Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Animal Science
versão On-line ISSN 2221-4062
versão impressa ISSN 0375-1589
S. Afr. j. anim. sci. vol.52 no.2 Pretoria 2022
http://dx.doi.org/10.4314/sajas.v52i2.8
Effects of feeding pellets, live earthworms and tilapia on the growth of African sharptooth catfish fingerlings
M.Y. MoroasuiI, #; A. Ampofo-YeboahII; M.A AntwiI
IDepartment of Agriculture and Animal Health, University of South Africa, P/Bag X 06, 1710, Florida Campus, Johannesburg, South Africa
IIDepartment of Fisheries and Aquatic Resources Management, Faculty of Biosciences, University for Development Studies, Nyankpala Campus, Box TL1882, Tamale, Ghana
ABSTRACT
The objective of the study was to evaluate the effects of feeding live earthworms (Eisenia foetida) (Savigny, 1826) (Musyoka et al., 2020) and tilapia (Oreochromis mossambicus) (Peters, 1852) (Russell et al., 2012) on the growth rate of African sharptooth catfish (Clarias gariepinus) (Burchell, 1822) (Dadebo et al., 2014) fingerlings. Approximately 300 African catfish fingerlings (5 g) were stocked in 16 circular tanks (1000 L) inside an indoor system. The tanks were divided into three groups of treatments (pellets, earthworms and tilapia) with four replicates. The fish were left to acclimatize for a week before the experiment began. The fish were weighed individually each week until the end of the experiment. The results showed that growth differed between catfish fed tilapia fish and those fed pellets and earthworm. There were no differences in average weight gain, specific growth rate, and food conversion ratio between treatment groups. Survival rates differed in catfish fed pellets compared with tilapia and earthworms. Catfish fed tilapia obtained high cumulative feed intake at the end of the experiment. At the end of the experiment, the fingerlings differed in weight between the treatments and their weight was correlated positively with depth. It was concluded that tilapia improved the growth of catfish fingerlings the most and could be a solution for rural farmers who have limited access to fishmeal and feed formulation expertise to maximize productivity.
Keywords: catfish fingerling, earthworm, feed, tilapia, growth
Introduction
The FAO (2017) indicated that significant efforts have been made to reduce aquaculture's dependency on fishmeal. Insects as live feed or as a source of protein might be an alternative (Ng et al., 2001; Bahadori et al., 2017; Belghit et al., 2019). It was critical that fish farmers could produce cheaper alternative feed that met the nutritional requirements of the fish raise to realise success (Iheanacho et al., 2019; Ogunji et al., 2020). African catfish (Clarias gariepinus) are indigenous to many African countries and feed on everything from crustaceans to fish and invertebrate prey (Bruton, 1979; Loveline et al., 2018). In Lake Sibiya, stomach contents of C. gariepinus included crabs (40%), small cichlids (32%), insects (12%), molluscs (10%) and small amounts of algae and diatoms (Minshull 1969, in Bruton, 1976). Bruton (1976) later found 164 specimens from the stomach of C. gariepinus were 33% fish remains and 9% cichlids.
Vermiculture has been practised for centuries and is being revisited for its potential benefits in agriculture and aquaculture (Mohanta et al., 2016). Earthworms (Eisenia foetida) have been identified as the best fishmeal replacement because they have high nutritional value, they grow and reproduce more quickly, and they can withstand all sorts of environmental conditions (Dedeke et al., 2001; Bahadori et al., 2017; Musyoka et al., 2020). However, the pprofitability of farming with earthworms as fish feed remains controversial because of the presence of anti-nutritional factors and incorrect processing methods (Musyoka et al., 2019; Musyoka et al., 2020). Although earthworms have been used widely as bait in commercial sport fisheries for long periods (Tacon et al., 1983), their moist sticky nature causes harvesting to be prolonged and time consuming, prompting earthworms to release foul-smelling coelom fluid, which causes unpalatability and toxicity to fish (Kobayashi et.al., 2001). They are made of 580-710 g/kg of crude protein and have a high concentration of lysine (Loh et al., 2005). Earthworms are conventional fish food and contain all the essential amino acids, including increased iron (Chakrabarty et al., 2009).
Rural farmers commonly farm with Mozambique tilapia (Oreochromis mossambicus). The species is limited by reproduction at an early age, causing famers to sit with overpopulations of undersized and unmarketable fish (Guerrero, 1975). This caused the species to lose value as most preferred species with which to farm in aquaculture (Russell et al., 2012). The solutions were to produce all-male tilapia because of their ability to reach market size more quickly (Wasserman & Afonso, 2003). Limited access to technology, stunting, and inefficient management strategies of small-scale farmers were prevalent in sub-Saharan Africa (Ampofo-Yeboa, 2013). A number of breeding techniques were reported and each had its own limitations (Mair & Little, 1991). However, most of these methods are expensive and escalate the cost of production for rural fish farming in Africa (Limbu et al., 2015). Many countries invested in controlling and eliminating the species because of its invasive nature of causing environmental and ecological problems (Canonico et al., 2005) and it is also listed in the top 100 invasive alien species in the planet because of its tolerance of wide environmental conditions, dietary requirements and rapid reproduction (Brummett, 1995; Russell et al., 2012). In South Africa, O. mossambicus are reported to spawn up to five times in a season under favourable or aquarium conditions (James & Bruton, 1992; Skelton, 2001), proving its enormous ability to reproduce. However, C. gariepinus are commonly used to police the population of Nile tilapia (Oreochromis niloticus) in ponds (Musa et al., 2013; Alfaro et al., 2014) and little is reported on the use of Oreochromis species to grow C. gariepinus and its economic potential.
The objective of the current study was to evaluate the effects of live earthworms and tilapia on the growth performance of African sharptooth catfish fingerlings.
Materials and Methods
The experiment was approved by the Ethics Committee of the University of South Africa, Florida Campus, Johannesburg (approval no 2017/CAES/009). It was conducted at Disaneng Village, 41 km from Mahikeng Town, North West, South Africa. A batch of 300 African catfish fingerlings (5 ± 0.01 g) were sourced from Aquaculture Innovations (Pretoria, South Africa). Earthworms (1000 per plastic bag per week) were sourced from Worm-farm (Witkoppen Wildflower Nursery, Randburg, South Africa). Mozambique tilapia were sourced from MyAquaponics Pty Ltd. (Johannesburg, South Africa) and from La Pieus Aqua Holdings (Roodeplaat, South Africa). One bag (25 kg) of starter fish diet was sourced from Aqua-Plus (AVI-Products, Cato Ridge, South Africa).
Circular indoor tanks with 1000 L capacity were supplied with water at a flow rate of 0.665 m3/s. Each tank was randomly allocated 20 African catfish (Clarius gariepinus) fingerlings for seven weeks. The tanks were divided into three groups of dietary treatments (pellets, tilapia and earthworms) with four replicates for each. The catfish, earthworms and tilapia were acclimatized to the new environment for seven days. The catfish fingerlings were fed two times daily (08h00 and 16h00) to apparent satiation. The amount of feed consumed was measured by weighing the feed containers before and after feeding. The basal diet (pellets) was formulated to provide at least 400 g/kg protein, 22 g/kg lysine, 50 g/kg fat and 7 g/kg phosphorus. It contained maximums of 30 g/kg of both crude fibre and calcium. The earthworms were washed with clean water to remove the debris from compost and weighed before feeding. The tilapia were scooped live from separate tanks, weighed on an electronic scale, and fed to the catfish. The amounts of feed provided to the catfish, and feedstuffs necessary to sustain the earthworms and tilapia are given in Table 1.
The tanks were cleaned every morning by siphoning out one third of the water. The dead fingerlings were removed, counted and recorded. Water quality parameters were monitored and recorded daily with a water quality meter. Dissolved oxygen ranged between 2.3 and 7.1 mg/L. Ammonia was <0.05 mg/L. Nitrite was between 0.05 and 2 mg/L. Nitrate was 250 mg/L. pH was between 6.8 and 8. Temperature was 24.7 °C to 29.8 °C. After seven days of acclimatization the catfish were weighed individually, then every week for seven weeks. The length was measured from the mouth of the fish to the end of the hypural bone, determined by bending the caudal fin, and the depth was measured between the dorsal and ventral fins (Skelton, 2001). Microsoft Excel (Microsoft Corp., Redmond, Washington, United States) was used for data management. Specific growth rate (SGR), feed conversion ratio (FCR), weight gain (WG), and survival rate (SR) were calculated following Duman (2020):

Economic analyses followed Gbai et al. (2018) and were based on these equations.
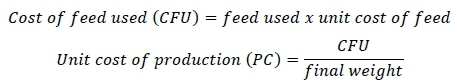
One way analysis of variance was used to determine the significance of differences among the treatments. Duncan's multiple range test was used to separate means of treatment groups. Significance was declared at probability level of P <0.05. The correlation of body depth with bodyweight was utilized to determine the strength of the relationship between these two variables.. Estimates of correlation greater than 0.8 were deemed to indicate a strong relationship and those less than 0.5 were deemed to indicate a weak relationship. All data was analysed with Statistical Package of Social Sciences version 25 (IBM Corp., Armonk, New York, USA).
Results and Discussion
The bodyweights of the catfish fed earthworms differed from week 0 to week 3 (P<0.001) compared with those fed pellets and tilapia (Table 2). There were no differences among the treatments (P >0.05) in bodyweight at week 4. However, in weeks 5 to 7 the catfish fed tilapia were heavier than those fed pellets and earthworms (P <0.001).
There were no differences (P >0.05) in AWG, SGR and FCR between groups (Table 3). Survival rate differed (P <0.05) in catfish fed the pelleted feed compared with tilapia and earthworms. These differences may be attributed to cannibalism, which is a common problem among catfish when they are stressed or short of food (Naumowicz et al., 2017).
Body length differed (P <0.001) in catfish fed earthworms compared with pellets and tilapia between week 0 and week 2. Further differences (P <0.05) in body length were observed in the tilapia group than in pelleted feed and earthworms in week 2. There were no differences (P >0.05) in body length between groups from week 3 to week 7 (Table 4).
Cumulative feed intake per fish was numerically higher in catfish fed tilapia and pellets and lower in the group fed earthworms in week 1 (Table 5). By the end of the experiment, cumulative feed intake in catfish was highest in tilapia, intermediate in pellets, and lowest in earthworms.
Weight differed and was correlated positively (r=0.857, P <0.001) with depth between treatments at end of this study (Table 6). Weight did not differ between groups, and was correlated negatively (r=0.032, P >0.05) with depth in the initial week of the experiment.
The economic analysis of catfish production when feeding pellets, tilapia, and earthworms is shown in Table 7. It was less expensive to produce a gram of catfish by feeding with pellets and tilapia than earthworms.
Aquaculture is an alternative supplier of fish to reduce pressure on the overfished oceans. However, the sustainability of aquaculture as an agricultural enterprise is threatened mostly by the high cost of fish feeds. A sustainable fish feed should meet all the nutritional requirements of the fish, be cost effective, palatable to the fish, digestible, maintain water quality, and improve disease resistance. These factors enhance growth performance, health and productivity, which would ensure improved yields and maximize profits. Every fish farmer's aim is to access good quality fish feeds at an affordable cost to realize economic value (Musyoka et al., 2020).
In this study, final growth was improved (P <0.001) in catfish fed tilapia (28.21 g) compared with pelleted feed and earthworms. This was consistent with improved growth observed in African catfish that swallowed tilapia whole during a pond poly-culture trial (Limbu et al., 2015). Tacon et al. (1983) also reported an interest of African catfish to prey on Oreochromis mossambicus fish in nature. Relative slow growth was observed in catfish fed earthworms in this study. This was not surprising since Rohu fry fed with whole frozen earthworms showed unsatisfactory performance because of the presence of the chitin layer from the hard cuticle (Mohanta et al., 2016). Ng et al. (2001) also reported reduced growth in catfish fed meal worms as a replacement for fish meal as a protein source. Furthermore, chitin decreased growth in Oreochromis niloticus crossed with blue tilapia (Oreochromis aures) (Shiau & Yu, 1999). Rainbow trout fed an increased amount of frozen worms showed supressed growth (Pereira & Gomes, 1995). Tacon et al. (1983) indicated that rainbow trout fed entirely on worm meal (Eisnia foetida) found the food unpalatable and exhibited depressed growth. On the contrary, Djissou et al. (2016) reported better growth in fish fed a mixture of earthworm- and maggot-based diets than those fed fish meal. Formulated feed had better growth performance than live feed in Tor tambroides larvae (Asaduzzaman et al., 2016), which is in agreement with the better performance attained in pellet feed than earthworms. There were no differences (P >0.05) in AWG, SGR and FCR between groups in this study. Gbai et al. (2018) reported lower weight gain in Nile tilapia fed an earthworm diet than a maggot diet. This was consistent with the current study, in which numerically lower weight gain was observed in catfish fed earthworms. Adámek et al. (1999) found lower SGR of 0.36 in catfish fed live fish, and attributed it to their losing too much energy in capturing the prey. Catfish fed tilapia in the present study obtained SGR of 0.64, which was higher than that reported by Adámek et al. (1999). The authors added that catfish consumed only the fish they had repeatedly injured and attacked on their own. This may be the case in this study as catfish showed high interest in preying on tilapia.
According to the literature, the standard FCR for aquatic animals raised intensively with commercial pellets was between 1.0 and 2.4 (Fry et al., 2018; Toutou et al., 2018) and 1.0 for Clarias gariepinus (Hetch et al., 1996). This study found the FCR of catfish fed commercial pellets was higher than reported, perhaps because the commercial diet was formulated for tilapia and was not catfish species specific. Limbu (2020) also reported that the continuous lack of suitable diets for African catfish was a limitation in developing countries. Tadesse (2019) reported a significantly lower FCR of 2.14 in catfish fed a mixture of earthworms, soybean and brewery waste, indicating a better consumption rate between treatments. Low FCR was also found in Nile tilapia fed earthworms (Gbai et al., 2018; Musyoka et al., 2020) and South America catfish (Arslan et al., 2009). No differences in FCR were found in in Atlantic salmon fed with insect meal (Belghit et al., 2016). The tilapia group in this study had the lowest FRC (2.58), which was almost within the desired ranges for aquaculture species. This differed from a higher FCR of 4.73 attained when catfish preyed on tilapia (Adámek et al., 1999).
Catfish fed the pelleted feed a showed higher survival ratio (P <0.05) than tilapia and earthworm groups in this study. Similarly, Asaduzzaman et al. (2016) reported 90.6% survival in formulated feed groups of Tor tambroides larvae. Djissou et al. (2019) also found a survival ratio of 93% fishmeal, 95% earthworm and 94% maggot catfish groups. An even higher survival rate (98.9%) was reported for catfish given a commercial diet (Toutou et al., 2018). However, fish in the earthworm group of the current study had a lower survival rate than was reported by Toutou et al. (2018). Complete survival was reported in all bass given a dietary protein during their study (Cai et al., 2020). Gbai et al. (2018) found low survival in an earthworm diet fed to Nile tilapia. This was consistent with the current study, in which catfish fed earthworms had a low survival ratio. In contrast, common carp fed earthworms had 100% survival rate (Ogunji et al., 2008). Survival rate was not affected by poultry viscera diets higher than 92% in Clarias gariepinus (Oke et al., 2016; Toutou et al., 2018). The low survival obtained in the current study was attributed to cannibalism, which is a natural behaviour of catfishes (Dadebo et al., 2014). During the larval, fry and fingerling stages of catfish growth cannibalism is a huge challenge (Hecht & Appelbaum, 1988; Kaiser et al., 1995) that results in economic losses (Smith and Reay, 1991). Significantly higher mortality as a result of cannibalism was observed in underfed catfish housed in concrete tanks (Al-Hafedh & Ali, 2004). In contrast, Djissou et al. (2019) recommended that earthworm meal and maggot meal could enhance survival of C. gariepinus. because they are omnivorous and can utilize an animal protein base to meet energy requirements (NRC, 2011). Al-Hafedh & Ali (2004) found that lower size variation and optimum feeding of juvenile catfish at 6% bodyweight per day could reach 64 g without mortality. However, reduction in food increased territorial behaviour and aggression (Hetch & Pienaar, 1993), which explained the decline in survival in the earthworm group in this study. For catfish to reach market size, this behaviour should be managed by manipulating the colour of food so as to improve consumption, lessen aggression and promote survival (Kawamura et al., 2017).
In this study the cost was low when feeding tilapia or pellets and high when feeding earthworms. This was attributed to the limited timeframe of the study and inefficient procurement, which escalated the cost of commercial earthworms. These increased earthworm costs might be drastically reduced if they were self-cultured. In contrast, Gbai et al. (2018) reported the commercial diet in their study was expensive compared with earthworm and maggot diets. This was not surprising since fish meal and oil are costly in the marketplace (FAO, 2014). As a result, the use of earthworms and maggots as alternative sources of protein reduced the cost of feed and improved production of C. gariepinus (Djissou et al., 2019). T Musyoka et al. (2020) found improved economic returns in replacing fish meal with earthworm bedding in the diet of Nile tilapia because of cheaper cost of production. Gbai et al. (2018) found feeding Oreochromis niloticus with earthworm meal and maggot meal resulted in lower cost/kg of feed and cost/kg of fish. Thus, the inclusion of 100% of maggot meal was recommended to reduce cost/kg of feed and improve profit (Ali et al., 2015). The use of earthworms as feed was recommended in areas where the components of commercial fishmeal diets were scarce and costly to reduce the pressure of overfishing (Kouba et al., 2018). Further 30% reduction of feed cost was reported when C. gariepinus was fed diets with produced on farm (Limbu, 2020). Musyoka et al. (2020) found that profit improved significantly with increasing earthworm inclusion in the diet.
Conclusion
The growth of catfish fingerlings was enhanced by feeding tilapia and was cost effective. Lower growth was obtained in catfish fed live earthworms, perhaps because of chitin, which could be removed in future studies to enhance palatability. Earthworms must be self-cultured on farm or procured at an appropriate price to reduce production costs. Farmers who lack access to fishmeal and feed formulation expertise could attain improved growth of catfish with locally accessible Mozambique tilapia fingerlings and earthworms to improve productivity. However, expertise in vermiculture may need to be developed. Further studies could be conducted on the use of mixed species to feed catfish and also to determine its meat quality.
Acknowledgements
The authors thank the National Research Foundation (Grant No. 235487) for funding this study and acknowledge B.L. Mogoje for assisting with the statistical analysis. The authors also acknowledge M.H. Mabilo; L.K. Modise and K.E. Tabane for assisting with data collection and the North West Department of Agriculture.
Authors' contribution
All authors were involved in the design, implementation, analysis and interpretation of the outcomes.
Conflict of interest
There is no conflict of interest associated the publication of the manuscript and no major financial support that could have influenced the outcomes.
References
Adámek, Z., Fašaić, K. & Siddiqui, M.A., 1999. Prey selectivity in wels (Silurus glanis) and African catfish (Clarias gariepinus). Ribarstvo 57, 47-60. [ Links ]
Ali, A.E., Mekhamar, M.I., Gadel-Rab, A.G. & Osman, A.G.M., 2015. Evaluation of growth performance of Nile tilapia Oreochromis niloticus fed Piophila casei maggot meal (magmeal) diets. American J. Life. Sci. 3, 24-29. DOI: 10.11648/j.ajls.s.2015030601.14 [ Links ]
Alfaro, A.T., Biluca, F.C., Marquetti, C., Tonial, I.B. & de Souza, N.E., 2014. African catfish (Clarias gariepinus) skin gelatin: Extraction optimization and physical-chemical properties. Food Research Int. 65, 416-422. http://dx.doi.org/10.1016/j.foodres.2014.05.070 [ Links ]
Al-Hafedh, Y.S. & Ali, S.A., 2004. Effects of feeding on survival, cannibalism, growth and feed conversion of African catfish, Clarias gariepinus (Burchell) in concrete tanks. J. Appl. Ichthyology 20, 225-227. https://doi.org/10.1111/j.1439-0426.2004.00544.x [ Links ]
Ampofo-Yeboa, A., 2013. Effect of phytogenic feed additives on gonadal development in Mozambique tilapia (Oreochromis mossambicus). PhD dissertation, University of Stellenbosch. South Africa. [ Links ]
Asaduzzaman, Md., Kader, Md.A., Bulbul, M., Abol-Munafi., A.B., Ghaffer, M. Abd. & Verdegem, M., 2016. Biochemical composition and growth performances of Malaysian Mahseer Tor tambroides larvae fed with live and formulated feeds in indoor nursery rearing system. Aquaculture Reports 4, 156-163. http://dx.doi.org/10.1016/j.aqrep.2016.09.003 [ Links ]
Arslan, B.M., Dabrowski, K. & Portella, M.C., 2009. Growth, fat content and fatty acid profile of South American catfish, Surubim (Pseudoplatystoma fasciatum) juveniles fed live, commercial and formulated diets. J. Appl. Ichthyology 25, 73-78. DOI: 10.1111/j.1439-0426.2008.01154.x [ Links ]
Bahadori, Z., Esmaielzadeh, L., Karimi-Torshizi, M.A., Seidavi, A., Olivares, J., Rojas, S., Salem, A.Z.A., Khusro, A. & López, S., 2017. The effect of earthworm (Eisenia foetida) meal with vermin-humus on growth performance, hematology, immunity, intestinal microbiota, carcass characteristics, and meat quality of broiler chickens. Livest. Sci. 202, 74-81. https://doi.org/10.1016/j.livsci.2017.05.010 [ Links ]
Belghit, I., Liland N.S., Gjesdal, P., Biancarosa, I., Menchetti, E., Li, Y., Waagb0, R., krogdahl, A. & Lock, E-J., 2019. Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar). Aquaculture 503, 609-619. https://doi.org/10.1016/j.aquaculture.2018.12.032 [ Links ]
Brummett, R.E., 1995. Environmental regulation of sexual maturation and reproduction in tilapia. Rev. Fisheries Sci. 3, 231-248. https://doi.org/10.1080/10641269509388573 [ Links ]
Bruton, M.N., 1979. The food and feeding behaviour of Clarias gariepinus (Pisces: Clariidae) in Lake Sibaya, South Africa, with emphasis on its role as a predator of cichlids. Transactions of Zoological Society of London 35, 47-114. https://doi.org/10.1111/j.1096-3642.1979.tb00057.x [ Links ]
Bruton, M.N. & Boltt, R.E., 1975. In: M.N. Bruton (ed). The food and feeding behaviour of Clarias gariepinus (Pisces: Clariidae) in Lake Sibaya, South Africa, with emphasis on its role as a predator of cichlids. Transactions of Zoological Society of London 35, 47-114. https://doi.org/10.1111/j.1096-3642.1979.tb00057.x [ Links ]
Cai, Z.N., Qian, X.Q. & Xie, X.Q., 2020. Optimal dietary protein concentrations for largemouth bass (Micropterus salmoides) of different sizes (10-500g). Aquaculture Int. 28, 831-840. https://doi.org/10.1007/s10499-019-00498-9 [ Links ]
Canonico, G.C., Arthington, A., McCrary, J.K. & Thieme, M.L., 2005. The effects of introduced tilapias on native biodiversity. Aquatic Conserv: Mar. Freshw. Ecosyst. 15, 463-483. https://doi.org/10.1002/aqc.699 [ Links ]
Chakrabarty, D., Das, K., Das, M. & Biswas, P., 2009. Application of vermitechnology in aquaculture. Dynamic Soil. Dynamic. Plant 3, 41-44. [ Links ]
Dadebo, E., Aemro, D. & Tekle-Giorgis, Y., 2014. Food and feeding habits of the African catfish Clarias gariepinus (Burchell, 1822) (Pisces: Clariidae) in Lake Koka, Ethiopia. Afr. J. Ecol. 52, 471-478. https://doi.org/10.1111/aje.12146 [ Links ]
Dedeke, G.A., Owa S.O. & Olurin, K.B., 2001. Amino acid profile of four earthworms species from Nigeria. Agric. Biol. J. North America. 1, 97-102. [ Links ]
Djissou, A.S.M., Adjahouinou, D.C., Koshio, S. & Fiogbe, E.D., 2016. Complete replacement of fishmeal by other animal protein sources on growth performance of Clarias gariepinus fingerlings. Int. Aquat. Res. 8, 333-341. DOI: 10.1007/s40071-016-0146-x [ Links ]
Duman, S., 2020. Effect of concrete pond and net-cage culture systems on growth performance and haematological parameters of Siberian sturgeon (Acipenser baerii). Turkish J. Vet. Anim. Sci. 44, 624-631. https://doi.org/10.3906/vet-1910-41 [ Links ]
FAO (Food and Agriculture Organization), 2014. La situation mondiale des pêches et de I'aquaculture. FAO, Rome, Italy. [ Links ]
FAO (Food and Agriculture Organization) 2017. Aquaculture newsletter 56 (April). FAO, Rome, Italy. [ Links ]
FAO (Food and Agriculture Organization), 2019. The state of world fisheries and aquaculture 2020, safeguarding against economic slowdowns and downturns. FAO, Rome, Italy. https://doi.org/10.4060/ca9229en [ Links ]
Fry, J.P., Mailloux, N.A., Love, D.C., Milli, M.C. & Cao, L., 2018. Feed conversion efficiency in aquaculture: Do we measure it correctly? Enviro. Research Letters 13, 024017. https://iopscience.iop.org/article/10.1088/1748-9326/aad007 [ Links ]
Gbai, M., Ouattara, N., Bamba, Y., Ouattara, M., Ouattara, A. & Yao, K., 2018. Substitution of fish meal by earthworm and maggot meal in the feed of Nile tilapia Oreochromis niloticus reared in freshwater. Int. J. Fish. Aquac. 10, 77-85. DOI: 10.5897/IJFA2018.0682 [ Links ]
Guerrero, R.D., 1975. Use of androgens for the production of all-male tilapia aurea (Steindachner). Transactions American Fisheries Society, 104: 342-348. https://doi.org/10.1577/1548-8659(1975)104<342:UOAFTP>2.0.CO;2 [ Links ]
Hecht, T., Appelbaum, S., 1988. Observations on intraspecific aggression and coeval sibling cannibalism by larval and juvenile Clarias gariepinus (Clariidae: Pisces) under controlled conditions. J. Zool. 214, 21 -44. DOI:10.1111/J.1469-7998.1988.TB04984.X [ Links ]
Hetch, T., Oellermann, L. & Verheust, L., 1996. Perspectives on clariid catfish culture in Africa. Aquatic Living Resources 9, 197-206. DOI: 10.1051/ALR:1996054 [ Links ]
Iheanacho, S.C., Ogueji, E., Igberi, C., Avwemoya, F., Amadi-Eke, A., Yaji, A. & Mbah, C., 2019. Suitability of discarded cashewnut (Anacardium occidentale) meal as replacement of soybean meal (Glycine max) in the diet of juvenile African catfish Clarias gariepinus (Burchell, 1822). Indian J. Fisheries 66, 78-86. DOI: 10.21077/ijf.2019.66.3.89214-10 [ Links ]
James, N.P.E. & Bruton, M.N., 1992. Alternative life-history traits associated with reproduction in Oreochromis mossambicus (Pisces: Cichlidae) in small water bodies of the Eastern Cape, South Africa. Environ. Biol. Fishes 34, 379-392. https://doi.org/10.1007/BF00004742 [ Links ]
Kaiser, H., Weyl, O., Hecht, T., 1995. Observations on agonistic behaviour of Clarias gariepinus larvae and juveniles under different densities and feeding frequencies in a controlled environment. J. Appl. Ichthyology 11, 25-36. DOI: 10.1111/J.1439-0426.1995.TB00003.X [ Links ]
Kawamura, G., Bagarinao, T.U., Asmad, M.F.B. & Lim, L.S., 2017. Food colour preferences of hatchery-reared juveniles of African catfish Clarias gariepinus. Appl. Anim. Behaviour Sci. 196, 119-112. http://dx.doi.org/10.1016/j.applanim.2017.06.013 [ Links ]
Kobayashi, H., Ohtomi, M., Sekizawa, Y. & Ohta, N., 2001. Toxicity of coelomic fluid of the earthworm Eisenia foetida to vertebrates but not invertebrates: probable role of Sphingomyelin. Comparative Biochemistry Phys. Part C: Toxi. Pharmacology 128, 401-411. https://doi.org/10.1016/S1532-0456(00)00213-1 [ Links ]
Kouba, A., Lunda, R., Hlaváč, D., Kuklina, I., Hamáčková, J., Randák, T., Kozák, P., Koubová, A. & Buřič, M.,, 2018. Vermicomposting of sludge from recirculating aquaculture system using Eisnia andrei: Technological feasibility and quality assessment of end products. J. Clean. Prod. 177, 665-673. https://doi.org/10.1016/jJclepro.2017.12.216 [ Links ]
Limbu, S.M., 2020. The effects of on-farm produced feeds on growth, survival, yield and feed cost of juvenile African sharptooth catfish (Clarius gariepinus). Aquaculture Fisheries 5, 58-64. https://doi.org/10.1016/j.aaf.2019.07.002 [ Links ]
Limbu, S.M., Shoko, A.P., Lamtane, H.A., Shirima, E.D., Kishe-Machumu, M.A., Mgana, H. F. & Mgaya, Y.D. 2015. Effect of initial stocking of the predatory African sharptooth catfish (Clarius gariepinus) on recruits, growth performance, survival, yield of mixed-sex Nile tilapia (Oreochromis niloticus) in concrete tank culture system. Int. Aquat. Res. 7, 63-73. DOI: 10.1007/s40071-014-0093-3 [ Links ]
Loh, T.C., Lee, Y.C., Liang, J.B. & Tan, D., 2005. Vermicomposting of cattle and goat manure by Eisenia foetida and their growth and reproduction performance. Bioresour. Technol. 69, 111-114. doi: 10.1016/j.biortech.2003.03.001 [ Links ]
Loveline, O.C., Samuel, P.O., Arimoro, F.O., Ayanwale, A.V., Auta, Y.I. & Muhamed, A., 2018. Effects of lead nitrate on catalase production levels in post juvenile Clarius gariepinus (Burchell, 1822). Int. J. Fish. Aquat. 10, 1-7. DOI: 10.5897/IJFA2016.0611 [ Links ]
Mair, G.C. & Little, D.C., 1991. Population control in farmed tilapia. NAGA, ICLARM Quarterly. 17, 8-13. [ Links ]
Minshull, J.L., 1969. An introduction of the food web of Lake Sibiya, Northern Zululand. In: M.N. Bruton, (ed.) The food and feeding behaviour of Clarias gariepinus (Piscess clariidae) in Lake Sibaya South Africa, with emphasis on its role as a predator of cichlids. Zool. Soc. London 35, 47-114. [ Links ]
Mohanta, K.N., Subramanian, S. & Korikanthimath, V.S., 2016. Potential of earthworm (Eisenia foetida) as dietary protein source for rohu (Labeo rohita) advanced fry. Cogent Food & Agriculture 2, 1138594. http://dx.doi.org/10.1080/23311932.2016.1138594 [ Links ]
Musa, S.M., Aura, C.M., Ogello, E.O., Omondi, R., Charo-Karisa, H. & Munguti, J.M., 2013. Haematological response of African catfish (Clarias gariepinus Burchell 1822) fingerlings exposed to different concentrations of tobacco (Nicotiana tobaccum) leaf dust. ISRN Zool. 2013, 1-7. http://dx.doi.org/10.1155/2013/492613 [ Links ]
Musyoka, S.N., Liti, D.N., Ogello, E. & Waidbacher, H., 2019. Utilisation of the earthworms, Eisenia foetida (Savigny, 1826) as an alternative protein source in fish feeds processing: A review. Aquaculture Research 50, 2301-2315. DOI: 10.1111/are.14091 [ Links ]
Musyoka, S.N., Liti, D., Ogello, E.O., Meulenbroek, P.M. & Waidbacher, H., 2020. Earthworm, Eisenia foetida, bedding meal as potential cheap fishmeal replacement ingredient for semi-intensive farming of Nile tilapia, Oreochromis niloticus. Aquaculture Research 51, 2359-2368. DOI: 10.1111/are.14579 [ Links ]
Naumowicz, K., Pajdak, J., Terech-Majewska, E. & Szarek, J., 2017. Intracohort cannibalism and methods for its mitigation in cultured freshwater fish. Rev. Fish Biol. Fish. 27, 193-208. https://doi.org/10.1007/s11160-017-9465-2 [ Links ]
Ng, W-K., Liew, F-L., Ang A-L. & Wong, K-W., 2001. Potential of mealworm (Tenebrio molitor) as alternative protein source in practical diets for African catfish, Clarias gariepinus. Aquaculture Research 32, 273-280. https://doi.org/10.1046/j.1355-557x.2001.00024.x [ Links ]
NRC, 2011. Nutrient requirements of fish and shrimp. National Academy Press, Washington D.C. P. 405. [ Links ]
Ogunji, J.O., Iheanacho, S.C., Abe, G.A. & Ikeh, O. R. 2020. Assessing effects of substituting dietary fish meal with boiled donkey and cow blood meal on growth performance and digestive enzyme activities of Clarias gariepinus juvenile. World Aquaculture Society 51, 1066-1079. [ Links ]
Ogunji, J., Toor, R.S., Schulz, C. & Kloas, W., 2008. Growth performance, nutrient utilization of Nile Tilapia Oreochromis niloticus fed housefly maggot meal (Magmeal) diets. Turkish J. Fish. Aquat. Sci. 8, 141-147. [ Links ]
Oke, V., Odountan, H.O. & Abou, Y., 2016. Chicken viscera meal as a main component in diet for African catfish Clarias gariepinus (Burchell 1822) reared in earthen ponds. J. Food Nutri. Res. 4, 799-805. DOI: 10.12691 /jfnr-4-12-6 [ Links ]
Pereira, J.O. & Gomes, E.F., 1995. Growth of Rainbow trout fed a diet supplemented with earthworms, after chemical treatment. Aquac. Int. 3, 36-42. https://doi.org/10.1007/BF00240919 [ Links ]
Russell, D.J., Thuesen, P.A. & Thomson, F.E., 2012. A review of the biology, ecology, distribution and control of Mozambique tilapia Oreochromis mossambicus (Peters 1852) (Pisces: Cichlidae) with particular emphasis on invasive Australian populations. Rev. Fish Biol. Fish. 22, 533-554. https://doi.org/10.1007/s11160-011-9249-z [ Links ]
Shiau, S-Y. & Yi, Y-P., 1999. Dietary supplementation of chitin and chitosan depresses growth in tilapia, Oreochromis niloticus X O. aureus. Aquac. 97, 439-446. https://doi.org/10.1016/S0044-8486(99)00177-5 [ Links ]
Skelton, P.H., 2001. A complete guide to the freshwater fishes of Southern Africa. Struik, Cape Town, South Africa. P. 395 [ Links ]
Smith, C. & Reay, P., 1991. Cannibalism in teleost fish. Rev. Fish Biol. Fish. 1, 41-61. https://doi.org/10.1007/BF00042661 [ Links ]
Tacon, A.G.J., Stafford, E.A. & Edwards, E.A., 1983. A preliminary investigation of the nutritive value of three terrestrial lumbricid worms for rainbow trout. Aquaculture 3, 187-199. https://doi.org/10.1016/0044-8486(83)90090-X [ Links ]
Tadesse, Z., 2019. Effect of different levels of protein diets on growth performance of African catfish (Clarias gariepinus, Burchell, 1822) fingerlings in tanks. Ethopian J. Biol. Soc. 18, 109-122. [ Links ]
Toutou, M.M., Soliman, A.A., Abd Elnabi, H.E., Abouelwafa, A.E. & Abdel Rahim, M.M., 2018. Does feeding African catfish, Clarias gariepinus vinegar immersed poultry viscera affect it growth performance, hygienic status and pathogenic bacteria load. Egyptian J. Aquat. Biol. Fish. 22, 61-76. DOI: 10.21608/EJABF.2018.7982 [ Links ]
Wasserman, G.J. & Afonso, L.O.B., 2003. Sex reversal in Nile tilapia (Oreochromis niloticus) Linnaeus by androgen immersion. Aquac. Res. 34, 65-71. https://doi.org/10.1046/j.1365-2109.2003.00795.x [ Links ]
Submitted 19 July 2021
Accepted 5 December 2021
Published 25 April 2022
# Corresponding author: mymanganeng@yahoo.com














