Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Animal Science
On-line version ISSN 2221-4062
Print version ISSN 0375-1589
S. Afr. j. anim. sci. vol.52 n.4 Pretoria 2022
http://dx.doi.org/10.4314/sajas.v52i4.14
Effects of biomacromolecules on growth, digestibility, digestive enzyme activity, antioxidation, and immunity in broilers
R.F. WangI, II; Y. WangI, II; X.P. AnI, II; X. JiI; J. DuI, II; Y.C HuI, II; T. GuoI, II; J. ZhangI, II; A.Q. GaoI,#; J.W. QiI,II,#
ICollege of Animal Science, Inner Mongolia Agricultural University, Hohhot 010018, China
IIInner Mongolia Herbivorous Livestock Feed Engineering Technology Research Center, Hohhot 010018, China
ABSTRACT
This study aimed to evaluate the effects of two biomacromolecules, fermented wheat bran polysaccharides (FWBPs) and sodium humate (SH), on growth performance, nutrient digestibility, digestive enzyme activity, antioxidant status, and immunity of broilers. A total of 144 male, one-day-old Arbor Acres broilers were randomly divided into three dietary groups: control (CON), 0.4% FWBPs, and 0.1% SH, with six replicates of eight birds. The FWBPs and SH groups had a greater body weight (BW) at 21 and 42 d, average daily weight gain (ADG) in the starter period, average daily feed intake (ADFl) in the overall period, and feed-to-gain ratio (F:G) in the grower period. However, the ADFI was decreased by FWBPs supplementation and increased by SH supplementation in the grower period. The dry matter (DM) and organic matter (OM) digestibility were higher in the SH group at 21 d. At 21 d, the FWBPs group had an increased duodenal trypsin and serum glutathione peroxidase (GSH-Px) activity and immunoglobulin M (IgM) concentration, and a decreased liver malondialdehyde (MDA) concentration. The SH group had increased duodenal lipase activity, serum IgM, and interleukin-2 concentrations but decreased liver MDA concentrations. At 42 d, FWBPs and SH supplementation decreased duodenal trypsin and chymotrypsin and serum superoxide dismutase activity. Jejunum chymotrypsin activity and liver MDA content were decreased in the SH group. In conclusion, dietary FWBPs or SH supplementation during the starter period can improve growth performance and nutrient digestibility and enhance antioxidant capacity and immunity of broilers.
Keywords: antioxidant status, digestive ability, fermented wheat bran, immunological function, polysaccharide, sodium humate
Introduction
Over the past few decades, antibiotic growth promoters (AGPs) have been widely used to enhance growth performance and improve feed efficiency with the intensification of livestock and poultry farming (Gadde et al., 2017). Because of growing concern about the transmission and proliferation of resistant bacteria via food chains, the European Union has imposed a complete ban on the use of antibiotics in animal feed as growth promoters in livestock and poultry since 2006 (Hassan et al., 2010).
China has issued requirements to ban the use of growth-promoting feed drug additives since 1 January 2020 (Tian et al., 2021). These changes have stimulated nutritionists and feed manufacturers to search for new, safer alternatives to antibiotics to improve poultry production. Thus, in recent years, several additives (such as plant polysaccharides, oligosaccharides, organic acids, probiotics, and immunostimulants) have been used as growth promoters in broilers. Among the alternatives, some biomacromolecules such as plant polysaccharides (Liu et al., 2021) and humic substances (Arif et al., 2019) have been recommended as growth promoters, owing to their environmentally friendly attributes.
Polysaccharides, as a primary class of biomacromolecule, are composed of monosaccharides and are distributed widely among animals, plants, algae, and fungi (Ohta et al., 2016). Numerous plant polysaccharides (not only those from medicinal plants and grasses, but also from cereals) have been demonstrated to possess a broad spectrum of bioactive properties such as antioxidant, anti-cancer, and immune-modulating activities (Yu et al., 2014; Fadel et al., 2018), and have been tentatively used in poultry farming (Akhtar et al., 2012). Wheat is the most widely-grown cereal in the world (Slafer & Satorre, 1999). Wheat bran, the outer layer of the kernel, is produced in abundance as a byproduct during the milling process. It contains the bulk of high nutritional value components, such as non-starch polysaccharides, fatty acids, tocopherols, and phenolic compounds (Stevenson et al., 2012). The major constituents of wheat bran are non-starch polysaccharides; arabinoxylans account for 70% of non-starch polysaccharides (Maes & Delcour, 2002). In recent years, wheat bran polysaccharides (WBPs) have been extensively demonstrated to have many beneficial roles in human nutrition and health, such as immunostimulatory (Shen et al., 2017), antioxidant (Malunga & Beta, 2015), anticomplementary, and antitumor effects (Hromadkova et al., 2013). Wheat bran, fermented wheat bran, and its main polysaccharides (arabinoxylans) have been reported to play beneficial roles in poultry farming, including the improvement of growth performance and intestinal morphology (Chu et al., 2017; Elmasry et al., 2017; Li et al., 2018), increasing intestinal microflora abundance (Courtin et al., 2008; Feng et al., 2020), and immunostimulatory and protective effects against coccidiosis (Akhtar et al., 2012).
Humic substances are complex, natural biomacromolecules that are decomposed primarily from organic matter by bacteria in the soil. The major components are humin, humic, fulvic, and ulmic acids, and trace minerals (Stevenson, 1994). Humic substances have been used extensively in agriculture, industry, environment, and biomedicine. Because of the specific properties of humates, humic acid and its sodium salt are permitted for oral use in horses, ruminants, swine, and poultry for the treatment of diarrhoea, dyspepsia, and acute intoxication in veterinary practices in Europe (EMEA, 1999). During the last two decades, many studies have found that humates included in the feed and water can confer a number of advantages to health and productive performance in poultry, such as improved nutrient digestibility (Windisch et al., 2008), food conversion efficiency, enhanced growth performance (Kamel et al., 2015), improved gut health, and modulated immune (Kamel et al., 2015; Aristimunha et al., 2019; Mudronova et al., 2020) and antioxidant functions (Ipek et al., 2008).
Although in most studies, humic substances and WBP supplementation have shown a positive influence on poultry, the results are sometimes controversial. Other studies have noted non-significant (Smeets et al., 2018) and even negative effects (Rath et al., 2006; Samudovska et al., 2018) on broiler chickens. The objective of the current study was to determine the effect of a single application of polysaccharides extracted from fermented wheat bran (FWB) and sodium humate (SH) on growth performance, nutrient digestibility, digestive enzyme activity levels, antioxidant status, and immunity of broiler chickens.
Materials and Methods
The study was conducted according to the institutional guidelines for the care and use of laboratory animals in China (The State Science and Technology Commission of China, 1988) and approved by the Institutional Animal Care and Use Committee of Inner Mongolia Agricultural University (protocol number 2020066).
A total of 144 one-day-old, male, Arbor Acres broiler chickens (initial body weight (BW): 44.40 ± 0.51 g) were purchased from a commercial hatchery and randomly placed in cages with eight birds per cage, and six replications per treatment. The chicks were housed in an environmentally-controlled facility and had ad libitum access to feed and water throughout the experiment. The room temperature was maintained at 34 °C for the first 3 d, after which the temperature was gradually reduced by 2-3 °C per week to 21 °C as the birds advanced in age. This temperature was maintained until the end of the 42-day experiment. The photoperiod was set at 23 L:1 D during the 1st week, a 20 L:4 D lighting regimen was maintained from the second to third weeks, and a 23 L:1 D photoperiod was used for the remaining trial period. All chicks were inoculated with inactivated Newcastle disease vaccine on day seven and an inactivated infectious bursal disease vaccine on day 14.
The chicks received different diets in the starter (1-21 d) and grower (22-42 d) periods. The control diet composition is represented in Table 1 and was analysed according to the AOAC (1994). Lysine and methionine were calculated according to the NRC (1994). Two experimental diets were prepared by adding 4 g/kg FWBP or 1 g/kg SH to the basal diet. The levels of supplementation of FWBP and SH were selected according to the manufacturer's recommendations and information obtained from previous experiments (unpublished data). All diets were formulated to meet the NRC (1994) nutrient requirements.
Commercial SH was supplied by Beijing Sloan Biological Technology Co., Ltd, (Beijing, China). Wheat bran was fermented using Saccharomyces cerevisiae CGMCC 2.119 and Bacillus subtilis CGMCC 1.0892 obtained from China General Microbiological Culture Collection Centre (Beijing, China) under solid-state fermentation (Wang et al., 2020). Polysaccharides were extracted from powdered material of fermented wheat bran using the same method (Wang et al., 2020). The monosaccharide composition of FWBPs showed xylose (35.38%) as the dominant sugar, followed by glucose (29.15%), arabinose (24.29%), galactose (5.70%), mannose (3.17%), and rhamnose (2.31%), according to PMP-high performance liquid chromatography (Zhang et al., 2016). All birds were weighed individually after arrival at the laboratory. They were also weighed on days 21 and 42 after a 12-h fast. Birds in each cage were monitored daily for abnormal health and mortality. The feed was provided daily, and any leftover feed was weighed and recorded weekly to calculate the net feed intake. The feed intake of birds in each cage was recorded to determine the average daily weight gain (ADG) and average daily feed intake (ADFI), and the feed-to-gain ratio (F:G) was calculated as ADFI/ADG.
Acid insoluble ash (AIA) was used to determine digestibility. During the last 3 d (18-21 d and 3942 d) of each experimental diet and period, droppings were collected from the plates placed under each cage. Total tract digestibility was evaluated per cage using the total collection of excreta method. Fresh feed and water were available ad libitum during the excreta collection. The total fresh excreta per cage were weighed daily, frozen at -20 °C and lyophilized. Excreta collected per cage during the 3-d collection period were pooled for further analysis. Diet and excreta were homogenized, ground to pass through a 1-mm mesh, and used to determine proximate composition. Dry matter, organic matter, ether extract (EE), and crude protein (CP, determined as 6.25 * Kjeldahl nitrogen) were carried out according to AOAC (1994) procedures. Acid insoluble ash determinations were performed after ashing the samples and treating the ash with boiling 4 M hydrochloric acid (Siriwan et al., 1993). All analyses were performed in duplicate. All values were expressed on a dry matter (DM) basis. The apparent nutrient digestibility was computed as:
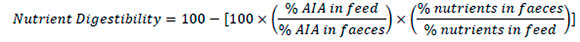
The formula was used to calculate dry matter, crude protein, ether extract, and organic matter digestibility.
One bird per cage with a BW similar to the mean BW of the full replicate cohort was randomly selected, marked, and weighed. After a 12 hour fast (water was offered ad libitum), six birds per group were euthanized by CO2 asphyxiation. Blood samples were obtained via heart puncture. The blood was allowed to clot at room temperature for 2 h and then centrifuged at 1, 917 * g for 10 min at 4 °C to obtain serum, which was then stored at -20 °C until analysis of antioxidant enzyme activities, immunoglobulin, and cytokine levels. Immediately after blood sampling, the birds were slaughtered by cervical dislocation, and liver tissue samples were harvested, stored at -80 °C, and then used for antioxidant enzyme activity level assays. The duodenal and jejunal digesta were obtained to determine digestive enzyme activities.
The activity of four digestive enzymes [alpha-amylase (AMS, C016-1-1), lipase (LPS, A054-1-1), trypsin (TPS, A080-2-2), and chymotrypsin (CTS, A080-3-1)], and of three antioxidant enzymes [superoxide dismutase (SOD, A001-3-2), glutathione peroxidase (GSH-Px, A005-1-2), and catalase (CAT, A007-1-1)], as well as malondialdehyde (MDA, A003-1-2) was determined using commercial colorimetric diagnostic kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), according to the manufacturer's instructions.
The concentrations of immunoglobulin A (IgA), immunoglobulin G (IgG), and immunoglobulin M (IgM), interleukin-2 (IL-2), interleukin-6 (IL-6), interleukin-10 (IL-10), and TNF-a in the serum were measured with chicken-specific ELISA kits (Colorful Gene Biological Technology Co., Ltd, Wuhan, China). All measurements were conducted in triplicate according to the manufacturer's instructions.
All data were analysed using one-way analysis of variance. When a significant effect occurred, data were further compared using the Duncan's multiple comparison procedure. Analyses were performed using SPSS (version 16.0) and statistical significance was defined as P <0.05. A statistical trend was defined as 0.05< P <0.1. All data are expressed as mean ± standard error (SE).
Results and Discussion
The effects of dietary FWBPs and SH supplementation on broiler growth performance at different phases are shown in Table 2. The initial BW did not differ between treatments (P >0.05). Fermented wheat bran polysaccharides induced higher BW on days 21 and 42 (P <0.05), and dietary SH produced a higher BW on day 21 (P <0.05), compared with the CON. Average daily gain increased after dietary supplementation with FWBPs and SH, compared with birds fed the basal diet from 1-21 d (P <0.05), and this trend (0.05< P <0.1) was also observed during the experiment. In the grower period, the ADFI was reduced in the FWBPs group and increased in the SH group compared with the CON (P <0.05). However, it increased in the two additive treatments compared with the control group from 1-42 d (P <0.05). From 22-42 d, the addition of FWBPs and SH increased the F:G ratio compared with the control (P <0.05).
Growth performance is the key index for evaluating feed additives for farmers. The current study showed that FWBPs has beneficial nutritional effects in broiler chickens. The growth-promoting effects of WBP in chickens as a feed supplement were supported by Akhtar et al. (2012), who found that orally administered wheat bran arabinoxylan in chickens produced higher live weight gain compared to the control group. The beneficial impact of WBP on the growth performance of chickens has been corroborated by many studies, which have reported that a diet containing wheat bran (Li et al., 2018) or fermented wheat bran (Chu et al., 2016; Elmasry et al., 2017) produces better growth than the corresponding unsupplemented diet. In these studies, the growth-promoting effects of wheat bran were attributed to the prebiotic effect of its main components (arabinoxylan). Based on the monosaccharide composition analysis, FWBPs are formed mainly from arabinoxylan.
Therefore, these studies support the findings of the current study. The increased growth performance after FWBP supplementation in the starter period could be associated with an improvement in feed utilization rate and absorption capability, because the authors observed elevated digestive enzyme activity levels (lipase and trypsin) at 21 d. Furthermore, the improved growth performance may be associated partly with enhanced antioxidative capacity and immune function, because higher GSH-Px activity and IgM levels in the serum and lower MDA concentrations in the liver were observed at 21 d. Notably, the growth-promoting effects of FWBPs in the grower period seemed less obvious, even negative, compared to the starter period. This is because a decreased ADFI and increased F:G were observed during the grower period.
Some researchers have reported increased growth performance as a consequence of humic acid or humate inclusion in broiler diets (Kocabagli et al., 2002; Kamel et al., 2015). In the present study, 0.1% SH dietary supplementation improved BW on day 21, ADG in the starter and overall period, and ADFI in the grower and overall periods. Based on previous studies, the increased growth performance in broilers after HS supplementation can be attributed to an improvement in nutrient digestibility (Ozturk & Coskun, 2006; Taklimi et al., 2012), formation of a protective film on the mucus epithelium of the gastrointestinal tract, and the maintenance of gut microflora (Windisch et al., 2008). Although the authors did not investigate the intestinal structure and gut microflora, DM and OM digestibility, and lipase and trypsin activity levels did increase. Therefore, the slight growth-promoting effects in the grower period and over the entire experiment could be attributed to a higher feed intake. These results indicate that the effects of HS on broilers can also be associated with the administration period, and administration of SH in the starter period may exert a more pronounced effect. However, inconsistent results were reported by Kocabagli et al. (2002,) who found that feeding humate during the grower period had the most beneficial effects on broiler performance in terms of growth and feed conversion. Eren et al. (2000) also reported that dietary humate supplementation at 1.5 and 2.5 g/kg improved the live weights of broilers at 42 d substantially, but there was no difference in performance at 21 d. Some studies have reported that humic acid exerts a negative effect on the growth performance of broiler chickens (Rath et al., 2006; Samudovska et al., 2018). These inconsistent results may be attributable principally to the chemical composition and concentration of the types of HS, supplementation levels, administration period, and the poultry species and ages in these studies.
Diets supplemented with SH increased the digestibility of DM and OM on day 21 (P <0.05) but decreased the digestibility remarkably on day 42 (P <0.05), compared with the control. The digestibility of DM, CP, and EE showed a decreasing trend (0.05< P <0.1) in the FWBPs group on day 42 (Table 3).
Dietary or drinking water supplementation of SH improves nutrient digestibility in poultry and has been reported extensively in the literature (Ozturk et al., 2014; Arif et al., 2019). Increased digestibility of DM and OM with SH administration in the current study was observed at 21 d. Dietary supplementation with SH can improve nutrient digestibility mainly because of its positive effects on maintaining gut microflora (Schepetkin et al., 2003; Windisch et al., 2008) and improving gut structure and function (Taklimi et al., 2012). Further studies are needed to investigate the changes in the microbial community and histological morphology in the gut of broilers after dietary SH supplementation. In contrast to the result at 21 d, reduced ether extract digestibility was also found at 42 d after dietary SH supplementation in the present study.
The effects of dietary FWBPs and SH supplementation on duodenum and jejunum digesta digestive enzyme activities in broilers at 21 d and 42 d are shown in Table 4. At 21 d, supplementation with FWBPs in the broiler diets increased trypsin activity and slightly increased lipase activity (0.05< P <0.1) but decreased chymotrypsin activity in the duodenal digesta (P < 0.05). Dietary supplementation with SH increased lipase activity (P <0.05) and slightly increased trypsin activity in duodenal digesta (0.05< P <0.1). Amylase, lipase, and trypsin activities in the jejunal digesta increased after dietary FWBPs supplementation, and lipase and trypsin activity levels in jejunal digesta increased after SH supplementation, although not significantly. At 42 d, FWBP supplementation in the broiler diets decreased trypsin and chymotrypsin activity levels in the duodenal digesta (P <0.05), whereas supplementation with SH decreased trypsin and chymotrypsin activity levels in the duodenal digesta and chymotrypsin activity in the jejunal digesta (P <0.05), compared with the control group.
The fundamental concept in improving digestive ability is enhancing the activity of digestive enzymes in the intestines to speed up the degradation of nutrients in the diet (Feng et al., 2020). Therefore, the determination of digestive enzyme activity levels is important in evaluating the effects of additives in the poultry breeding industry. The current results showed that the addition of FWBPs improved trypsin and lipase activities in the duodenal digesta of broilers at 21 d. Dietary prebiotic supplementation improving the digestive enzyme activity levels of broiler chickens has also been reported with Lycium barbarum polysaccharides (Long et al., 2019) and fructooligosaccharides (Xu et al., 2003). This beneficial effect of prebiotic supplementation on digestive enzyme activity levels may be attributable to changes in the gut microbial community (Xu et al., 2003). Further studies that use 16S rRNA sequencing to confirm the prebiotic effects of dietary FWBPs on broilers may be a next step.
Dietary humic substance supplementation that improves digestive enzyme activity was reported in broilers (Mao et al., 2019) and fish (Oreochromis niloticus) (Deng et al., 2019). Similar to these studies, the current data showed that supplementation with SH improved lipase and trypsin activity levels in the duodenal digesta of 21-day-old broilers, which indicated that SH could induce the expression of digestive enzymes and improve the digestive capacity of broilers. Indeed, this result is consistent with improved DM and OM digestibility and ADG in this study. The beneficial effect of SH supplementation on digestive enzymes activity might also be associated with changes in the gut microbial community. A previous study showed that humic substances could alter the intestinal microflora by increasing the counts of beneficial bacteria (Schepetkin et al., 2003). Thus, the bacteria could also secrete exo-enzymes and make a small contribution to the total enzyme activity of the intestine. However, the activity of amylase and chymotrypsin was not affected by SH in the current study and the reasons for this remain unclear. Notably, in contrast to the starter period, supplementation with FWBPs and SH reduced the digestive enzyme activity level in the duodenal and jejunal digesta in 42-day-old broilers. This negative effect of FWBPs and SH on intestinal digestive enzymes in broilers is consistent with its nonsignificant effect on growth improvement and decrease in digestibility.
At 21 d, broilers fed diets supplemented with FWBPs had an increased serum GSH-Px activity (P <0.05) and decreased liver MDA concentration (P <0.05) (Table 5). In addition, broilers fed diets supplemented with SH showed a decrease in liver MDA concentration (P <0.05). No significant changes were observed in other antioxidant enzyme activities in the serum and liver. At 42 d, broilers fed diets supplemented with FWBPs and SH had a substantially decreased serum SOD activity. The concentration of MDA in the liver showed a decrease and SOD, CAT, and GSH-Px activities in the liver showed a decreasing trend (0.05< P <0.1) after SH supplementation. No significant changes were observed in other antioxidant enzyme activities in the serum and liver.
Excessive reactive oxygen species (ROS) production in vivo causes damage to cells and tissues. However, during evolution, living organisms have developed a fine defence network system against oxidative stress (Wang et al., 2011). Primary antioxidant enzymes, such as SOD, CAT, and GSH-Px, form part of this defence system and are capable of eliminating ROS and cytotoxic peroxides (Muthulakshmi & Saravanan, 2013). Lipid oxidation produces a number of secondary products; MDA is the principal and most studied product of polyunsaturated fatty acid peroxidation and is widely used as a biomarker of oxidative stress (Niki, 2008). Several studies have shown that natural antioxidants can protect hosts against oxidative damage. Wheat bran possesses good antioxidant capacity, which has been demonstrated extensively in vitro (Hromadkova et al., 2013; Malunga & Beta, 2015). It has been recommended as a potential natural antioxidant in the functional food, cosmetics, and pharmaceutical industries (Yan et al., 2015). In vivo, the antioxidative benefits of wheat bran xylooligosaccharides in rats have also been reported by Wang et al. (2011). Similar to these findings, the present study observed that dietary FWBP supplementation increased serum GSH-Px activity levels substantially and reduced liver MDA levels in 21-day-old broilers, suggesting that FWBPs had the potential to protect broiler chickens against damage from ROS and free radicals.
The present study showed that dietary SH reduced MDA content in the liver of broilers, although no significant differences were found in other antioxidant enzyme activity levels between treatments in 21-day-old broilers. Similar results were reported by Mao (2019), who found that broilers fed with diets containing fulvic acid exhibited higher SOD and GPx activity and lower MDA levels in the serum. Similarly, Kamel et al. (2015) reported that supplementation with HS in the drinking water of broiler chickens increased glutathione reductase, total antioxidant, and catalase activity in the blood. These findings, including those of the present study, demonstrate the antioxidant activity of SH in poultry production. Quinone, phenol, and other functional groups, especially the quinone group, play an important role in electron transport; the reduced quinone group is the main source of the antioxidant activity of humic acids (Aeschbacher et al., 2012). Therefore, the observed antioxidant effect of SH on broilers can be related to the structural features of HS. However, further research is required to elucidate this mechanism.
Notably, in contrast to the starter period, the negative effect of dietary SH on antioxidant enzyme activity was apparent at 42 d, reflected by a significant decrease in serum SOD and a slight decrease in the liver SOD, CAT, and GSH-Px activities. The antioxidant effects of SH appear to be highly variable. Ipek et al. (2008) reported that lower levels of humic acid did not have an influence on total antioxidant capacity, but high levels reduced total antioxidant capacity in Japanese quails. Tunç & Yörük (2017) found that humate additives had a positive effect on the suckling period of calves, which can lead to oxidative stress. Therefore, because of effects of SH and FWBPs on antioxidant enzymes and other parameters investigated in the present study at 21 d and 42 d, the inclusion level and administration period of the two additives should be investigated when they are used in broilers.
The effects of FWBPs and SH supplementation on IgA, IgG, and IgM concentration in the serum of broilers at 21 d and 42 d are shown in Table 6. An increase in IgM was observed in the dietary FWBPs and SH groups at 21 d (P <0.05). However, there was no significant difference in other immunoglobulin concentrations among the groups at 21 d or 42 d.
The immune system is the primary factor influencing an animal's health and performance. Serum immunoglobulins are important indicators of the humoral immune status of animals. The current results showed that FWBP supplementation promoted a humoral immune response, leading to a substantial increase in serum IgM levels at day 21. However, the serum IgA and IgG levels were not affected. Similar results were reported by Akhtar et al. (2012), who found that significantly higher total-Igs, IgG, and IgM titres on the seventh and fourteenth day post-primary and -secondary injections of sheep red blood cells in the experimental chickens orally administered wheat bran arabinoxylan. A previous study also reported that supplementation with humic acid in the drinking water increased lymphocyte and leukocytic counts, and serum globulin (a, p and y) concentration in broiler chickens (Salah et al., 2015). The impact of humic acid in improving the immune functions of poultry and its possible mechanism of action was reviewed by Arif et al. (2019). The results of the present study indicate that FWBP and SH supplementation can induce IgM production, and this response may play an important role in the struggle against pathogenic and nonpathogenic immune challenges during the starter period.
At 21 d, broiler chicks fed SH had higher serum levels of IL-2 than birds in the control group (Table 7). At 42 d, IL-2 concentrations in the FWBPs and SH groups and IL-6 concentrations in the SH group showed an increasing trend (0.05< P <0.1), compared to the control. There were no significant differences in other cytokine concentrations among the groups at 21 d or 42 d.
The immune response is controlled by a highly complex and intricate network of control elements. The prominent regulatory components are anti-inflammatory cytokines and specific cytokine inhibitors (Opal & DePalo, 2020). The immunomodulatory potential of humic substances has been confirmed by its extensive usage in both human and veterinary medicine for thousands of years for their detoxication (Arafat et al., 2017), antibacterial (Humin, 2004), antiviral (Chen et al., 2003), and anti-inflammatory (Joone & van Rensburg, 2004) effects. Data from the present study showed that dietary SH supplementation substantially increased serum IL-2 levels at day 21 and slightly increased serum IL-2 and IL-6 levels at day 42. This indicates that SH can activate immune function, leading to enhanced antibody production. In addition, it was observed that serum IL-2 level also showed an increased tendency compared with the control group on day 42 after dietary supplementation with FWBPs. Slightly elevated serum cytokine levels may partially demonstrate the immune-stimulatory effects of FWBPs in broiler chickens. Similar results were reported by Shen et al. (2017), who found that polysaccharides from wheat bran induced cytokine expression and prevented cyclophosphamide-induced immunosuppression.
In the current study, it is worth noting that some of the investigated parameters showed a decreasing trend or were even substantially reduced at 42 d, whereas those data were elevated at 21 d. These included a non-significant increase in ADG during the grower period, as well as substantially reduced DM, CP, and EE digestibility, decreased trypsin and chymotrypsin activity in gut digesta, and antioxidant enzyme activity in the serum and liver at 42 d. Similar to the present study, non-significant (even negative) effects during the grower period or over the entire period with a positive effect during the starter period, have also been reported in previous studies after dietary additive supplementation (Lee et al., 2003; Kong et al., 2010). This inconsistent result might be related to the development of the gut microflora. Gut dominant microbial communities become more complex as broilers grow (Lan et al., 2005). Thus, the effect of additives might become increasingly inconspicuous with the abundance of intestinal flora. Furthermore, prolonged use of dietary additives can also exert a negative effect. Previous studies have reported that chronic intake of humic acid is associated with human diseases (Rath et al., 2006), but the exact reason is unclear. Further studies are warranted to investigate the effects of FWBPs and SH on the growth performance and physiological function of broiler chickens in the different periods and to determine the optimal dietary inclusion level. Based on this study, we can conclude that FWBPs and SH can be useful additives in the feeding of broilers during the starter period.
Conclusions
Dietary FWBPs or SH supplementation during the starter period can improve growth performance, nutrient digestibility, antioxidant capacity, and immunity in broilers.
Acknowledgements
The research was supported by Double First-Rate Discipline Innovation Team Programme for the Talents by IMAU (NDSC2018-02), Major Science and Technology Program of Inner Mongolia Autonomous Region (2020ZD0004), Key Technology Project of Inner Mongolia Autonomous Region (2020GG0030), and the Initial Funding of Scientific Research for the Introduction of Talents by IMAU (NDGCC2016-10). The authors would like to thank Editage (www.editage.cn) for English language editing.
Authors' Contributions
Conceptualization, XA, AG, and JQ; data curation, formal analysis, and investigation, RW, XJ, JD, JZ, TG, YH; writing-original draft, RW; writing-review and editing, YW; funding acquisition, AG and JQ; All authors read and approved the final manuscript.
Conflict of interest declaration
The authors certify that there is no potential conflict of interest to disclose.
References
Aeschbacher, M., Graf, C., Schwarzenbach, R.P., & Sander, M., 2012. Antioxidant properties of humic substances. Environ. Sci. Technol. 46, 4916-4925. DOI: 10.1021/es300039h [ Links ]
Akhtara, M., Tariqa, A.F., Awais, M.M., Iqbal, Z., Muhammad, F., Shahid, M. & Hiszczynska-Sawickad, E., 2012. Studies on wheat bran arabinoxylan for its immunostimulatory and protective effects against avian coccidiosis. Carbohyd. Polym. 90, 333-339. DOI: 10.1016/j.carbpol.2012.05.048 [ Links ]
AOAC, 1994. Official Methods of Analysis. 14th edn. Association of Official Analytical Chemists. Washington, DC. [ Links ]
Arafat, R. Y., Khan, S.H., & Saima, I., 2017. Evaluation of humic acid as an aflatoxin binder in broiler chickens, Annu. Anim. Sci. 17, 241-255. DOI: 10.1515/aoas-2016-0050 [ Links ]
Arif, M., Rehman, A., El-Hack, M.E.A., Saeed, M., Khan, F., Akhtar, M., Swelum, A.A., Saadeldin, & Alowaimer, A.N., 2018. Growth, carcass traits, cecal microbial counts, and blood chemistry of meat-type quail fed diets supplemented with humic acid and black cumin seeds. Asian-Aust. J. Anim. Sci. 31, 1930-1938. DOI: 10.5713/ajas.18.0148 [ Links ]
Arif, M., Alagawany, M., Abd El-Hack, M.E., Saeed, M., Arain, M.A., & Elnesr, S.S., 2019. Humic acid as a feed additive in poultry diets. Iran. J. Vet. Res. 20, 167-172. [ Links ]
Aristimunha, P.C., Mallheiros, R.R., Ferket, P.R., Cardinal, K.M., Moreira Filho, A.L.B., Santos, E.T., Cavalcante, D.T., & Ribeiro, A.M.L., 2019. Effect of dietary organic acids and humic substance supplementation on performance, immune response, and gut morphology of broiler chickens. J. Appl. Poult. Res. 29(1), 85-94. DOI: 10.3382/japr/pfz031 [ Links ]
Chen, C H., Liu, J.J., Lu, F.J., Yang, M.L., Lee, Y., & Huang, T.S., 2003. The effect of humic acid on the adhesibility of neutrophils. Thromb. Res. 108, 67-76. DOI: 10.1016/S0049-3848(02)00384-5 [ Links ]
Chu, Y. T., Lo, C.T., Chang, S.C., & Lee, T.T., 2017. Effects of Trichoderma-fermented wheat bran on growth performance, intestinal morphology, and histological findings in broiler chickens. Ital. J. Anim. Sci. 16, 8292. DOI: 10.1080/1828051X.2016.1241133 [ Links ]
Courtin, C.M., Broekaert, W.F., Swennen, K., Lescroart, O., Onagbesan, O., Buyse, J., Decuypere, E., Van de Wiele, T., Marzorati, M., Verstraete, W. and Huyghebaert, G., 2008. Dietary inclusion of wheat bran arabinoxylooligosaccharides induces beneficial nutritional effects in chickens. Cereal Chem. 85(5), 607-613. DOI: 10.1094/CCHEM-85-5-0607. [ Links ]
Deng, J. M., Lin, B.B., Zhang, X.D., Guo, L., Chen, L.S., Li, G.B., Wang, Q.M., Yu, C.Q., & Mi, H.F., 2019. Effects of dietary sodium humate on growth, antioxidant capacity, non-specific immune response, and resistance to Aeromonas hydrophila in genetic improvement of farmed tilapia (GIFT, Oreochromis niloticus). Aquaculture. 520, 734788. DOI: 10.1016/j.aquaculture.2019.734788 [ Links ]
Elmasry, M., Elgremi, S.M.,Belal, E., Elmostafa, K.E., & Eid, Y., 2017. Assessment of the performance of chicks fed with wheat bran solid fermented by Trichoderma longibrachiatum (SF1). J. Sus. Agric. Sci. 43, 115-126. DOI: 10.21608/jsas.2017.1162.1008 [ Links ]
EMEA, 1999. Humic acids and their sodium salts: Summary report. Committee for Veterinary Medicinal Products. Eur. Agency Eval. Med. Prod. http://www.emea.eu.int/pdfs/vet/mrls/055499en.pdf [ Links ]
Eren, M., Deniz, G., Gezen, S.S., & Turkmen, I., 2000. Broyler yemlerine katilan humatlarin besi performansi, serum mineral konsantrasyonu ve kemik kulu uzerine etkileri (Effects of humates on fattening performance, serum mineral concentration, and bone ash of broilers). Ankara Universitesi Veteriner Fakultesi Dergisi 47, 255-263. [ Links ]
Fadel, A., Plunkett, A., Li, W.L.,Ranneh, Y.,Tessu, V.E., Gyamfi, Salmon, Y., Nyaranga, R., & Ashworth, J., 2018. Arabinoxylans from rice bran and wheat immunomodulatory potentials: A review. Nutr. Food Sci. 48, 97-110. DOI: 10.1108/NFS-06-2017-0111 [ Links ]
Feng, Y, L. Wang, A. Khan, R. Zhao, S., Wei, & Jing, X.Y., 2020. Fermented wheat bran by xylanase-producing Bacillus cereus boosts the intestinal microflora of broiler chickens. Poult. Sci. 99, 263-271. DOI: 10.3382/ps/pez482 [ Links ]
Gadde, U.,Kim, W.H., Oh, S.T., & Lillehoj H.J., 2017. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: A review. Anim. Health. Res. Rev. 18, 26-45. DOI: 10.1017/S1466252316000207 [ Links ]
Hassan, H. M. A., Mohamed, M.A. Youssef, A.W., & Hassan, E.R., 2010. Effect of using organic acids to substitute antibiotic growth promoters on performance and intestinal microflora of broilers. Asian-Australas J. Anim. Sci. 23, 1348-1353. DOI: 10.5713/ajas.2010.10085 [ Links ]
Hromadkova, Z., Paulsen, B.S., Polovka, M., Kostalova, Z., & Ebringerova, A., 2013. Structural features of two heteroxylan polysaccharide fractions from wheat bran with anti-complementary and antioxidant activities. Carbohyd. Polym. 93, 22-30. DOI: 10.1016/j.carbpol.2012.05.021 [ Links ]
Humin, T., 2004. Humin animal feed supplements and veterinary medicine and humic acid-based products. Humintech-Humintech GmbH, Heerdter Landstr. 189/D, D-40549 Dusseldrof, Germany. [ Links ]
Ipek, H., Avci, M., Iriadam, M., Kaplan, O., & Denek, N., 2008. Effects of humic acid on some hematological parameters, total antioxidant capacity, and laying performance in Japanese quails. Archiv fur Geflugelkunde. 72, 56-60. [ Links ]
Joone, G.K., van Rensburg, C.E.J., 2004. An in vitro investigation of the anti-inflammatory properties of potassium humate. Inflammation. 28, 169-174. DOI: 10.1023/B:IFLA.0000039563.90066.5d [ Links ]
Kamel, M.M., Elhady, M., El Iraqi, K.G., & Wahba, F.A., 2015. Biological immune stimulants effects on immune response, behavioural, and productive performance of broilers. Egypt Poult Sci J. 35, 691-702. [ Links ]
Kocabagli, N., Alp, M., Acar, N., & Kahraman, R., 2002. The effects of dietary humate supplementation on broiler growth and carcass yield. Poult. Sci. 81, 227-230. DOI: 10.1093/ps/81.2.227 [ Links ]
Kong, C., Lee, J.H., & Adeola O., 2011. Supplementation of b-mannanase to starter and grower diets for broilers. Can. J. Anim. Sci. 91, 389-397. DOI: 10.4141/cjas10066 [ Links ]
Lan, Y., Verstegen, M.W.A., Tamminga, S., & Williams, B.A., 2005. The role of the commensal gut microbial community in broiler chickens. World. Poult. Sci. J. 61, 95-104. DOI: 10.1079/WPS200445 [ Links ]
Lee, K.W., Everts, H., Kappert, H.J., Frehner, M., Losa, R., &Beynen, A.C., 2003. Effect of dietary essential oils on growth performance, digestive enzymes, and lipid metabolism in female broiler chickens. Br. Poult. Sci. 44,450-457. DOI: 10.1080/0007166031000085508 [ Links ]
Li, B., Leblois, J.L., Taminiau, B., Schroyen, M., Beckers, Y., Bindelle, J., & Everaert, N., 2018. The effect of inulin and wheat bran on intestinal health and microbiota in the early life of broiler chickens. Poult. Sci. 97, 31563165. DOI: 10.3382/ps/pey195 [ Links ]
Liu, W.C., Zhu, Y.R., Zhao, Z.H., Jiang, P., & Yin, F.Q., 2021. Effects of dietary supplementation of algae-derived polysaccharides on morphology, tight junctions, antioxidant capacity, and immune response of duodenum in broilers under heat stress. Animals. 11, 2279. DOI: 10.3390/ani11082279 [ Links ]
Long, L.N., Kang, B.J., Jiang, Q., & Chen, J.S., 2020. Effects of dietary Lycium barbarum polysaccharides on growth performance, digestive enzyme activities, antioxidant status, and immunity of broiler chickens. Poult. Sci. 99, 744-751. DOI: 10.1016/j.psj.2019.10.043 [ Links ]
Maes, C., & Delcour, J.A., 2002. Structural characterisation of water extractable and water-unextractable arabinoxylans in wheat bran. J. Cereal Sci. 3, 315-326. DOI: 10.1006/jcrs.2001.0439 [ Links ]
Malunga, L.N., & Beta,T., 2015. Antioxidant capacity of arabinoxylan oligosaccharide fractions prepared from wheat aleurone using Trichoderma viride or Neocallimastixpatriciarum xylanase. Food Chem. 167, 311-319. DOI: 10.1016/j.foodchem.2014.07.001. [ Links ]
Mao, Y.M., 2019. Modulation of the growth performance, meat composition, oxidative status, and immunity of broilers by dietary fulvic acids. Poul. Sci. 98(10), 4509-4513. DOI: 10.3382/ps/pez281. [ Links ]
Mudronova, D., Karaffova, V., Pesulova, T., Koscova, J., Cingel'ova Maruscakova, J., Bartkovsky, M., Marcincakova, D., Sevcfkova, Z., & Marcincak,S., 2020. The effect of humic substances on gut microbiota and immune response of broilers. Food Agr. Immunol. 31, 137-149. DOI: 10.1080/09540105.2019.1707780 [ Links ]
Muthulakshmi, S., & Saravanan, R., 2013. Protective effects of azelaic acid against high-fat diet-induced oxidative stress in liver, kidney, and heart of C57BL/6J mice. Mol. Cell. Biochem. 377, 23-33. DOI: 10.1007/s11010-013-1566-1 [ Links ]
Niki, E. 2008. Lipid peroxidation products as oxidative stress biomarkers. Biofactors. 34, 171-180. DOI: 10.1002/biof.5520340208 [ Links ]
NRC. 1994. Nutrient Requirements of Poultry. 9th ed. National Academy Press, Washington, DC. [ Links ]
Ohta, T., Kusano, K., Ido, A., Chiemi, M., & Miura, T.,2016. Silkrose: A novel acidic polysaccharide from the silkmoth that can stimulate the innate immune response. Carbohydr. Polym. 136, 995-1001. DOI: 10.1016/j.carbpol.2015.09.070 [ Links ]
Opal, S.M., & DePalo, D.V.A, 2020. Impact of basic research on tomorrow's medicine anti-inflammatory cytokines. Chest. 117, 1162-1172. [ Links ]
Ozturk, E., & Coskun, I., 2006. Effects of humic acids on broiler performance and digestive tract traits. Proceedings of the Book of Abstracts of the 57th Annual Meeting of the European Association for Animal Production. Antalya, Turkey. pp 301. [ Links ]
Ozturk, E., Coskun, I., Ocak, N., Erene, N., Dervisoglu, M., & Turhan, S., 2014. Performance, meat quality, meat mineral contents, and caecal microbial population responses to humic substances administered in drinking water in broilers. Br. Poult. Sci. 55, 668-674. DOI: 10.1080/00071668.2014.960807 [ Links ]
Purdue, E.M, in: G.R. Aiken, D.M. McKnight, R.L. Wershaw, P. McCarthy (eds.), Humic Substances in Soil, Sediment, and Water. Wiley, New York, 1985. pp. 493-526. [ Links ]
Rath, N. C., Huff, W.E., & Huff, G.R., 2006. Effects of humic acid on broiler chickens. Poult Sci. 85, 410-414. DOI: 10.1093/ps/85.3.410. [ Links ]
Salah, H., Mansour, E., Reham, R.R., & Abd El Hamid, E.S., 2015. Study on the effect of humic acid on growth performance, immunological, some blood parameters and control intestinal clostridium in broiler chickens. Zag. Vet. J. 43, 102-109. DOI: 10.21608/zvjz.2015.29352. [ Links ]
Samudovska, A.H.,Hudak, M., Marcin, A., Bujnak, L., &NaD, P., 2018. Effect of sodium humate on some production parameters in broiler chickens. NutriNet. 46-52. [ Links ]
Schepetkin, I.A., Khlebnikov, A.I., Ah, S.Y., Woo, S.B., Jeong, C.S., Klubachuk, O.N., & Kwon, B.S., 2003. Characterization and biological activities of humic substances from mumie. J. Agric. Food Chem. 51, 52455254. DOI: 10.1021/jf021101e [ Links ]
Shen, T., Wang, G.C., You, L., Zhang, L., Ren, H., Hu, W.C., Qiang, Q., Wang, X.F., Ji, L.L.,Gu, Z.Z., & Zhao, X.X., 2017. Polysaccharide from wheat bran induces cytokine expression via the toll-like receptor 4-mediated p38 MAPK signaling pathway and prevents cyclophosphamide-induced immunosuppression in mice. Food Nutr. Res. 61, 1-12. DOI: 10.1080/16546628.2017.1344523 [ Links ]
Siriwan, P., Bryden, W.L., Mollan, Y., & Annison, E.F., 1993. Measurement of endogenous amino acid losses in poultry. Br. Poult. Sci. 34, 939-949. DOI: 10.1080/00071669308417654. [ Links ]
Slafer, G.A., & Satorre, E.H., 1999. An introduction to the physiological-ecological analysis of wheat yield. In: E.H. Satorre, & G.A. Slafer (eds.) Wheat: Ecology and Physiology of Yield Determination. The Haworth Press, New York. pp 3-12. [ Links ]
Stevenson, F.J. 1994. Humus Chemistry Genesis, Composition, Reactions (2nd edn). John Wiley & Sons, New York, USA. [ Links ]
Stevenson, L., Phillips, F., O'Sullivan, O., & Walton, J., 2012. Wheat bran: Its composition and benefits to health, a European perspective. Int. J. Food Sci. Nutr. 63, 1001-1013. DOI: 10.3109/09637486.2012.687366 [ Links ]
Taklimi, S.M., Ghahri, H., Isakan, M.A., 2012. Influence of different levels of humic acid and esterified glucomannan on growth performance and intestinal morphology of broiler chickens. Agric. Sci. 3, 663-668. DOI: 10.4236/as.2012.35080 [ Links ]
Tian, M., He, X.M., Feng, Y.Z., Wang, W.T., Chen, H.S., Gong, M., Liu, D., Clarke, J.L., & Eerde. A.V., 2021. Pollution by antibiotics and antimicrobial resistance in livestock and poultry manure in China, and countermeasures. Antibiotics 10, 539. DOI: 10.3390/antibiotics10050539 [ Links ]
Tunç, M.A., & Yörük, MA., 2017. Effects of humate and probiotic on the number of Escherichia coli, blood, and antioxidant parameters in the suckling period of calves. Asian J. Anim. Vet. Adv. 12, 169-176. DOI: 10.3923/ajava.2017.169.176 [ Links ]
Van Campenhout, C., Delezie, E., & Niewold, T., 2018. Interactions between the concentration of nonstarch polysaccharides in wheat and the addition of an enzyme mixture in a broiler digestibility and performance trial. Poult. Sci. 97, 2064-2070. DOI: org/10.3382/ps/pey038 [ Links ]
Vasková, J., Patlevic, P., Zatko, D., Marcincák, S.,Vasko, L., Krempaská, K., & Nagy, J., 2018. Effects of humic acids on poultry under stress conditions. Slov. Vet. Res. 55, 245-253. DOI: 10.26873/SVR-469-2018 [ Links ]
Wang, J., Cao, Y.P., Wang, C.T., & Sun, B.G., 2011. Wheat bran xylooligosaccharides improve blood lipid metabolism and antioxidant status in rats fed a high-fat diet. Carbohyd. Polym. 86,1192-1197. DOI: 10.1016/j.carbpol.2011.06.014 [ Links ]
Wang. Y., Wang, R.F., Hao, X.R., Hu, Y.C., Guo, T., Zhang, J., Wang, W.W., Shi, X.Y., An, X.P., & Qi, J.W., 2020. Growth performance, nutrient digestibility, immune responses, and antioxidant status of lambs supplemented with humic acids and fermented wheat bran polysaccharides. Anim. Feed. Sci. Tec. 269, 114644. DOI: 10.1016/j.anifeedsci.2020.114644 [ Links ]
Windisch, W., Schedle, K., Plitzner, C., & Kroismayr, A., 2008. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 86, E140-E148. DOI: 10.2527/jas.2007-0459 [ Links ]
Xu, Z.R., Hu, C.H., Xia, M.S., Zhan, X.A., & Wang, M.Q., 2003. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora, and morphology of male broilers. Poult. Sci. 82,1030-1036. DOI: 10.1093/ps/82.6.1030 [ Links ]
Yan, X.G., Ye, R., &Chen, Y., 2015. Blasting extrusion processing: The increase of soluble dietary fiber content and extraction of soluble-fiber polysaccharides from wheat bran. Food Chem. 180, 106-115. DOI: 10.1016/j.foodchem.2015.01.127 [ Links ]
Yu, X.H., Yang, R.Q., Gu, Z.X., Lai, S.J., & Yang, H.S., 2014. Anti-tumor and immunostimulatory functions of two feruloyl oligosaccharides produced from wheat bran and fermented by Aureobasidium pullulans. BioResources. 9, 6778-6790. DOI: 10.15376/biores.9.4.6778-6790 [ Links ]
Zhang, Z., Kong, F., Ni, H., Mo, Z., Wan, J.B., Hua, D., & Yan, C., 2016. Structural characterization, a-glucosidase inhibitory, and DPPH scavenging activities of polysaccharides from guava. Carbohydr. Polym. 144, 106-114. DOI: 10.1021/acs.jafc.6b01272 [ Links ]
Submitted 12 July 2021
Accepted 10 May 2022
Published 3 November 2022
# Corresponding author: gaoaiqin999@163.com














