aragón Martínez, email: armandres@gmail.com














