Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Animal Science
On-line version ISSN 2221-4062
Print version ISSN 0375-1589
S. Afr. j. anim. sci. vol.52 n.2 Pretoria 2022
http://dx.doi.org/10.4314/sajas.v52i2.6
Effect of acute heat stress on production performance and egg quality in four strains of chickens
M. Ghoname; A. Elnaggar; S. Hassan; W. Habashy#
Department of Animal and Poultry Production, Faculty of Agriculture, Damanhour University, Damanhour 22511, Egypt
ABSTRACT
The objective of this study was to investigate differences between four strains of chickens on laying hen productivity and egg quality under acute heat stress (AHS). The study consisted of 44 hens from each of the Fayoumi (FY), Golden Sabahia (GS), White Leghorn (WL) and Lohman Brown (LB) strains that were 30 weeks old. They were allotted to two groups (a thermoneutral temperature regime (26.0 ± 1 °C) and the AHS regime (35 ± 1 °C) and 55 ± 5% relative humidity) for six hours. Egg number and feed intake were recorded. After imposition of the AHS, four hens per strain per treatment were randomly selected and slaughtered to calculate the relative mass of various organs and the number of follicles. Acute heat stress decreased feed intake of all strains significantly. There was a significant interaction between temperature treatment and strain on feed intake, egg weight, surface area of egg, yolk index, yolk weight, and albumen weight and on the relative weights of the various organs, except for the gizzard. Thus, the magnitude of the effects of AHS was strain dependent, with GS seemingly being less affected than the other strains.
Keywords: exposure, Fayoumi, Haugh unit, Lohman Brown, organ weight
Introduction
The environmental temperature increased by 1.53 °C from 2006 to 2015 compared with 1850 to 1900 (IPCC, 2019). As a result of global warming, extreme climatic events such as extreme heat will become more frequent and severe, particularly in tropical areas, and studies that examine the impact of climatic changes on animal production are essential. Heat stress has become one of the most important challenges affecting the chicken industry (Ayo et al., 2011). In the United States, loss in the layer industry because of heat stress is estimated to be 61.4 million dollars per year (St-Pierre et al., 2003). These losses occur because heat stress causes considerable reduction in egg weight, shell thickness, and rate of production in laying hens (Wolfenson et al., 2001; Huang et al., 2015; El-Kholy et al., 2017).
Responses to heat stress vary among chickens with different genetic origins (Franco-Jimenez et al., 2007; Star et al., 2008). Hy-Line W-98 laying hens consumed less feed and had lower mortality during heat stress than Hy-Line W-36 and Hy-Line Brown laying hens (Franco-Jimenez et al., 2007). The Hy-Line W-98 egg production strain also returned to baseline faster than the other strains when recovering from periods of heat stress.
Egypt has a diverse population of native chickens with a wide range of phenotypic traits such as differential growth rate, bodyweight, and reproductive performance. These local chicken breeds in tropical areas are more adaptable to harsh environmental conditions (El Nagar & Ibrahim, 2007). For example, FY and GS are native Egyptian dual-purpose breeds. The FY originated in the Fayoumi region, which is located southwest of Cairo and west of the Nile. An advanced intercross of FY and a commercial broiler line showed genomic regions attributable to FY, indicating adaptation to heat stress (Bjorkquist et al., 2015). Golden Sabahia chickens are a synthetic line that originated from crossing four local strains (Silver Montazah, Golden Montazah, Mandarah and Bahij) and one commercial strain Lohman Brown (LB) (Ghanem et al., 2017). Lohman Brown is a commercial breed that is raised specifically for egg production. White Leghorn is an exotic Mediterranean breed that is used for egg production and has been acclimated to the Egyptian climate for many years (Hosny, 2006). It accounts for at least 90% of the world's white-egg production. It is lightweight and has a large, red, single comb (Riggs et al., 2011).
Little is known about the differences in laying hen productivity and egg quality between commercial, standard, developed, and native laying hens during AHS. Therefore, the current research study was designed to investigate the effect of genetic variation on laying hen productivity and egg quality during AHS.
Material and Methods
All experimental procedures were approved by Animal and Poultry Production Scientific and Ethics Committee, Faculty of Agriculture, Damanhour University, Egypt. The handling and care of the animals were performed to maintain their welfare according to International guidelines for research involving animals (Directive 2010/63/EU).
A total of 176 laying hens (44 from each strain) were used in this study. The 30-week old hens were housed in individual laying cages (20 χ 45 χ 40 cm) by strain at the Poultry Unit of Faculty of Agriculture, Damanhour University. Within each strain, hens were randomly divided into two equal groups. Group 1 was kept under thermo-neutral temperature (26.0 ± 1 °C) and Group 2 was exposed to heat stress (35 ± 1 °C) with relative humidity (55 ± 5%) for six hours. During the egg production period, all birds were fed ad libitum a diet containing 18% crude protein and 2.8 Kcal ME/Kg feed, 3.5 % calcium and 0.5% available phosphorus. Light was provided 16 hours per day during laying period.
Feed intake was determined by the weight of a level scoop of feed provided to each bird. Feed refusal was recorded the second day of the AHS period. The feed conversion ratio was calculated by dividing the feed intake by the egg mass,. Eggs were collected the next day of heat stress and weighed between 0900 and 1000 hours each day. The egg production percentage was calculated by dividing the total number of eggs produced by the number of birds in the treatment at the beginning of the period, and egg mass was calculated by multiplying the average egg weight with egg production. Expected egg weight was calculated by regression analysis. Residual egg weight was calculated as the difference between observed egg weight and expected egg weight for each experimental hen using the PROC REG procedure of SAS 9.4 (SAS Institute Inc., Cary, North Carolina, USA).
On the second day, eight eggs were collected for each strain and used to determine egg weight, shell weight, and yolk weight. After the shell membrane had been removed, the thickness of the shell was measured with a micrometer at the blunt end, middle, and sharp point of the egg and these measures were averaged to determine shell thickness. To determine yolk colour, a Roche colour fan made up of 15 coloured plastic strips was used as a reference. A tripod micrometer was used to measure the height of the yolk and albumen. The albumen weight was calculated by subtracting the yolk and shell weights from the egg weight. Vernier callipers were used to measure egg length, egg width, and yolk width with a minimum count of 0.01 mm. (Duman et al., 2016). The egg shape index was computed following Duman et al. (2016). The yolk index indicated the proportional relationship of yolk height and yolk width. Monira et al.'s (2003) formula was used to calculate the average Haugh unit value. The surface area of egg was calculated according to Carter (1975):
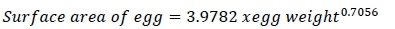
After six hours of heat stress, four hens per strain per treatment were weighed individually and slaughtered. Body organ measurements were calculated as a percentage of live bodyweight. After complete bleeding, the carcasses were eviscerated and the liver, spleen, heart, pancreas, intestine, proventriculus, gizzard, ovary and oviduct were weighed to the nearest 0.1 g. The organ weights were expressed as the percentage of live weight. The numbers of large, medium and small follicles were counted.
The statistical analysis was performed with the general linear model procedure of SAS version 9.4 (SAS Institute Inc., Cary, North Carolina, USA). The statistical model used in this study was:
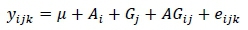
where: yijk= an observation from the kth bird, μ = general mean, Ai= the effect of the ith treatment, Gj = the effect of the jth strain, AGiy= the interaction between heat stress and strain, and eijk= the random error. Statistical significance of the effects was assessed at a P-value of 0.05. Means were separated using Tukey's test.
Results and Discussion
The interaction of treatment and strain was significant only for feed intake, with feed intake by the GS strain being less affected by AHS than the other strains (4.91% vs 16.18%, 12.43% and 27.48% in the LB, FY and WL birds, respectively. Previous studies also documented that, the consumption of feed and appetite are reduced in laying hens under exposure to heat stress (Attia et al., 2016; Franco-Jimenez et al., 2007; Mashaly et al., 2004). The observed reduction in feed intake may be because the hens attempt to maintain homoeostasis by reducing their heat production (Etches et al., 2008). Differences among strains were evident for the other traits. Table 1 shows the effects of thermal treatment and strain of laying hen on production performance.
The egg weight of LB hens was greater than for the other strains. Fayoumi hens had lower egg production, egg weight, egg mass and worse feed conversion than the other strains. In addition, egg production and egg mass had a tendency to decrease (P <0.05) under AHS. Although egg production was not affected significantly by the AHS treatment, egg production dropped 17.65% in LB, 23.21 % in GS, 15.15 % in FY and 28.57% in WL. Mack et al. (2013) also reported that there was no interaction between temperature and strain on egg production. Barrett et al. (2019) found heat stress lowered feed intake. With decreased feed intake, birds may divert energy from biological functions such as reproduction to maintain physiological integrity (Mumma et al., 2006). This could imply that laying hens subjected to AHS try to conserve metabolic energy to adapt. Sandercock et al. (1995) demonstrated that the degree of thermo-tolerance could vary depending on the strain, in agreement with the current findings.
The interaction of strain and thermal treatment was significant for five of the 15 characteristics of egg quality, namely egg weight, yolk weight, albumen weight, yolk index and surface area of the egg. Egg weight and surface area of the LB hens was depressed by AHS, but not changed significantly for the other strains (Tables 2 and 3). The yolk weight of eggs from LB hens was also depressed by AHS, but unchanged in GS and WL (Table 2). Decreased egg weight was observed to accompany changes in the eggshell (Rostagno, 2020; Nawab et al., 2018). Although the shell thickness of eggs was lower than control, reduction was least in Golden Sabahi hens (0.03 mm) compared with the 0.07 mm reduction in LB and FY under the same conditions Based on these findings, GS appeared to be more heat resistant than other genotypes. Interestingly, in FY the yolk weight was depressed by AHS, but the albumen weight was increased and GS also showed increased albumen weight Table 2). These changes resulted in FY having a reduced yolk index under AHS, whereas the other strains were not similarly affected (Table 3).
The strains also differed significantly in egg length and width, yolk width, yolk height and shell weight (Tables 2 and 3). Fayoumi hens produced smaller eggs with lighter shells and less internal content than the other strains. Some studies showed that genotype and heat stress interacted to affect egg quality (Franco-Jimenez et al., 2007; Sokotowicz et al., 2019; Sõzcü et al., 2021). Sokotowicz et al. (2019) confirmed the significant effect of genotype on egg quality by observing that commercial layers produced heavier eggs than native Greenleg Partridge hens. Franco-Jimenez et al. (2007) showed that Hy-Line W-36 hens produced smaller eggs, albumen, and shell weight than Hy-Line Brown and Hy-Line W-98 hens. The present findings revealed that the most of the egg quality traits of GS hens were most similar to LB. These results may be a consequence of the genetic makeup of GS, which was developed through a crossbreeding programme and subject to genetic selection. Although the egg quality was consistently lower of all traits in the AHS group, the effect was not significant (Table 2; Table 3).
The interaction of strains with the thermal environmental treatments was significant for all of the relative organ weights except the gizzard (Table 4). White Leghorn hens exposed to AHS had significantly heavier liver and pancreas weights relative to their bodyweight than the other groups. However, the LB strain showed an increase in relative weight of the pancreas under AHS. The relative weight of the intestine in LB increased under AHS, whereas in FY it decreased and was unchanged in GS and WL. Lohman Brown hens showed a relatively larger increase in the relative weight of the proventriculus compared with GS, FY and WL. The heart increased in size as a proportion of bodyweight in LB, GS, and WL birds that were subject to AHS, but decreased in relative size in FY in response to the AHS treatment. Fayoumi and WL hens exposed to AHS had a significantly greater increase in abdominal fat percentage under AHS than LB and GS.
Golden Sabahi and White Leghorn kept under control and Fayoumi hens kept under AHS had significantly heavier ovary percentages. Golden Sabahia hens under control and Fayoumi hens under AHS had higher numbers of large follicles than the other groups (Table 5). Golden Sabahia hens kept under AHS had a significantly larger number of medium follicles than the other groups. Lohman Brown, GS and FY hens kept under control had significantly larger numbers of small follicles than the other groups. The data indicated that heat stress had detrimental effects on the physiology of laying hens because of genetic variations.
The relative weight of internal organs differed significantly among chicken breeds (Dror et al., 1977; Al-Marzooq et al., 2020). The present study indicated that the differences among strains may in part be due to the environmental situation in which they are raised. In poultry, the liver has an essential role in synthesizing fatty acids (Emami et al., 2020) and maintaining homeostasis (Jastrebski et al., 2017). Increased transport of very low density lipoprotein to adipose tissue appears to match the increased rate of lipogenesis during the acute adaptation to heat stress (Lu et al., 2019). Based on the results of the present study, it seems that increased liver percentage under AHS might be because of increased lipogenesis. Osman & Tanios (1983) reported that under heat stress the pancreas increases biosynthesis and decreases the rate of secretion of amylase to the intestine. From this experiment, the relative weight of the pancreas increased after six hours of AHS, suggesting an increase in the biosynthesis of amylase to increase the metabolic rate. Furthermore, the increase in relative weight of the heart in hens exposed to AHS may be a result of the need for increased blood circulation in order to release heat.
Similar to the metabolic organs, the reproductive organs were greatly influenced by the interaction of the imposed thermal treatments and the strains of birds that were used in this study (Table 5). Lohman Brown hens kept under AHS had a significantly higher oviduct percentage than the other groups. The effect of AHS was to decrease the count of small yellow follicles markedly in all strains except WL. These small follicles are the basis for the hierarchy of follicular development (Rangel et al., 2014) and can affect the rate of future egg production. In GS the count of medium-sized follicles increased with AHS. However, the numbers of medium sized follicles were not greatly affected in the LB, FY and WL strains. The large follicle count was greatly reduced with AHS in GS and WL, but increased in LB and FY. Thus, based on the present results, the slightly reduction of egg production by LB and GS might be expected because of AHS, but FY and WH would probably be less affected.
Nitta et al. (1991) reported that more than 80% of total ovarian oestrogen is produced by small follicles, and thus these follicles regulate reproductive tract growth and development (Campbell et al., 2003). In laying chickens, granulose cells in large follicles release progesterone (Porter et al., 1991), which is highly correlated with egg production (Adeyinka et al., 2007). Reduced reproductive efficiency in hens exposed to AHS may be caused by problems with ovarian function.
Conclusions
Responses to AHS varied by strain. Acute heat stress had a negative impact on egg production and quality in four genetically diverse strains. Lohman Brown exhibited the greatest response to AHS in feed intake, egg quality and the number of small follicles, with the other strains showing lower responses. The underlying molecular and cellular mechanisms should be investigated further.
Acknowledgments
The authors are grateful for the support by the Science, Technology and Innovation Funding Authority (STDF) (Project Accession No. 42872).
Authors' Contributions
Conceptualization and design of study, WSH (ORCID ID 0000-0002-2009-5145); methodology, formal analysis, and data curation, WSH, ASE, MGG and SSH; original draft preparation, WSH; review and editing, WSH, ASE and SSH. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest Declaration
The authors have declared that there are no competing interests.
References
Adeyinka, F.D., Eduvie, L.O., Adeyinka, I.A., Jokthan, G.E. & Orunmuyi, M., 2007. Effect of progesterone secretion on egg production in the Grey Helmet Guinea Fowl (Numida meleagris galleata). Pakistan J. Biol. Sci. 10, 998-1000. DOI: 10.3923/pjbs.2007.998.1000 [ Links ]
Al-Marzooq, W., Al-Kharous, K., El Tahir, Y. & Johnson, E.H., 2020. Microbial community dynamics in the gastrointestinal tract of indigenous Omani chickens. Int. J. Poult. Sci. 19, 309-320, DOI: 10.3923/ijps.2020.309.320 [ Links ]
Attia, Y.A., Abd El-Hamid, A., Abedalla, A.A., Berika, M.A., Al-Harthi, M.A., Kucuk, O., Sahin, K. & Abou-Shehema, B.M., 2016. Laying performance, digestibility and plasma hormones in laying hens exposed to chronic heat stress as affected by betaine, vitamin C, and/or vitamin E supplementation. SpringerPlus 5(1), 1619. https://doi.org/10.1186/s40064-016-3304-0. [ Links ]
Ayo, J.O., Obidi, J.A. & Rekwot, P.I., 2011. Effects of heat stress on the well-being, fertility, and hatchability of chickens in the northern Guinea savannah zone of Nigeria: A review. ISRN Vet. Sci. 2011,838606, https://doi.org/10.5402/2011/838606 [ Links ]
Barrett, N.W., Rowland, K., Schmidt, C.J., Lamont, S.J., Rothschild, M.F., Ashwell, C.M. & Persia, M.E., 2019. Effects of acute and chronic heat stress on the performance, egg quality, body temperature, and blood gas parameters of laying hens. Poult. Sci. 98(12),6684-6692. DOI: 10.3382/ps/pez541. PMID: 31573614. [ Links ]
Bjorkquist, A.G., Rothschild, M.F., Persia, M.E., Ashwell, C., Schmidt, C. & Lamont, S.J., 2015. Genetic markers found for response to heat stress in chickens. Animal Industry Report AS 661. https://doi.org/10.31274/ans_air-180814-1318 [ Links ]
Campbell, J.R., Kenealy, M.D. & Campbell, K.L., 2003. Physiology of egg laying. Animal sciences: The biology, care and production of domestic animal, 4th edition. Waveland Press, Long Grove, Illinois, USA. Pp. 283-294. [ Links ]
Carter, T.C., 1975. The hen's egg: Estimation of shell superficial area and egg volume, using measurements of fresh egg weight and shell length and breadth alone or in combination. Brit. Poult. Sci. 16, 541-543. [ Links ]
Dror, Y., Nir I. & Nitsan Z., 1977. The relative growth of internal organs in light and heavy breeds. Br. Poult. Sci. 18, 493496. https://doi.org/10.1080/00071667708416389 [ Links ]
Duman, Μ ., Sekeroglu, A., Yildmm, A., Eleroglu, H.& Camci, Ö., 2016. Relation between egg shape index and egg quality characteristics. Europ. Poult. Sci. 80, 1-9. DOI: 10.1399/eps.2016.117 [ Links ]
El Nagar, A. & Ibrahim, A., 2007. Case study of the Egyptian poultry sector. https://www.fao.org/ag/AGAInfo/home/events/bangkok2007/docs/part1/1_6.pdf [ Links ]
El-Kholy, M.S., El-Hindawy, M.M., Alagawany, M., Abd El-Hack, M.E. & El-Sayed, S.A.A., 2017. Dietary supplementation of chromium can alleviate negative impacts of heat stress on performance, carcass yield, and some blood hematology and chemistry indices of growing Japanese quail. Biol. Trace Elem. Res. 179(1),148-157. DOI: 10.1007/s12011-017-0936-z [ Links ]
Emami, N.K., Jung, U., Voy, B. & Dridi, S., 2020. Radical response: Effects of heat stress-induced oxidative stress on lipid metabolism in the avian liver. Antioxidants 10(1),35. DOI: 10.3390/antiox10010035 [ Links ]
Etches, R.J., John, T.M. & Gibbins, A.M.V., 2008. Behavioral, physiological, neuroendocrine and molecular responses to heat stress. In: N.J. Daghir (ed). Poultry production in hot climates. CABI, Cambridge, UK. [ Links ]
Franco-Jimenez, D.J., Scheideler, S.E., Kittok, R.J., Brown-Brandl, T.M., Robeson, L.R., Taira, H. & Beck M. M., 2007. Differential effects of heat stress in three strains of laying hens. J. Appl. Poult. Res. 16, 628-634. DOI:10.3382/japr.2005-00088 [ Links ]
Ghanem, H.H., EL-Turky, A.I., Abou, El-Ghar, R.Sh., Aly, O.M., Nawar, A.N., Shalan, H.I. & Mahmoud, T.H., 2017. Golden S a b ahia ... a new strain of chickens. E g y pt. Poult. Sci. 37, 57-63. [ Links ]
Hester, P.Y., Muir, W.M., Craig, J.V. & Albright, J.L., 1996a. Group selection for adaption to multi-hen cages: Production traits during heat and cold exposures. Poult. Sci. 75, 1308-1314, DOI: 10.3382/ps.0751308 [ Links ]
Hester, P.Y., Muir, W.M., Craig, J.V. & Albright, J.L., 1996b. Group selection for adaptation to multiple-hen cages: Hematology and adrenal function. Poult. Sci. 75, 1295-1307, DOI: 10.3382/ps.0751295 [ Links ]
Hosny, F.A., 2006. The structure and importance of the commercial and village based poultry systems in Egypt. Poult. Sect. Count. Rev. 1, 39. https://www.fao.org/3/ai355e/ai355e.pdf [ Links ]
Huang, C., Jiao, H., Song, Z., Zhao, J., Wang, X.& Lin, H., 2015. Heat stress impairs mitochondria functions and induces oxidative injury in broiler chickens. J. Anim. Sci. 93, 2144-2153, DOI: 10.2527/jas.2014-8739 [ Links ]
IPCC (Intergovernmental Panel on Climate Change), 2019. Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems (SR2). Working Group III Technical Support Unit, Imperial College, London, England. https://www.ipcc.ch/site/assets/uploads/2018/07/sr2_background_report_final.pdf [ Links ]
Jastrebski, S.F., Lamont, S.J. & Schmidt, C.J., 2017. Chicken hepatic response to chronic heat stress using integrated transcriptome and metabolome analysis. PLoS ONE 12(7), e0181900. https://doi.org/10.1371/journal.pone.0181900 [ Links ]
Lu, Z., He, X.F., Ma, B.B., Zhang, L., Li, J.L., Jiang, Y., Zhou, G.H.& Gao, F., 2019. Increased fat synthesis and limited apolipoprotein B cause lipid accumulation in the liver of broiler chickens exposed to chronic heat stress. Poult. Sci. 98, 3695-3704, https://doi.org/10.3382/ps/pez056 [ Links ]
Mack, L.A., Felver-Gant, J.N., Dennis, R.L. & Cheng, H.W., 2013. Genetic variations alter production and behavioral responses following heat stress in two strains of laying hens. Poult. Sci. 92(2), 285-294. DOI: 10.3382/ps.2012-02589 [ Links ]
Mashaly, M.M., Hendricks, G.L. 3rd, Kalama, M.A., Gehad, A.E., Abbas, A.O. & Patterson, P.H., 2004. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult. Sci. 83(6), 889-894. DOI: 10.1093/ps/83.6.889 [ Links ]
Monira, K.N., Salahuddin, M. & Miah, G., 2003. Effect of breed and holding period on egg quality characteristics of chicken. Int. J. Poult. Sci. 2(4), 261-263. https://scialert.net/abstract/?doi=ijps.2003.261.263 [ Links ]
Mumma, J.O., Thaxton, J.P., Vizzier-Thaxton, Y. & Dodson, W.L., 2006. Physiological stress in laying hens. Poult. Sci. 85,761-769. https://doi.org/10.1093/ps/85.4761 [ Links ]
Nawab. A., Ibtisham, F., Li, G., Kieser, B., Wu, J., Liu, W., Zhao, Y., Nawab,, Y., Li K., Xiao, M. & An, L., 2018. Heat stress in poultry production: Mitigation strategies to overcome the future challenges facing the global poultry industry. J. Therm. Biol. 78, 131-139. https://doi.org/10.1016/j.jtherbio.2018.08.010 [ Links ]
Nitta, H., Osawa, Y. & Bahr, J.M., 1991. Immunolocalization of steroidogenic cells in small follicles of chicken ovary, anatomical arrangement and location of steroidogenic cells change during follicular development. Domest. Anim. Endocrinol. 32,190-200. DOI: 10.1016/0739-7240(91)90028-i [ Links ]
Osman, A.M. & Tanios, N.I., 1983. The effect of heat on the intestinal and pancreatic levels of amylase and maltase of laying hens and broilers. Comp. Biochem. Physiol. 75A, 563-567. DOI: 10.1016/0300-9629(83)90421-8 [ Links ]
Porter, T.E., Hargis, B.M., Silsby, J.L. & El Halawani, M.E., 1991. Characterization of dissimilar steroid production by granulosa, theca internal and theca external cells during follicular maturation in the turkey (Meíeagris gaííopavo). Gen. Comp. Endocrinol. 84, 1-8. DOI: 10.1016/0016-6480(91 )90058-e [ Links ]
Rangel, P.L., Rodríguez, A., Gutiérrez, K., Sharp, P.J.& Gutierrez, C.G., 2014. Subdominant hierarchical ovarian follicles are needed for steroidogenesis and ovulation in laying hens (Gaííus domesticus). Anim. Reprod. Sci. 147(3-4)144-153. DOI: 10.1016/j.anireprosci.2014.04.011 [ Links ]
Riggs, P., Willis, K. & Ludlow, R., 2011. Keeping chickens for dummies. John Wiley & Sons UK. https://www.wiley.com/en-us/Keeping+Chickens+For+Dummies%2C+UK+Edition-p-9781119994183 [ Links ]
Rostagno, M.H., 2020. Effects of heat stress on the gut health of poultry. J. Anim. Sci. 98, skaa090. DOI: 10.1093/jas/skaa090 [ Links ]
Sandercock, D.A., Mitchell, M.A., & MacLeod, M.G. 1995. Metabolic heat production in fast and slow growing broiler chickens during acute heat stress. Brit. Poult. Sci. 36(5), 868. [ Links ]
Sokotowicz, Z., Dykiel, Μ ., Krawczyk, J. & Aug ustynska-Prejsnar, A., 2019. Effect of layer genotype on physical characteristics and nutritive value of organic eggs. CyTA - J. Food 17,(1), 11-19, DOI:10.1080/19476337.2018.1541480 [ Links ]
Sözcü, A., Iρek, A., Oguz, Z., Gunnarsson, S. & Riber, A.B., 2021. C om ρ arison of ρ erformance, e g g quality, and yolk fatty acid profile in two Turkish genotypes (Atak-S and Atabey) in a free-range system. Animals 11, 1458. https://doi.org/10.3390/ani11051458. [ Links ]
Star, L., Kemp B., van den Anker I. & Parmentier, H.K., 2008. Effect of single or combined climatic and hygienic stress in four layer lines. 1. Performance. Poult. Sci. 87, 1022-1030, https://doi.org/10.3382/ps.2007-00142 [ Links ]
St-Pierre, N.R., Cobanov, B. & Schnitkey, G., 2003. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 86, 52-77. https://doi.org/10.3168/jds.S0022-0302(03)74040-5 [ Links ]
Wolfenson, D., Bachrach, D., Maman, M., Graber, Y. & Rozenboim, I., 2001. Evaporative cooling of ventral regions of the skin in heat-stressed laying hens. Poult. Sci. 80, 958-964. https://doi.org/10.1093/ps/80.7.958 [ Links ]
Submitted 28 October 2021
Accepted 17 January 2022
Published 19 April 2022
# Corresponding author: walidh55@gmail.com; Walid.habashi@agr.dmu.edu.eg














