Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Animal Science
On-line version ISSN 2221-4062
Print version ISSN 0375-1589
S. Afr. j. anim. sci. vol.52 n.4 Pretoria 2022
http://dx.doi.org/10.4314/sajas.v52i4.04
Does dietary inclusion of defatted yellow mealworm (Tenebrio molitor) affect growth and body composition of juvenile common carp (Cyprinus carpio)?
A. GebremichaelI, #; Z.J. SándorII; B. KucskaI
IDepartment of Applied Fish Biology, Hungarian University, Agricultural and Life Sciences, Kaposvár Campus, H-7400 Kaposvár, Guba S. u. 40
IIResearch Centre of Aquaculture and Fisheries, Hungarian University, Agricultural and Life Sciences, H-5540 Szarvas, Anna-Liget. u. 35
ABSTRACT
The objective of this study was to evaluate the effects of total and partial replacement of fishmeal with defatted yellow mealworm (Tenebrio molitor) meal on one-summer-old common carp (Cyprinus carpio) with an initial bodyweight of 97.54 g ± 51.0 g. Fish were kept in an experimental recirculating aquaculture system (9 x 250 L tanks) and fed with two experimental diets formulated with mealworm meal replacements and one control, which had 100 g/kg fishmeal without mealworm meal (MWM0). In the first treatment, 50% of fishmeal was replaced with mealworm meal (MWM50). In the second treatment, fishmeal was replaced totally with mealworm meal (MWM100). Fish were fed three times per day at 3.0% of fish biomass. After six weeks of the experimental period, growth performance, nutrient utilization, body composition, and biometric indices were compared. The results revealed that the growth performance of common carp was not affected significantly by the inclusion level of mealworm meal. However, the highest weight gain was observed in MWM100, where specific growth rate was 0.76 ± 0.10 g/day. Crude fat content of the fish body differed significantly between experimental groups and the control. This investigation demonstrated that MWM could be used as an alternative dietary protein source to replace fishmeal without adverse effects on the growth performance of one-summer-old common carp._
Keywords: aquafeed, dietary protein sources, insect meal
Introduction
Common carp (Cyprinus carpio) is considered a significant species for commercial aquaculture in Asia and some European countries as it has high adaptive capability to the environment and food (Rahman, 1996; Soltani et al., 2010; Manjappa et al., 2011). Feed in aquaculture accounts for 50-70% of production costs (Rana et al., 2009). Feeding technology in the leading carp producer countries is based mainly on formulated feeds. Until recently fish meal was one of the main dietary protein sources.
Overfishing pressure, the finite nature of fishmeal, climate change, and increased demand, especially in the commercial fish production industry, have resulted in a supply shortage, with the concomitant price increase in fish meal as an optimal protein source in fish diet (Rana et al., 2009; Oliva-Teles et al., 2015; Golden et al., 2016; Hua et al., 2019). It is vital to reduce the use of fish meal in fish diets by replacing it with local sustainable protein sources or, as in the poultry sub-sector, with an amino acid supplement instead of crude protein (Hafez & Attia, 2020) to minimize production costs and maximize the profitability of the fish farming sub-sector.
Insect meals are considered one of the most promising, sustainable, novel protein sources in aquaculture feed. As part of the natural diet of many fish species, insect meals have favourable nutritional composition, especially in protein content and amino acid profile, and their production leaves a small ecological footprint (need less land and water) and is eco-friendly (Oonincx & de Boer, 2012; Magalhães et al., 2017; Hua et al., 2019). Mealworm (Tenebrio molitor) is a widely used and approved food and animal feed by the European Union (EU) (Schiavone et al., 2014; Shafique et al., 2021; Bovera et al., 2015; Bovera et al., 2016; Tran et al., 2022). Based on experimental data, MWM seems suitable for partial or total replacement of fish meal in aqua feeds for a variety of marine and freshwater fish species in terms of growth performance and nutrient utilization (Ng et al., 2001; Belforti et al., 2015; Henry et al., 2015; Gasco et al., 2016; laconisi et al., 2019; Hoffman et al., 2020; Basto et al., 2021). The replacement level of MWM has been studied for various fish species and 75% is recommended for yellow catfish (Pelteobagrus fulvidraco) (Su et al., 2017); 67-100% for rainbow trout (Oncorhynchus mykiss) (Belforti et al., 2015; Rema et al., 2019; Chemello et al., 2020; Jeong et al., 2020); 32% for mandarin fish (Siniperca scherzeri) (Sankian et al., 2018), 36% for European sea bass (Dicentrarchus labrax L) (Gasco et al., 2016), 38% for rockfish (Sebastes schlegeli) (Khosravi et al., 2018), and 100% for red seabream (Pagrus major) (Ido et al., 2019). However, there are few studies of MWM inclusion level for cyprinid species such as the common carp. However, data are available for the utilization of mealworm oil in mirror carp diet (Xu et al., 2020). Mealworm meal as a fishmeal replacement in the diet of ornamental cyprinids was assessed by Mamuad et al. (2021), who confirmed that 30% Ptecticus tenebrifer (Walker) and 30% MWM increased the length of common carp, but the result was not conclusive because the amounts of both insects were increased from 0-18% and 0-12%.
Evaluating the effect of replacement with insects in aqua feeds would be important in determining the optimum inclusion level of scarce resources and monitoring the health status and effect on performance (Henry et al., 2015; Chemello et al., 2020; Tran et al., 2022). Therefore, the aim of the current study was to investigate the effect of partial or total replacement of fish meal with MWM on growth performance, feed efficiency, and body composition of one-summer-old common carp.
Materials and Methods
The experiments were approved ethically by the institutional Animal Care and Use Committee of the Research Institute of Aquaculture and Fisheries (licence number BE/25/4302-3/2017).
The basal diet was set at 35.2% crude protein and 6.7% crude fat and contained 10% fish meal (Table 1). In Treatment 1, 50% of fish meal was replaced with MWM (MWM50), and in Treatment 2, fish meal was totally replaced by MWM (MWM100). The diets were isonitrogenous and isoenergetic. Yellow MWM was imported by Hecron-Agro Kft., Hungary (Table 2), from Berg & Schmidt Pty Ltd, Singapore in dried and processed form.
The diets were prepared manually by mixing dried ingredients with oil and slightly warm water. Carboxymethyl cellulose was used as binder. The homogenized and moisturized ingredients were then pelleted with a grinder and dried with cold ventilation for 48 hours.
The six-week experiment was carried out at the Department of Applied Fish Biology, Hungarian University of Agriculture and Life Science, Kaposvári Campus, with a recirculating aquaculture system (RAS).
A completely randomized design was used to set up the experiment. The fish were stocked in an experimental RAS facility with 250 L tanks. All tanks were connected and contained a drum filter, moving bed biofilter, and aeration. Experimental fish (n: 135) (w0: 97.54 ± 15.0 g) were distributed randomly to the three groups (MWM0, MWM50, MWM100) in triplicate (15 fish per tank) and acclimatized for one week with experimental feed before the nutritional trial. The fish were fed manually three times a day at 3% of bodyweight. Water parameters were checked regularly, dissolved oxygen was measured daily and kept close to saturation, and water temperature was kept at 24.0 ± 0.5 °C. Water parameters such as ammonium, nitrite, nitrate, and pH were measured weekly. Fish weight and length were determined individually before and at the end of the trial, and tank biomass was measured weekly to adjust the daily feed portions for fish.
The proximate composition of the MWM, feeds, and whole fish body were analysed using standard methods of the AOAC (1998) (Tables 1 and 2). The Kjeldahl method was used for protein analysis with conversion factors of N χ 6.25 for fish and N χ 4.76 for mealworm diets. Soxhlet extraction was used for crude fat content analysis with petroleum ether (40-60 °C) as solvent. Ash was determined by measuring 2 g ground and homogenized samples in a crucible and burning in a muffle furnace set at 550 oC for three hours. Moisture content was established with the oven set at 105 oC for four hours. Amino acid content was analysed with an amino acid analyser (Waters Acquity UPLC H-Class with AccQ UPLC BEH C18 2.1 χ 100 mm), 1.7 μηι column and AccQ Tag Ultra Eluent A, B, and water in gradient mode. The chitin was assessed as the difference between ash-free acid detergent fibre (ADF) and protein linked to ADF (chitin% = ADF% - acid detergent insoluble protein%) according to Finke (2007) and Marono et al. (2015). Acid detergent fibre was determined on a Gerhardt Fibretherm FT12 apparatus with the protocol provided by the manufacturer.
At the end of trial, 18 fish (two individuals per tank, six fish per treatment) were taken randomly and dissected for somatic indices and body proximate composition. The samples were kept in a fridge under -20 °C until they were sent to Central Laboratory Department of Food and Feed Safety, Hungarian University of Agriculture and Life Sciences, for proximate composition analyses in duplicates.
These parameters were calculated at the end of the trial to determine growth performance and nutrient utilization:
Relative growth rate (RGR) = 100 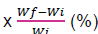 (Wannigamma et al., 1985)
(Wannigamma et al., 1985)
Specific growth rate (SGR) = 100  (g/day) (Duman, 2020)
(g/day) (Duman, 2020)
where wf = final average weight at the end of the experiment (g); wi = initial average weight at the beginning of the experiment (g); t = experimental time in days.
Weight gain (WG) = wf - wi (g)
where wf = final total biomass (g); wi = initial total biomass (g)
Protein efficiency ratio (PER) =  (Abdel-Tawab, 2012)
(Abdel-Tawab, 2012)
Feed conversion ratio (FCR) = (Duman, 2020)
(Duman, 2020)
Survival rate (SR, %) = 100 x  (Duman, 2020)
(Duman, 2020)
Hepatosomatic index: (HSI, %) =100 χ LW/FBW (%) (Chemello et al., 2020) Viscera somatic index: VSI (VSI, %) =100 χ VW/FBW (%) (Chemello et al., 2020) Condition factor = 100 χ total FBW/FTL3 (g/cm3) (Chemello et al., 2020) Relative gut length (RGL, %) = 100 χ GL/FTL (Chemello et al., 2020) where VW = visceral weight (g)
LW = liver weight (g)
FBW = final total bodyweight (g)
FTL = final total length (cm)
GL = gut length (cm)
Gross energy (GE, kJ/g or MJ/kg) =17.2 χ TC + 23.6 χ protein + 39.5 χ lipid,
where total carbohydrate (TC, %) = 100 - (crude protein% + crude fat% + crude fibre% + crude ash%) (Halver & Hardy, 2002).
The data was analysed with Rcmdr version 4.0.2 with one-way ANOVA at confidence interval level 95% considering P <0.05 significance (Gebremichael et al., 2021).
Results and Discussion
All diets were highly palatable to common carp juveniles, which accepted the feed voluntarily throughout the experiment. All fish were active and appeared healthy. The growth parameters did not differ significantly between MWM50 and MWM100 and the control, but the highest values of FBW, SGR, and weight gain were obtained with MWM50 (Table 3). The feed utilization (FCR, PER) parameters showed a decreasing tendency with rising inclusion level of MWM. This finding is in agreement with the reports of Mamuad et al. (2021). Growth performance showed a decreasing pattern as the replacement level of fishmeal with MWM increased.
The better growth performance in MWM50 than MWM100 might be because the high dietary chitin content of MWM impaired the digestibility of other nutrients; most fish have limited chitinolytic action (Lindsay et al., 1984; Ng et al., 2001; Krogdahl et al., 2005; Kroeckel et al., 2012; Abro et al., 2014). Chitin contributed to increased bulk, reduced faecal retention time, and lowered enzyme accessibility to substrates (Zhang et al., 2014).
Studies with silkworm meal by Nandeesha et al. (2000) and Rahman et al. (1996) confirmed that partly replacing fish meal in common carp diets did not affect performance. In laconisi et al. (2019) and Piccolo et al. (2017), full-fat MWM was replaced by up to 49% and 74% without negative effects on growth performance and FCR in 18- and 23-week feeding experiments with red sea bream and gilthead sea bream. Total replacement of fish meal with defatted MWM did not affect FCR and increased growth performance after four weeks of feeding of red sea bream (Ido et al., 2019).
In another study, MWM replaced fishmeal successfully in practical diets of Nile tilapia (Oreochromis niloticus) juveniles (Tubin et al., 2020). The authors observed greater growth (P <0.05) at the highest inclusion level MWM. Su et al. (2017) stated that after five weeks of feeding, total replacement of fishmeal with MWM did not affect the growth performance of yellow catfish (Pelteobagrus fulvidraco). Moreover, Chemello et al. (2020) reported up to 100% replacement of fishmeal with MWM without negative influence on the performance of rainbow trout. Similarly, Gebremichael et al. (2021) reported improved FCR and PER of common carp fed at 100% replacement of fishmeal with black soldier fly meal. These discrepancies might be the result of variations in the nutritional properties of mealworm larvae reared on different substrates (Jajic et al., 2019; Liu et al., 2020), particularly in chitin content, which could affect the crude protein digestibility of MWM (Marono et al., 2015); in lack of micronutrients and essential amino acids (Nogales-Merida et al., 2018); in low digestibility (Adesulu & Mustapha, 2000); and in the processing methods of mealworm (Tschirner & Simon 2015; Huang et al., 2019), although the size of the fish could contribute to growth and nutrient utilization results.
Biometric indices indicate the physiological condition of specimens based on fat accumulation, gonadal development, general wellbeing, and adaptation to the environment (Nikolsky, 1963). The condition factor (K) expresses the ratio between fish bodyweight and length, reflecting the interactions between biotic and abiotic variables in its physiological condition (Le Cren, 1951). In this study, biometric parameters such as total body length, bodyweight, hepatosomatic index (HSI), and condition factor were not affected significantly by partial or total replacement of fish meal with MWM (Table 4). This result agrees with the findings of Gebremichael et al. (2021) and Hoffmann et al. (2020), who reported that HSI and condition factor of common carp and sea trout larvae (Salmo trutta) were not affected significantly by partial or total replacement of fish meal with black soldier fly meal and MWM, respectively.
Similarly, Sankian et al. (2018) confirmed that there were no significant differences in the survival rate, condition factor, HSI, and VSI values among mandarin fish fed a diet in which up to 30% fish meal was replaced with MWM. Chemello et al. (2020) reported the absence of significant differences among treatments for condition factor and somatic indices of rainbow trout fed up to 100% replacement of fish meal with MWM, whereas Gasco et al. (2014) observed that the lowest hepatosomatic indices were observed in rainbow trout fed 25% and 50% yellow mealworm replacement diets. These differences might be because of the age of the fish, the gonadal maturity stage, and processing methods of MWM (Chemello et al., 2020; Rizzo & Bazzoli, 2020).
The proximate composition of the eviscerated carp was not affected significantly by inclusion level of MWM, except that crude fat content was substantially higher at MWM50 and MWM100% (Table 5). Tubin et al. (2020) also observed a rise in crude fat content of Nile tilapia as the level of MWM replacement increased (P <0.05). Rema et al. (2019) observed no significant difference in whole body composition of rainbow trout fed various levels of MWM, which agreed with the findings of Iaconisi et al. (2018). There were no differences in the proximate composition of fillets (raw and cooked), whereas fatty acid profile was affected strongly by the diet containing insect meal. Ng et al. (2001) reported differences, although non-significant, in the lipid composition of African catfish fillets with various inclusion levels of MWM.
Because of variations among fish and insect species, their rearing conditions, processing methods of insect meal, duration of trials, and water temperature, the direct comparison of results among studies would be difficult and the optimal fish meal replacement levels could vary (Osimani et al., 2016; Iaconisi et al., 2019; Jeong et al., 2020). In addition, standardization of insect protein production is central to assuring the correct formulation of diets for fish and other targeted animal species.
Conclusions
Mealworm meal in the diet of common carp juveniles did not significantly affect growth performance, feed utilization, or biometric indices up to six weeks of feeding. The body composition of the fish might indicate differences in nutrient utilization. Thus, further investigation should be undertaken on nutrient digestibility, chitinolytic enzyme activity, and chitin level in mealworm diets for common carp juveniles. The current results indicated that in the longer term MWM50 in carp diet should be optimal.
Acknowledgments
The work was supported by Stipendium Hungaricum Scholarship Program of the Hungarian Government. The publication was supported by the Tématerületi Kiválósági Program (2020-4.1.1-TKP2020) of the Hungarian National Research, Development, and Innovations Office.
Authors' contributions
AG: data collection, analysis and interpretation, drafting manuscript; ZJS: chemical analysis of the diet and mealworm meal, revision of the manuscript; BK: conception and design of the experiment, revision of the manuscript
Conflict of interest
The authors declare that there is no conflict of interest concerning this manuscript
References
Abdel-Tawwab, M., 2012. Effects of dietary protein levels and rearing density on growth performance and stress response of Nile tilapia, Oreochromis niloticus (L.). Inter. Aqua. Res. 1-11. [ Links ]
Abro, R., Sundell, K., Sandblom, E., Sundh, H., Brannas, E., Kiessling, A., Lindberg, J.E. & Lundh, T., 2014. Evaluation of chitinolytic activities and membrane integrity in gut tissues of Arctic charr (Salvelinus alpinus) fed fish meal and zygomycete biomass. Comp. Biochem. Physiol. 175, 1-8. [ Links ]
Adesulu, E.A. & Mustapha, A.K., 2000. Use of housefly maggots as a fishmeal replacer in tilapia culture: A recent vogue in Nigeria. Abstracts of 5th international Symposium on Tilapia Aquaculture, Rio de Janeiro, Brazil, pp. 138. [ Links ]
Basto, A., Calduch-Giner, J., Oliveira, B., Petit, L., Sá, T., Maia, M.R.G., Fonseca, S.C., Matos, E., Pérez-Sánchez, J. & Valente, L.M.P., 2021. The use of defatted Tenebrio molitor larvae meal as a main protein source is supported in European sea bass (Dicentrarchus labrax) by data on growth performance, lipid metabolism, and flesh quality. Frontiers in Physiology. DOI: 10.3389/fphys.2021.659567 [ Links ]
Belforti, M., Gai, F., Lussiana, C., Renna, M., Malfatto, V., Rotolo, L., De Marco, M., Dabbou, S., Schiavone, A., Zoccarato, I. & Gasco, L., 2015. Tenebrio molitor meal in rainbow trout (Oncorhynchus mykiss) diets: Effects on animal performance, nutrient digestibility, and chemical composition of fillets. Ital. J. of Anim. Sci. 14, 670-675. [ Links ]
Bovera, F., Loponte, R., Marono, S., Piccolo, G., Parisi, G., Iaconisi, V., Gasco, L. & Nizza, A., 2016. Use of Tenebrio molitor larvae meal as protein source in broiler diet: Effect on growth performance, nutrient digestibility, and carcass and meat traits. Journal of Animal Science 94(2), 639-647. DOI:10.2527/jas.2015-9201 [ Links ]
Bovera, F., Piccolo, G., Gasco, L., Marono, S., Loponte, R., Vassalotti, G., Mastellone, V., Lombardi, P., Attia, Y.A. & Nizza, A., 2015. Yellow mealworm larvae (Tenebriomolitor, L.) as a possible alternative to soybean meal in broiler diets. British Poultry Science 56(5), 569-575. DOI:10.1080/00071668.2015.1080815 [ Links ]
Chemello, G., Renna, M., Caimi, C., Guerreiro, I., Oliva-Teles, A., Enes, P., Biasato, I., Schiavone, A., Gai, F. & Gasco, L., 2020. Partially defatted Tenebrio molitor larva meal in diets for grow-out rainbow trout, Oncorhynchus mykiss (Walbaum): Effects on growth performance, diet digestibility and metabolic responses. Animals 10. DOI:10.3390/ani10020229 [ Links ]
Duman, S., 2020. Effect of concrete pond and net-cage culture systems on growth performance and hematological parameters of Siberian sturgeon (Acipenser baerii). Turk. J. Vet. Anim. Sci. 44(3), 624-631. DOI:10.3906/vet-1910-41 [ Links ]
Finke, M.D., 2007. Estimate of chitin in row whole insects. Zoo Biology 26, 105-115. [ Links ]
Gasco, L., Belforti M., Rotolo, L., Lussiana, C., Parisi G., Terova, G., Roncarati, A. & Gai, F., 2014. Mealworm (Tenebrio molitor) as a potential ingredient in practical diets for rainbow trout (Oncorhynchus mykiss). In: Proceedings of the 1st International Conference, Insects to Feed the World, Wageningen University, Wageningen, The Netherlands, 14-17 May 2014, pp 78. [ Links ]
Gasco, L., Henry, M., Piccolo, G., Marono, S., Gai, F., Renna, M., Lussiana, C., Antonopoulou, E., Mola, P. & Chatzifotis, S., 2016. Tenebrio molitor meal in diets for European sea bass (Dicentrarchus labrax L.) juveniles: Growth performance, whole body composition, and in vivo apparent digestibility. Anim. Feed Sci. Technol. 220, 34-45. [ Links ]
Gebremichael, A., Hancz, C. & Kucska, B., 2021. Effect of total or partial replacing of fishmeal with black solider fly (Hermetia illucens) meal on growth performance and body condition indices of common carp (Cyprinus carpio). AACL Bioflux 14(4), 2280-2286. [ Links ]
Golden, C.D., Allison, E.H., Cheung, W.W., Dey, M.M., Halpern, B.S., McCauley, D.J., Smith, M.,Vaitla, B., Zeller, D., Myers, S.S., 2016. Nutrition: Fall in fish catch threatens human health. Nature 534 (7607), 317-320. DOI: 10.1038/534317a [ Links ]
Hafez, M.H. & Attia, Y.A., 2020. Challenges to the poultry industry: Current perspectives and strategic future after the COVID-19 outbreak. Front. Vet. Sci. DOI: 10.3389/fvets.2020.00516 [ Links ]
Henry, M., Piccolo, G. & Fountoulaki, E., 2015. Review on the use of insects in the diet of farmed fish. Past and future. Anim. Feed Sci. Technol. 203, 1-22. [ Links ]
Hua, K., Cobcroft, J.M., Cole, A., Condon, K., Jerry, D.R., Mangott, A., Praeger, C., Vucko, M.J., Zeng, C., Zenger, K. & Strugnell, J.M., 2019. The future of aquatic protein: Implications for protein sources in aquaculture diets. One Earth 1, 316-329. [ Links ]
Huang, C., Feng, W., Xiong, J., Wang, W., Wang, T., Wang, C., Yang, F., 2019. Impact of drying method on the nutritional value of the edible insect protein from black soldier fly (Hermetia illucens L.) larvae: Amino acid composition, nutritional value evaluation, in vitro digestibility, and thermal properties. European Food Research and Technology 245, 11-21. [ Links ]
Hoffmann, L., Rawski, M., Nogales-Merida, S. & Mazurkiewicz, J., 2020. Dietary inclusion of Tenebrio molitor meal in sea trout larvae rearing: Effects on fish growth performance, survival, condition, and GIT and liver enzymatic activity. Ann. Anim. Sci. 8, 10807 [ Links ]
Iaconisi, V., Bonelli, A., Pupino, R., Gai, F. & Parisi, G., 2018. Mealworm as dietary protein source for rainbow trout: Body and fillet quality traits. Aquaculture 484, 197-204. [ Links ]
Iaconisi, V., Secci, G., Sabatino, G., Piccolo, G., Gasco, L., Papini, A.M. & Parisi, G., 2019. Effect of mealworm (Tenebrio molitor L.) larvae meal on amino acid composition of gilthead sea bream (Sparus aurata L.) and rainbow trout (Oncorhynchus mykiss W.) fillets. Aquaculture 513, 734403. [ Links ]
Ido, A., Hashizume, A., Ohta, T., Takahashi, T., Miura, C. & Miura, T., 2019. Replacement of fish meal by defatted yellow mealworm (Tenebrio molitor) larvae in diet improves growth performance and disease resistance in red seabream (Pargus major). Animals 9(3),100. [ Links ]
Jajič, I., Popovic, A., Uroševic, M.,Krstović, S., Petrović, M., Guljas, D.,, 2019. Chemical composition of mealworm larvae (Tenebrio molitor) reared in Serbia. Contemporary Agriculture 68 (1-2), 23-27 [ Links ]
Jeong, S.M., Khosravi, S., Mauliasari, I.R. & Lee, S.M., 2020. Dietary inclusion of mealworm (Tenebrio molitor) meal as an alternative protein source in practical diets for rainbow trout (Oncorhynchus mykiss) fry. Fish Aquatic Science 23, 12. [ Links ]
Kroeckel, S., Harjes, A., Roth, I., Katz, H., Wuertz, S., Susenbeth, A. & Schulz, C., 2012. When a turbot catches a fly: Evaluation of a pre-pupae meal of the black soldier fly (Hermetia illucens) as fishmeal substitute - Growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 364-365, 345-352. [ Links ]
Khosravi, S., Kim, E., Lee, Y.-S. & Lee, S.-M., 2018. Dietary inclusion of mealworm (Tenebrio molitor) meal as an alternative protein source in practical diets for juvenile rockfish (Sebastes schlegeli). Entomol. Res. 48, 214-221. [ Links ]
Krogdahl, A. & Bakke-McKellep, A., 2005. Fasting and refeeding cause rapid changes in intestinal tissue mass and digestive enzyme capacities of Atlantic salmon (Salmo salar L.). Comp. Biochem. Physiol. 141, 450-460. [ Links ]
Le Cren, C.D., 1951. The length-weight relationship and seasonal cycle in gonad weight and condition in perch, Perca fluviatilis. Journal of Animal Ecology 20, 201-219. [ Links ]
Lindsay, G.J.H., Walton, M.J., Adron, J.W., Fletcher, T.C., Cho, C.Y. & Cowey, C.B., 1984. The growth of rainbow trout (Salmo gairdneri) given diets containing chitin and its relationship to chitinolytic enzymes and chitin digestibility. Aquaculture 37, 315-334 [ Links ]
Liu, C., Masri, J., Perez, V., Maya, C. & Zhao, J., 2020. Growth performance and nutrient composition of mealworms (Tenebrio molitor) fed on fresh plant materials-supplemented diets. J. Foods 9, 151. [ Links ]
Magalhães, R., Sánchez-López, A., Leal, R.S., Martínez-Llorens, S., Oliva-Teles, A. & Peres, H., 2017. Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diets for European seabass (Dicentrarchus labrax). Aquaculture 476, 79-85. [ Links ]
Mamuad, L., Lee, S.H., Jeong, C.D., Ramos, S., Miguel, M., Son, A.R., Kim, S.H., Cho, Y.I. & Lee, S.S., 2021. Ornamental fish, Cyprinus carpio, fed with fishmeal replacement Ptecticus tenebrifer and Tenebrio molitor. Aquac. Res. 52, 980-990. [ Links ]
Manjappa, K., Keshavanath, P. & Gangadhara, B., 2011. Influence of sardine oil supplemented fish meal free diets on common carp (Cyprinus carpio) growth, carcass composition, and digestive enzyme activity. Journal of Fish Aquatic Science 6, 604-613. [ Links ]
Marono, S., Piccolo, G., Loponte, R., Di Meo, C., Attia, Y.A., Nizza, A. & Bovera, F., 2015. In vitro crude protein digestibility of Tenebrio molitor and Hermetia illucens insect meals and its correlation with chemical composition traits. Ital. J. of Anim. Sci. 14, 338-343. [ Links ]
Nandeesha, M. C., Gangadhara, B., Varghese, T. J. & Keshavanath, P., 2000. Growth response and flesh quality of common carp, Cyprinus carpio fed with high levels of non-defatted silkworm pupae. Asian Fish Science 13, 235-242. [ Links ]
Ng, W.K, Liew, F.L, Ang, L.P. & Wong, K., 2001. Potential of mealworm (Tenebrio molitor) as an alternative protein source in practical diets for African catfish, Clarias gariepinus. Aqua. Res. 32(1), 273-280. [ Links ]
Nikolsky, G.V., 1963. The Ecology of Fishes. Academic Press, London. [ Links ]
Nogales-Merida, S., Gobbi, P., Jozefiak, D., Mazurkiewicz J., Dudek K., Rawski M., Kieronczyk, B. & Jozefiak, A., 2018. Insect meal in fish nutrition. Rev. in Aquac. 11, 1080-1103. DOI: 10.1111/raq.12281 [ Links ]
Oliva-Teles, A., Enes, P. & Peres, H., 2015. Replacing fishmeal and fish oil in industrial aquafeeds for carnivorous fish In: D.A. Davis (ed). Feed and Feeding Practice in Aquaculture. Woodhead, Cambridge. pp. 203-233. [ Links ]
Oonincx, D.G.A.B. & de Boer, I.J.M., 2012. Environmental impact of the production of mealworms as a protein source for humans - a life cycle assessment. PLoS One 7, 1-5. [ Links ]
Osimani, A., Garofalo, C., Milanovic, V., Taccari, M., Cardinali, F., Aquilanti, L., Pasquini, M., Mozzon, M., Raffaelli, N., Ruschioni,S., Riolo, P., Isidoro, N. & Clementi, F., 2016. Insight into the proximate composition and microbial diversity of edible insects marketed in the European Union. Eur. Food Res. Technol. 243, 1157-1171. DOI: 10.1007/s00217-016-2828-4 [ Links ]
Rahman, M.A., Zaher, M., Mazid, M.A., Haque, M.Z. & Mahata, S.C., 1996. Replacement of costly fish meal by silkworm pupae in diet of mirror carp (Cyprinus carpio L.). Pakistan J. Sci. Ind. Res. 39 (1/4), 64-67. [ Links ]
Rana, K.J., Siriwardena S. & Hasan M.R., 2009. Impact of rising feed ingredient prices on aquafeeds and aquaculture production. FAO Fisheries and Aquaculture Technical Paper No. 541, FAO, Rome. Pp. 63. [ Links ]
Rema, P., Saravanan, S., Armenjon, B., Motte, C. & Dias, J., 2019. Graded incorporation of defatted yellow mealworm (Tenebrio molitor) in rainbow trout (Oncorhynchus mykiss) diet improves growth performance and nutrient retention. Animals 9. DOI:10.3390/ani9040187 [ Links ]
Rizzo, E. & Bazzoli N., 2020. Reproduction and embryogenesis. In: Biology and physiology of freshwater neotropical fish. https://www.researchgate.net/publication/338808928_Reproduction_and_embryogenesis. Accessed May, 2022. [ Links ]
Sankian, Z., Khosravi, S., Yi-Oh, K. & Sang-Min, L., 2018. Effects of dietary inclusion of yellow mealworm (Tenebrio molitor) meal on growth performance, feed utilization, body composition, plasma biochemical indices, selected immune parameters, and antioxidant enzyme activities of mandarin fish (Siniperca scherzeri) juveniles. Aquaculture 496, 79-87. [ Links ]
Schiavone, A., De Marco, M., Rotolo, L., Belforti, M., Martinez Mirò, S., Madrid Sanchez, J., Hernandez Ruiperez, F., Bianchi, C., Sterpone, L., Malfatto, V., Katz, H., Zoccarato, I., Gai, F. & Gasco, L., 2014. Nutrient digestibility of Hermetia illucens and Tenebrio molitor meal in broiler chickens. Proceedings of the 1st International Conference Insects to Feed the World, Wageningen University, Wageningen, The Netherlands. 14-17 May 2014. pp. 73. [ Links ]
Shafique, L., Abdel-Latif, H.M.R., Hassan, F.U., Alagawany, M., Naiel, M.A.E., Dawood, M.A.O., Yilmaz, S. & Liu, Q., 2021. The feasibility of using yellow mealworms (Tenebrio molitor); Towards a sustainable aquafeed industry. Animals 11, 1-38. [ Links ]
Soltani, M., Sheikhzadeh, N., Ebrahimzadeh-Mousavi, H.A. & Zargar, A., 2010. Effects of Zataria multiflora essential oil on innate immune responses of common carp (Cyprinus carpio). J. of Fish Aquac. Science 5, 191-199. [ Links ]
Su, J., Gong Y., Cao,S., Lu, F., Han, D., Liu, H.,Jin,J.,Yang,Y., Zhu, X. & Xie, S., 2017. Effects of dietary Tenebrio molitor meal on the growth performance, immune response, and disease resistance of yellow catfish (Pelteobagrus fulvidraco). Fish & Shellfish Immunology 69, 59-66. DOI:10.1016/j.fsi.2017.08.008 [ Links ]
Tschirner, M. & Simon, A., 2015. Influence of different growing substrates and processing on the nutrient composition of black soldier fly larvae destined for animal feed. J. of Insects as Food and Feed 1(4), 249-259. [ Links ]
Tran, H.Q., Prokešová, M., Zare, M., Matoušek, J., Ferrocino, I., Gasco, L. & Stejskal, V., V., 2022. Production performance, nutrient digestibility, serum biochemistry, fillet composition, intestinal microbiota, and environmental impacts of European perch (Perca fluviatilis) fed defatted mealworm (Tenebrio molitor). [ Links ]
Tubin, J.S.B., Paiano, D., Hashimoto, G.S. de O., Furtado, W.E., Martins, M.L., Durigon, E. & Emerenciano, M.G.C., 2020. Tenebrio molitor meal in diets for Nile tilapia juveniles reared in biofloc system. Aquaculture, 519. DOI:10.1016/j.aquaculture.2019.734763 [ Links ]
Wannigama, D. N., Weerakon, D. E.M., Muthukumarana, G., 1985. Cage culture of Oreochromis niloticus in Sri Lanka: Effect of stocking density and dietary crude protein levels on growth. In: Finish Nutrition in Asia. International Development Research Centre, Ottawa, Canada, [ Links ]
Xu, X., Ji, H., Belghit, I. & Sun, J., 2020. Black soldier fly larvae as a better lipid source than yellow mealworm or silkworm oils for juvenile mirror carp (Cyprinus carpio var. specularis). Aquaculture 527, 735453. [ Links ]
Zhang, Y., Zhou, Z., Liu, Y., Cao, Y., He, S., Huo, F., Qin, C., Yao, B. & Ringo, E., 2014. High-yield production of a chitinase from Aeromonas veronii B565 as a potential feed supplement for warm-water aquaculture. Appl. Microbiol. Biotechnol. 98, 1651-1662. [ Links ]
Submitted 20 January 2022
Accepted 30 May 2022
Published 14 October 2022
# Corresponding author: Askale Gebremichael, onan_ag@yahoo.com














